Substance & Its Nature | Famous Books for UPSC Exam (Summary & Tests) PDF Download
Introduction
The branch of science that studies the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions. The French chemist Antoine Lavoisier, who lived from 1743 to 1793, is often called the father of modern chemistry because of his contributions to the field.
Substance and Its Nature
Matter refers to anything that occupies space, has mass, and can be detected by our senses.
- Ancient Indian philosophers believed that matter is made up of five basic elements, known as the Panch Tattva. air, earth, fire, sky, and water. They thought that everything in the universe, both living and non-living, is composed of these elements. Similarly, ancient Greek philosophers had a comparable understanding of matter.
- Indian sage Maharishi Kanada was one of the first to suggest that all matter is made up of tiny particles called anu, which can be further divided into even smaller particles called paramanu.
- The Greek philosopher Democritus later referred to these small particles as atoms, derived from the Greek word atomos, meaning "uncut" or "indivisible." This concept implies that matter is composed of tiny, indivisible particles known as atoms.
- There are three primary states of matter: solid, liquid, and gas. Additionally, there are more advanced states of matter, such as plasma and Bose-Einstein condensate.
Plasma State
- The plasma state is made up of highly energetic and excited particles.
- These particles exist in the form of ionized gases.
- Plasma can occur when matter is heated to a very high temperature.
- At elevated temperatures, atoms lose electrons and become ions, resulting in the formation of plasma, which consists of free, highly energetic charged particles.
- Fluorescent tubes and neon sign bulbs contain inert gases. when an electric current passes through these gases, they create glowing plasma, which exhibits a characteristic color depending on the type of gas.
- The glow of CFL tubes is due to the presence of plasma.
Bose-Einstein Condensation State
- In 1920, Satyendra Nath Bose proposed the concept of a fifth state of matter through statistical calculations.
- Subsequently, scientists in America were able to successfully create this state.
- The Bose-Einstein state is observed when a dilute gas of bosons is cooled to temperatures close to absolute zero, at approximately one hundred thousandth of the density of normal air.
- This phenomenon is known as Bose-Einstein Condensation, and the resulting state of matter is called Bose-Einstein Condensate (BEC).
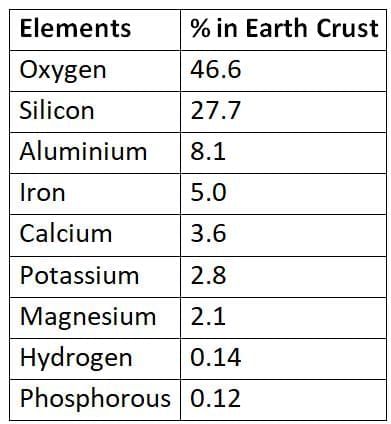
Approximate Relative Abundance of Some Elements in the Earth’s Crust
Pure Substances
- A pure substance is a type of matter that is uniform and consistent throughout, and it cannot be separated into different kinds of matter by any physical means.
- There are two main categories of pure substances: elements and compounds.
Elements
- An element is the simplest form of matter that cannot be broken down into simpler substances by ordinary physical or chemical processes. Elements are the building blocks of all matter.
Classification of Elements
1. Metals
- Metals are typically solid at room temperature, with the exception of mercury, which is a liquid.
- They are generally hard and have a shiny appearance.
- Metals melt and boil at high temperatures.
- They are excellent conductors of electricity.
- Metals can be easily shaped (malleability) and stretched into wires (ductility).
- Examples of metals include: Iron, Copper, Silver, Gold, Aluminium, Zinc, and more.
2. Non-metals
- Non-metals can exist in all states of matter. For instance:
- Gases: Oxygen and Nitrogen
- Solids: Sulphur and Carbon
- Liquid: Bromine
- Non-metals are usually soft and do not have a shiny appearance.
- They lack the metallic properties of malleability, ductility, and conductivity, with the exception of graphite, which can conduct electricity.
- Examples of non-metals include: Hydrogen, Carbon, Oxygen, Iodine, Sulphur, Phosphorus, and others.
Metalloids
In chemistry, metalloids and their compounds are crucial because they play a key role in many chemical reactions. Metalloids are elements that have characteristics of both metals and non-metals. Some examples of metalloids include Arsenic, Antimony, and Bismuth.
Compounds
Compounds are substances that consist of two or more different elements combined in a specific ratio by mass. The properties of a compound are distinct from those of the individual elements that make it up. For instance, water, various sugars like glucose, salt, chloroform, alcohol, and ethers are all examples of compounds.
Classification of Compounds
- Organic Compounds: These compounds originate from living organisms. However, the term "organic" now also encompasses hydrocarbons and their derivatives. Examples include carbohydrates, proteins, oils, and fats.
- Inorganic Compounds: These are compounds sourced from non-living entities such as rocks and minerals. Examples include common salt, marble, and washing soda.
Understanding Mixtures
A mixture is created when two or more substances are combined in any proportion, and the properties of each substance remain unchanged. Examples of mixtures include milk, seawater, petrol, paint, glass, cement, and wood.
Mixtures can be classified into two types:
- Homogeneous Mixture. In this type of mixture, the composition is uniform, and there are no visible boundaries between its components. The individual parts cannot be seen, even under a microscope. Examples. Common salt dissolved in water, sugar dissolved in water, iodine dissolved in carbon tetrachloride, benzene in toluene, methyl alcohol in water.
- Heterogeneous Mixture. This mixture does not have a uniform composition and displays visible boundaries between its different parts. The components can be seen with the naked eye. Examples. A mixture of sulfur and sand, a mixture of iron filings and sand.
Methods for Separating Mixtures
Here are some methods for separating mixtures:
- Sublimation: This method involves the process where a solid changes directly into vapor without passing through the liquid state. It is effective for separating materials that can sublimate from those that cannot. For instance, camphor and iodine can sublimate, whereas salt and sand cannot.
- Filtration: Filtration is a technique used to remove solid particles from a liquid by passing the mixture through filter paper. This method is commonly used to separate chalk powder from water, sand from water, and in the preparation of tea where tea leaves are filtered out.
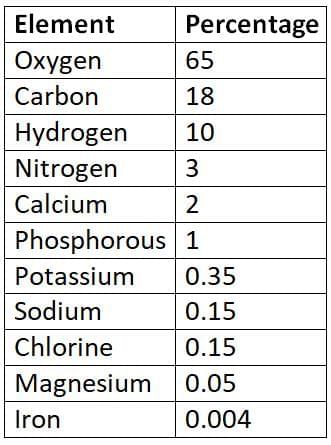
Approximate percentage of elements in the human body
The human body is primarily composed of the following elements in the given approximate percentages:
- Oxygen: 65%
- Carbon: 18%
- Hydrogen: 10%
- Nitrogen: 3%
- Calcium: 1.5%
- Phosphorus: 1%
- Potassium: 0.35%
- Sulphur: 0.25%
- Sodium: 0.15%
- Chlorine: 0.15%
- Magnesium: 0.05%
Additionally, there are trace elements present in minute quantities, such as: Iron, Manganese, Copper, Iodine, Zinc, Cobalt, Molybdenum, Selenium, Fluorine.
Evaporation and Crystallization
Evaporation is the process of transforming a liquid into its vapor at room temperature, leading to a cooling effect. For instance, during summer, water evaporates from ponds, wells, and lakes. Additionally, common salt is produced from seawater through the processes of evaporation and subsequent crystallization.
Crystallization is a technique used primarily for separating and purifying solid substances. The process involves heating an impure solid or mixture with a suitable solvent, such as alcohol, water, acetone, or chloroform, until the solute dissolves. The resulting clear solution is then cooled slowly below its boiling point, allowing pure solid crystals to form. These crystals are collected through filtration and dried.
In cases of more complex mixtures, fractional crystallization is employed. This method separates components based on their varying solubilities, causing them to crystallize at different temperatures.
Distillation
Distillation is a process used to separate a liquid from a mixture by heating it to create vapour and then cooling the vapour to form a liquid again. This process includes two main steps: vaporization and condensation.
Distillation = Vaporization + Condensation
Distillation is useful for separating liquids with different boiling points or for separating a liquid from non-volatile solids that are dissolved or suspended in it.
Example
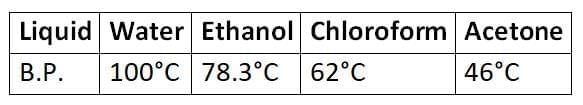
- For instance, a mixture of copper sulfate and water or a mixture of water (boiling point 100°C) and methyl alcohol (boiling point 45°C) can be separated using distillation. (B.P. stands for boiling point)
Fractional Distillation
Fractional distillation is an advanced method of separation that utilizes a fractionating column to separate two or more liquids with different boiling points. This technique is particularly effective for liquids that are close in boiling temperatures.
- Example 1: Methyl alcohol (boiling point = 337 K) and acetone (boiling point = 329 K) can be effectively separated using fractional distillation due to their differing boiling points.
- Example 2: Fractional distillation is employed to separate various components from crude petroleum, including petrol, diesel oil, kerosene oil, and heavy oil.
- Example 3: This process is also used to separate gases such as oxygen, nitrogen, rare gases, and carbon dioxide from liquid air.
Chromatography
Chromatography is a technique used to separate and analyze the components of a mixture based on their different affinities for an absorbent material. The word "chromatography" comes from the Latin word "chroma," meaning "color," as the method was initially used to separate colored substances. In chromatography, the components of a mixture are carried by a solvent or gas and interact with an absorbent material at different rates, allowing them to be separated and identified.
Types of Chromatography
- Column (absorption) chromatography
- Thin layer chromatography
- Paper chromatography
- High-performance liquid chromatography (HPLC)
- Ion-exchange chromatography
- Gas chromatography
Sedimentation and Decantation
Sedimentation and Decantation are methods used to separate mixtures of liquids and insoluble solids.
- Sedimentation occurs when a mixture, like muddy water, is left undisturbed. The heavier insoluble solids, such as dirt, clay, and sand, settle at the bottom of the container due to gravity.
- Decantation is the process of carefully pouring off the clear liquid that sits on top of the settled solids after sedimentation. This method is effective because the insoluble solid, being heavier, sinks to the bottom, allowing the liquid to be separated easily.
Change in State
- Melting Point: The melting point is the specific temperature at which a solid transforms into a liquid under normal pressure. For instance, ice melts at 0°C, while Sodium Chloride (NaCl) melts at around 801°C. Impurities can significantly alter the melting point of a substance. For example, a mixture of ice and salt, known as a freezing mixture, has a melting point of –15°C.
- Boiling Point: The boiling point is the temperature at which a liquid changes into vapour under normal atmospheric pressure. When atmospheric pressure is lower, the boiling point decreases. Soluble impurities can raise the boiling point of a liquid.
- Freezing Point: The freezing point is the constant temperature at which a liquid solidifies by releasing heat energy. For water, the freezing point is 0°C.
- Evaporation: Evaporation is the process by which a liquid changes into vapours at room temperature. This process cools the liquid because higher-energy molecules escape from the surface, lowering the average energy of the remaining molecules. Factors affecting evaporation include the nature of the liquid, temperature, and surface area.
- Vapour Pressure: Vapour pressure is the pressure exerted by the vapours of a liquid in equilibrium with the liquid at a specific temperature. It depends on the nature of the liquid. A higher vapour pressure indicates weaker intermolecular forces in the liquid and increases with temperature.
|
743 videos|1444 docs|633 tests
|
FAQs on Substance & Its Nature - Famous Books for UPSC Exam (Summary & Tests)
| 1. What is the definition of substance in a scientific context? |  |
| 2. How does the nature of substances differ between elements and compounds? |  |
| 3. What are the physical and chemical properties of substances? |  |
| 4. What role do substances play in chemical reactions? |  |
| 5. How can substances be classified based on their physical states? |  |
















