Surface Tension, Surface Energy & Angle of Contact | Physics Class 11 - NEET PDF Download
Surface Tension
- Surface tension is the phenomenon observed when the surface of a liquid interacts with another phase, which could also be another liquid. It causes liquids to minimize their surface area, making their surface resemble an elastic sheet.
- Imagine a line XY on the surface of the liquid in equilibrium; at every point on this line, equal and opposite forces act, stretching the surface uniformly in both directions.
- In equilibrium, the force acting per unit length on an imaginary line on the liquid's surface, perpendicular to the line and in the direction of the surface's tangent line, is termed as Surface Tension.
- Surface tension is typically represented by symbols like σ or T.
Cohesion and Surface Tension
- Surface Tension arises in liquids due to cohesion forces between their molecules. Cohesion force represents the attractive force between particles in solids and liquids, essential for maintaining their structural integrity.
- Surface Tension, a property of substances, is a result of cohesion forces and acts to uphold the surface structure against external forces.
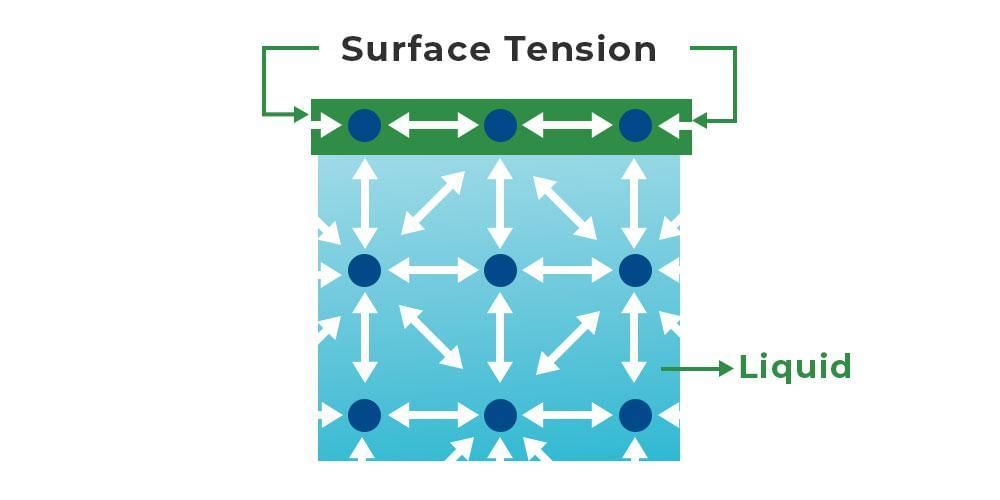
Surface Tension at Molecular Level
- Water molecules exhibit cohesion forces, causing them to adhere to one another. In a liquid, bottom-layer molecules have surrounding molecules for cohesion, while top-layer molecules lack this support.
- Internal liquid molecules experience balanced forces from all sides, leading to force cancellation. In contrast, surface molecules encounter a predominant inward force, contributing to the high surface tension of water.
Formula for Surface Tension Surface tension, mathematically defined, represents the force (F) acting on a surface divided by the length (l) of that surface:
T = F / l
Another perspective on surface tension is as the ratio of work done (W) to the change in the surface area (A):
T = W / A
Unit of Surface Tension Surface tension is essentially the ratio of the force applied to the length of the surface. Consequently, its SI unit is N/m, where force is measured in Newtons (N) and length in meters (m). In the CGS system, the unit of surface tension is Dyne/cm.
Dimension of Surface Tension Considering that the dimension formula of force is [MLT-2] and the dimension of length is [L], the dimensional formula of surface tension can be expressed as [M L0 T-2]. This signifies the relationship between force, length, and surface tension.
What is the Causes of Surface Tension?
- The phenomenon of surface tension arises due to the cohesive forces present among liquid molecules.
- At the surface of a liquid, molecules lack like molecules in both directions, leading them to align more closely with those at the surface.
- This alignment creates a cohesive "film" on the surface, which hinders the movement of objects across it compared to when fully submerged.
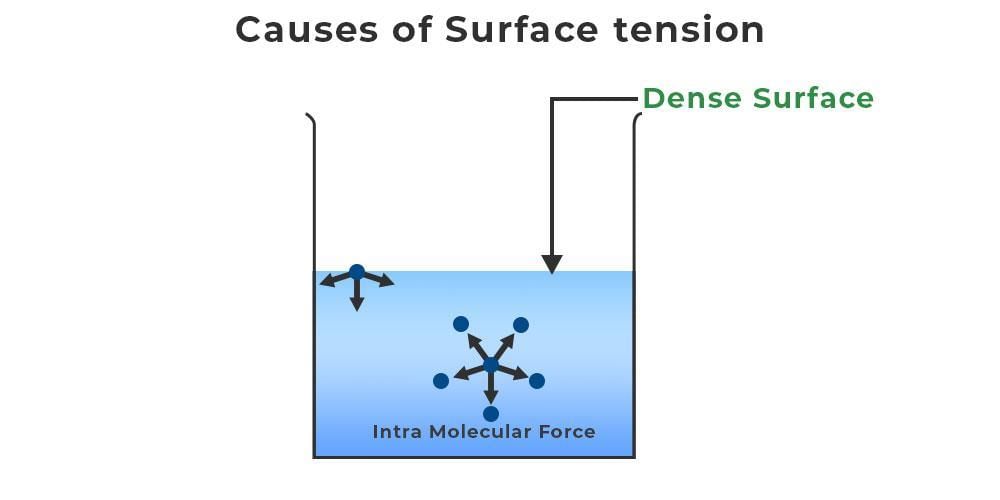
Explanation:
- Consider a jar filled with water; the water molecules exist in two locations - beneath the water and at the water's surface.
- Since there are no molecules above those at the surface, these surface molecules are unbalanced, attracting only the molecules below.
- This imbalance leads to the formation of a thin layer at the liquid's upper surface, creating a state of stress known as Surface Tension.
- This phenomenon can also be understood in terms of energy.
How to Calculate Surface Tension?
- The process of determining the surface tension of a substance can be easily carried out by utilizing the provided surface tension formula, as elaborated in the preceding content. To illustrate this calculation, let's consider the following example.
Example: Determining Surface Tension
Let's solve for the surface tension of a liquid when subjected to a dragging force of 12 N over a length of 4 m.
Given:
- Dragging Force (F) = 12 N
- Length (L) = 4 m
By applying the Surface Tension formula, T = F/L, we calculate:
- T = 12/4
- T = 3 N/m
Methods of Measurement
- Various techniques are employed to measure the surface tension of liquids, including:
- 1. Capillary Rise Method
- 2. Bubble Pressure Method
- 3. Spinning Drop Method
- 4. Du Noüy–Padday Method
- 5. Du Noüy Ring Method
- 6. Stalagmometric Method
- 7. Sessile Drop Method
Additionally, apart from these methods, there exist several other approaches for determining the surface tension of liquids.
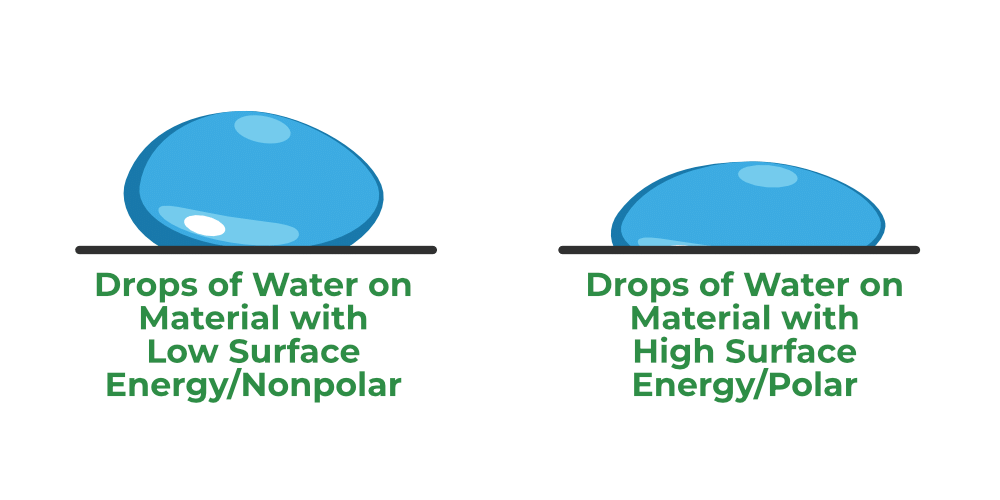
What is Surface Energy?
- Surface energy, also known as surface-free energy or interfacial-free energy, quantifies the disruption of intermolecular bonds during the creation of a surface. It is characterized as the work performed per unit area by the force responsible for forming the new surface.
The concept of surface energy can be illustrated by considering the surface of water molecules. When the surface area of a liquid expands, an opposing force against surface tension must be applied. This effort results in potential energy being stored on the liquid surface, which is termed surface energy.
Mathematically, surface energy is expressed as the product of surface tension and the change in surface area:
Surface energy = Surface tension × Change in surface area
ES = T × ΔA
The standard unit of surface energy is Newton per square meter (Nm-2), with a dimensional formula of [MT-2].
What is Angle of Contact?
The angle of contact refers to the angle formed between the tangents drawn at the liquid surface and the solid surface within the liquid at their point of contact (θ).
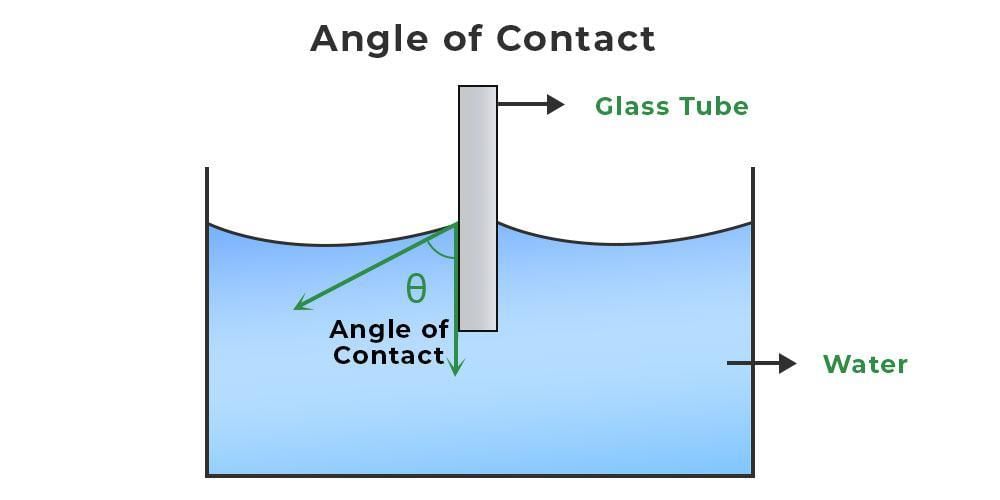
The angle of contact is influenced by various factors:
- The type of liquid and the solid it interacts with.
- The environment above the liquid's surface.
- An increase in liquid temperature leads to a rise in the angle of contact.
- The addition of soluble impurities to a liquid causes a decrease in the angle of contact.
Examples of Surface Tension
- Walking on Water: Certain insects demonstrate the concept of surface tension by effortlessly walking on water. This phenomenon occurs because their weight is insufficient to break the water's surface.
- Floating Needle: Despite being denser than water, a steel needle can float on the water's surface owing to surface tension forces that prevent it from sinking.
- Spherical Shape of Water Droplets: Tiny fluid droplets assume a spherical shape due to the cohesive forces of surface tension. Molecules at the surface possess higher energy and tend to minimize surface area, resulting in a spherical shape commonly seen in water and raindrops.
- Fire Polishing of Glass: Fire polishing, a technique utilized in glass or thermoplastic finishing, involves heating the material until it softens. Surface tension then smoothens the surface, creating a flat and polished finish. This method is particularly effective on flat surfaces and is commonly employed in acrylic plastic fabrication for its efficiency and cleanliness.
Soaps and Detergents
Soaps and detergents are effective for cleaning clothes because they reduce the surface tension of water, making it easier to penetrate and remove grease and soil particles.
Rise of Liquids in Capillary Tubes
A capillary tube, characterized by a small and uniform radius, demonstrates the rise of liquids when immersed in them. The liquid ascends to a certain height within the capillary tube due to capillary action.
Factors Affecting Surface Tension
Several factors influence the surface tension of a liquid:
- If a solute exhibits high solubility in the fluid, the surface tension of the liquid increases. Conversely, if the solute's solubility in the fluid is low, the surface tension decreases.
- The presence of dust particles or lubricants on the liquid's surface causes a reduction in surface tension.
- Elevating the temperature of the liquid leads to a decrease in surface tension, while reducing the temperature results in an increase in surface tension.
FAQs on Surface Tension
Question 1: What is Surface Tension?
The force on the surface of a liquid per unit length caused by intermolecular attraction among the liquid particles is known as Surface Tension.
Question 2: What is the Unit of Surface Tension?
The unit of Surface Tension is N/m in the SI system and dyn/cm in the CGS system.
Question 3: How does temperature affect the surface tension of water?
Increasing the temperature reduces the surface tension of water. This occurs because higher temperature leads to greater kinetic energy in the water molecules, weakening intermolecular attraction.
Question 4: What causes Surface Tension?
Surface Tension originates from the cohesive forces between liquid molecules, creating the phenomenon that resists external forces.
Question 5: Why is soap effective in cleaning clothes?
Soap is beneficial for washing clothes as it lowers the surface tension of water. This property enables water to access small areas in fabrics, facilitating the removal of stains.
|
95 videos|367 docs|98 tests
|





















