Surface Tension: Exploring the Definition, Units, and Examples of Surface Tension | Famous Books for UPSC Exam (Summary & Tests) PDF Download
| Table of contents |

|
| Introduction |

|
| Units of Surface Tension |

|
| Surface Tension Dimensional Formula |

|
| Surface Tension Examples |

|
| Conclusion |

|
Introduction
Surface tension refers to the property of a liquid's surface that allows it to resist external forces by virtue of the cohesive forces between its molecules. This phenomenon arises from electrostatic forces among liquid molecules, which act to minimize the liquid's surface area. An intriguing aspect of surface tension is its ability to enable certain objects to float on the surface of water, despite being denser than water itself. For instance, water striders and basilisks can effortlessly run on water due to the presence of surface tension.
Surface Tension Formula:
The surface tension formula expresses the relationship between the surface force (F) and the length of the acting force (L). It can be mathematically represented as follows:
Surface Tension = F / L
Units of Surface Tension
When measured in SI units, the surface tension unit is expressed as N/m (newton per meter). However, the more commonly used unit is the cgs unit, which is Dyne/cm (dyne per centimeter). In certain thermodynamic contexts, it is also useful to consider surface tension in terms of work per unit area, denoted by the unit J/m² (joules per meter squared) or the cgs unit erg/cm².
Surface Tension Definition:
Surface tension manifests as a phenomenon where the surface of a liquid acts as a thin elastic sheet upon contact with a gas, typically the air. This term is primarily used when the liquid surface interfaces with gas. When two different liquids come into contact, such as water and oil, the interfacial tension is referred to as "interface tension."
Surface Tension of Water:
Water exhibits surface tension due to the mutual attraction between its molecules. As each water molecule forms bonds with its neighboring molecules, the surface layer experiences a decrease in the number of molecules to cling to. To compensate for this, the surface layer forms stronger bonds with nearby molecules, resulting in the formation of surface tension. The surface tension of water is approximately 0.07275 joules per square meter. Comparatively, organic liquids like benzene and alcohol possess lower surface tensions, while mercury has a higher surface tension.
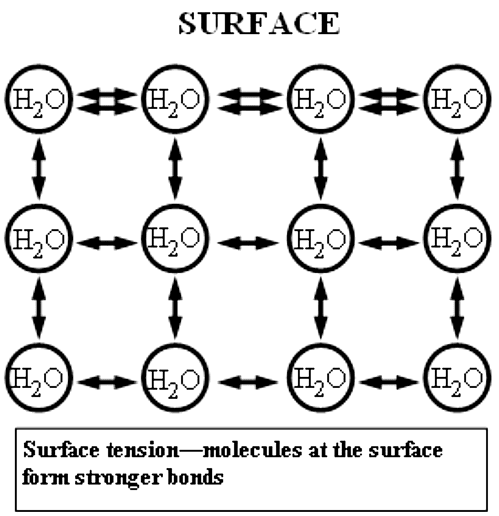
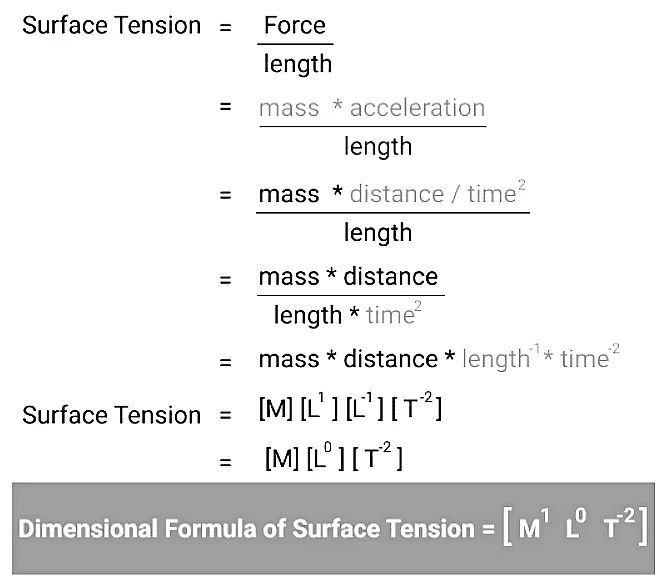
Surface Tension Dimensional Formula
The dimensional formula for surface tension can be expressed as follows:
[T] = [M] [L] [T]⁻²
Here, [T] represents time, [M] represents mass, and [L] denotes length.
Surface Tension Examples
a. Shape of liquid droplets:
Cohesive forces within the surface layer of a liquid result in water droplets adopting a spherical shape. These cohesive forces draw the droplets inward, maintaining their integrity.
b. Spherical shape of bubbles:
Surface tension plays a crucial role in the formation of bubbles. The surface tension of water provides the necessary wall tension for bubbles to take on a spherical shape. The tendency to reduce wall tension naturally leads to the formation of spherical bubbles.
c. Easy washing of clothes in hot water:
Hot water is often preferred for washing clothes due to its lower surface tension, making it a more effective wetting agent. The decreased surface tension allows hot water to penetrate pores and soiled areas more easily, aiding in the removal of dirt.
d. Test for jaundice:
The surface tension of normal urine is approximately 66 dynes/centimeter. However, in the presence of bile (a test for jaundice), the surface tension decreases to about 55 dynes/centimeter. This change is exploited in the Hay test, where powdered sulfur is sprinkled on the urine's surface. The sulfur floats in normal urine but sinks when the surface tension is reduced by the presence of bile.
e. Walking on water:
Water striders and various small insects can walk on water due to their weight being insufficient to penetrate the surface. The surface tension of water effectively supports these organisms, allowing them to glide across the water's surface.
f. Floating needle:
Despite being denser than water, a small needle can float on the surface of water, thanks to the phenomenon of surface tension. The cohesive forces within the water's surface layer enable the needle to rest on the water's surface.
g. Disinfectants and soaps:
Disinfectants are typically low surface tension solutions. This characteristic enables them to spread out and disrupt bacterial cell walls effectively. Similarly, soaps and detergents aid in cleaning clothes by lowering the surface tension of water, enhancing its wetting properties and facilitating the removal of stains.
Conclusion
Surface tension is a captivating property that governs the behavior of liquids at their interfaces with gases. By resisting external forces, surface tension showcases remarkable characteristics, such as the ability to support floating objects and facilitate the formation of spherical shapes. Understanding the concept of surface tension, its formula, units, and various examples, provides valuable insights into the intricate dynamics of this fascinating property of liquids.
|
743 videos|1444 docs|633 tests
|















