UPSC Exam > UPSC Notes > Famous Books for UPSC Exam (Summary & Tests) > Thermodynamics - Physics
Thermodynamics - Physics | Famous Books for UPSC Exam (Summary & Tests) PDF Download
Definition
Thermodynamics is the study of energy
Laws of Thermodynamics
First Law of Thermodynamics
- Energy can be changed from one form to another, but it cannot be created or destroyed.
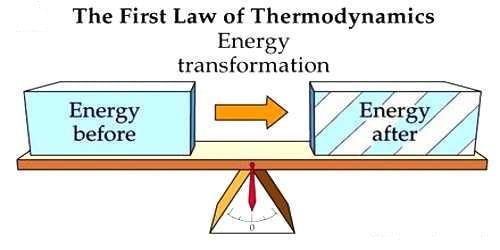 First Law of Thermodynamics
First Law of Thermodynamics
The Second Law of Thermodynamics
- It states that "in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state."This is also commonly referred to as entropy.
- One of the important fields of thermodynamics is heat transfer, which relates to transfer of heat between two media.
- There are three modes of heat transfer:
- conduction,
- convection and
- radiation.
- The concept of heat transfer is used in wide range of devices like heat exchangers, evaporators, condensers, radiators, coolers, heaters, etc.
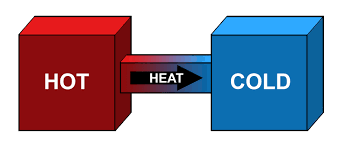 Second Law of Thermodynamics
Second Law of Thermodynamics
1. Conduction Heat Transfer :
- In this process heat transferred without bodily movement of the particles of medium.
2. Convection Heat Transfer :
- In this process heat is transferred by the bodily movement of particles of the medium due to difference in densities of different parts of the medium.
- All liquids and gases are heated by convection.
3. Radiation Heat Transfer :
- Quickest way of transmission of heat is known as radiation.
- In this mode of heat transmission heat is transferred from one place to another without effecting the intervening medium.
- Heat comes to us from the sun by radiation.
Perfectly Black Body
- Perfectly black body is that which neither reflects nor transmits the radiations falling on it, inspite of it the black body absorbs all radiations falling on it and hence it appears black.
- It is clear that, when heated, the black body will radiate all the energy absorbed by it.
Memorable Points
- All metals are good conductors of heat. Silver is the best conductor.
- Good conductors of heat are good conductors of electricity.
- Mica is an exception which although being a good conductor of heat and a bad conductor of electricity.
- Cooking utensils are provided with wooden or ebonite handles, since wood or ebonite is a bad conductor of heat.
- In winter, wooden chair appears hotter than the metal one because metal is a good conductor of heat while wood is a bad conductor of heat.
- In a room, ventilators are provided at ceiling, to escape the hot air by convection.
- Principle of chimney used in the kitchen or a factory is based on the convection.
- Land and sea breezes are due to the convection.
- Temperature of the upper of the flame is more then the temperature on the sides, because the currents of air carry the heat upwards.
- Highly polished surfaces are bad absorbers and bad emitters but they are good reflectors.
- If a thermous flask containing tea is shaken vigorously, temperature of the tea rises because of increase in internal energy.
- If the door of refrigerator is kept open, it will not cool the room, it will increases the temperature of the room because heat rejected by the refrigerator to the room will be more than the heat taken by the refrigerator from the room.
- If heat rejection portion of the refrigerator is outside the closed room, the opening of refrigerator door will cool the room gradually.
Latent Heat
- It is defined as the amount of heat absorbed or given out by a body during the change of state. It is of two types :
(1) Latent Heat of Fusion
- The latent heat of fusion of a substance is quantity of heat required to change unit mass of solid substance from solid state to liquid state.
(2) Latent Heat of Vaporization
- It is the quantity of heat required to change unit mass of liquid substance from liquid to vapour state while temperature remaining constant.
- The latent heat of vaporization of water is 536 cals
- Latent heat of vaporization is maximum at 0°C and decreases on increasing temperature.
- Latent heat of vaporization of water decreases on increasing pressure or we may say that the latent heat of vaporization decreases with increase in boiling point.
- Ice of 0°C feels colder than water at 0°C.
- Hot water (100°C) burns are less serve than steam burns (100°C), because steam has high latent heat.
- The melting point of solids which contract on melting decreases with increase in pressure e.g., ice and cast iron. These solids float on their corresponding liquid.
- The melting point of solids which expand on melting, increase with increase in pressure e.g., wax, glass, gold etc.,
- Boiling point increases on increasing pressure.
- Cooling a liquid below freezing point without turning it to a solid is called super-cooling. Water can be super – cooled to temperature as low as -12°C.
- Heating a liquid above its boiling point without converting it vapour state is called super – heating. Water can be heated up to 137°C without boiling.
Temperature
- Temperature is the property of a body which determines whether it is in thermal equilibrium with the neighbouring with the neighbouring body or not.
Mercury thermometer
- they generally contain two types of scale: Celsius scale (from 0oC to 100oC) and Fahrenheit scale(from 32oF 212oF).
- They are related as-
C = F-32
100 180
- In mercury thermometer, mercury is placed in a small spherical bulb attached to a long, thin glass capillary tube sealed at the top mercury is used because it undergoes uniform thermal expansion
Kelvin or absolute scale
- on this scale the freezing point of water is 273.15k (or 273k) and boiling point of water is 372K.
- K = 273 + oC
- OC = K – 273
- If C = - 273o then K = 0.
- This temperature is called absolute zero.
Thermal expansion
- The expansion of substances on heating is called thermal expansion.
- This expansion may be in length, area and volume. They are respectively called liner expansion, superficial expansion and volume expansion.
- Greensand all the liquids expand In volume when they are heated but water decreases in volume when heated from 0oC to 4oC and then after 4oC, it increases in volume on further heating.
- The volume of a fixed mass of water is minimum at 4oC, i.e., its density is maximum at oC (1.000 X 103 kg/ m3) with further rise in temperature its density decreases.
Anomalous expansion of water
- Greensand all the liquids expand In volume when they are heated but water decreases in volume when heated from 0oC to 4oC and then after 4oC, it increases in volume on further heating.
- The volume of a fixed mass of water is minimum at 4oC, i.e., its density is maximum at oC (1.000 X 103 kg/ m3) with further rise in temperature its density decreases.
The document Thermodynamics - Physics | Famous Books for UPSC Exam (Summary & Tests) is a part of the UPSC Course Famous Books for UPSC Exam (Summary & Tests).
All you need of UPSC at this link: UPSC
|
1243 videos|2222 docs|849 tests
|
FAQs on Thermodynamics - Physics - Famous Books for UPSC Exam (Summary & Tests)
| 1. What are the three laws of thermodynamics? |  |
Ans. The three laws of thermodynamics are:
1. The first law, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transferred or transformed from one form to another.
2. The second law, also known as the law of entropy, states that the entropy of an isolated system will always increase over time.
3. The third law, also known as the law of absolute zero, states that it is impossible to reach a temperature of absolute zero through any finite number of processes.
| 2. What is a perfectly black body? |  |
Ans. A perfectly black body is an idealized object that absorbs all incoming radiation without reflecting or transmitting any of it. It does not exist in reality, but it is used as a theoretical reference for studying the behavior of radiation and heat transfer.
| 3. What is latent heat? |  |
Ans. Latent heat is the amount of heat energy required or released during a phase change of a substance without a change in temperature. It is associated with the conversion of a substance between solid, liquid, and gaseous states. The latent heat of fusion is the heat energy required or released during the change between solid and liquid states, while the latent heat of vaporization is the heat energy required or released during the change between liquid and gaseous states.
| 4. How does a mercury thermometer work? |  |
Ans. A mercury thermometer works based on the principle of thermal expansion of liquids. The glass tube of the thermometer contains a small bulb at one end filled with mercury. As the temperature changes, the mercury expands or contracts, causing it to rise or fall in the narrow capillary tube. The temperature is read by observing the level at which the mercury stands on the scale marked on the tube.
| 5. What is the anomalous expansion of water? |  |
Ans. The anomalous expansion of water refers to the unusual behavior of water when it is heated or cooled. Most substances contract when cooled and expand when heated, but water behaves differently. It contracts when cooled from room temperature to around 4 degrees Celsius, but below 4 degrees Celsius, it starts to expand again. This expansion continues until it freezes into ice. This unique property of water has important implications for various natural processes, such as the formation of ice and the circulation of water in lakes and oceans.
Related Searches






















