Wave-Particle Duality | Physics Optional Notes for UPSC PDF Download
| Table of contents |

|
| Understanding Wave-Particle Duality |

|
| What is Wave-Particle Duality? |

|
| History of Wave-Particle Duality |

|
| Wave-Particle Duality Theory |

|
Understanding Wave-Particle Duality
The principle of wave-particle duality is a fundamental concept in quantum theory. It posits that matter exhibits both wave-like and particle-like characteristics, similar to light. This dual behavior has been observed in both simple and complex particles, including atoms and molecules.
This article aims to clarify the idea of wave-particle duality and the supporting theories behind it.
What is Wave-Particle Duality?
Material particles can act like waves under certain circumstances. The dual nature of matter is referred to as wave-particle duality. To substantiate this assertion, many experiments were conducted by various scientists. For instance, in some situations, light may be considered to also be a wave and a particle. Observing interference, diffraction, and reflection processes allow us to observe how light behaves as a wave. In contrast, it is found that light behaves like a particle when examining phenomena like the photoelectric effect.
Wave-Particle Duality in Light
Even though we can’t observe both simultaneously, the wave-particle duality of light theory asserts that light has both wave and particle qualities. The majority of the time, light behaves as a wave, but it may also be conceptualized as a collection of tiny energy packets called photons.
Wave-particle duality has been confirmed by a large number of experiments, but there are a few early experiments in particular that put an end to the controversy over whether light is made up of waves or particles.
Wave-Particle Duality in Matter
According to De Broglie, since radiant energy (light) has both a wave and a particle character, then a wave must be connected to the motion of a particle (matter). He thought that there must be some symmetry between energy and matter. The idea of the wave-particle duality of matter was born as a result. Certain conditions cause material particles to behave like waves. The concept of the wave-particle duality of matter describes this phenomenon.
History of Wave-Particle Duality
Isaac Newton and Christiaan Huygens both put forth rival hypotheses regarding the behaviour of light in the 1600s. Newton’s Corpuscular (particle) theory of light was opposed by Huygens’ Wave theory of light. Newton’s hypothesis predominated for more than a century since Huygens’ theory had some difficulties correlating with observation and Newton’s reputation provided backing for it.
The corpuscular hypothesis of light ran into issues in the early nineteenth century. One observation that it struggled to adequately explain was diffraction. It appears that Thomas Young’s double-slit experiment strongly supported the wave theory of light over Newton’s particle theory by producing clear wave behaviour.
A wave must often travel through some sort of medium in order to propagate. Huygens had suggested luminiferous aether as the medium (or in more common modern terminology, ether). James Clerk Maxwell assumed exactly such an ether as the medium of propagation when he quantified a set of equations (known as Maxwell’s Laws or Maxwell’s equations) to explain electromagnetic radiation (including visible light) as the propagation of waves, and his predictions were in line with experimental findings.
Because no such ether had ever been discovered, the wave theory had a problem. The ether would also need to be stationary in relation to a moving Earth, according to astronomical findings made in stellar aberration by James Bradley in 1720. Throughout the 1800s, attempts were attempted to directly detect the ether or its motion, and the Michelson-Morley experiment served as its climax. As the twentieth century got underway, there was a lot of discussions because none of them actually managed to detect the ether. Was light a particle or a wave?
Albert Einstein postulated that light moved as distinct bundles of energy in his paper explaining the photoelectric effect, which was published in 1905. The frequency of the light was correlated with the energy of a photon. The photon theory of light eventually gained popularity (although the word photon wasn’t invented until decades later). The ether was no longer necessary for the propagation of photons, but the strange mystery of why wave behaviour was observed remained. The Compton effect and the quantum fluctuations of the double-slit experiment, which appeared to support the particle interpretation, were even odder.
The daring de Broglie hypothesis, which expanded on Einstein’s work to link the observed wavelength of matter to its momentum, attempted to answer the question of whether such duality also manifested itself in the matter. In 1927, experiments proved the theory, and de Broglie was awarded the Nobel Prize in 1929 for his work. It appeared that matter, like light, displayed both wave and particle qualities under the appropriate conditions.
Wave-Particle Duality Theory
The wave-particle duality has many theories. These theories are explained below in brief.
Einstein’s Wave-Particle Theory:
Albert Einstein, a physicist, came up with a brand-new electromagnetic radiation theory in 1905. The wave-particle theory is a common name for the concept. It clarifies how electromagnetic radiation can act as a wave and a particle simultaneously. According to Einstein, electromagnetic energy is released as a distinct “packet” of energy when an electron drops to a lower energy level and emits it. Such a bundle of energy is now referred to as a photon. A photon resembles a particle yet moves like a wave, according to Einstein.
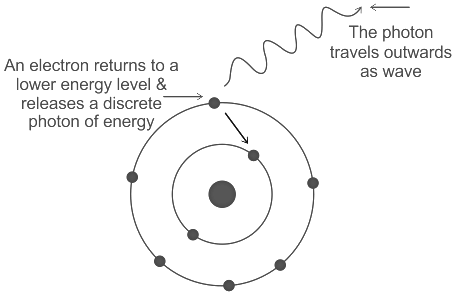
De Broglie Wavelength:
Louis de Broglie proposed in 1923 that matter can also have wave-particle duality. When electron and neutron beams were focused on a crystal and diffraction patterns were seen, de Broglie’s prediction that a particle would have wave qualities was validated. He goes on to say that any material particle with momentum (p) will also have a wavelength (λ) associated with it:
λ = h/p.
A photon is likewise subject to the same equation. He continues by saying that little items can be used to observe the wave qualities of matter. De Broglie’s Idea was further supported by Albert Einstein’s theory of the photoelectric phenomenon, which demonstrated that particles and waves can intersect.
Huygens Wave Theory:
Each point on a wavefront serves as a source for spherical wavelets that propagate ahead at the speed of light. These spherical wavelets are added together to generate the wavefront.
Huygens penned this hypothesis in 1678. Each point on a light waveform could be viewed as the source of a spherical wave, according to the theory’s postulated premise. Other physicists like Fresnel and Kirchhoff have tremendously benefited from this principle in their development of the wave theory of light.
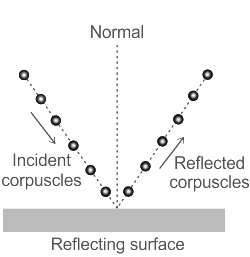
Corpuscular Theory:
Newton postulated that a source of light or a luminous body continuously emits corpuscles, which are minuscule, massless (or having a negligibly little mass). They move at the speed of light in straight lines throughout a homogeneous medium.
According to this idea, corpuscles make light. These luminous corpuscles move straight ahead. When light reflects off a planar surface, the law of reflection explains why it behaves like waves. However, in the instance of refraction, it has been claimed that light moves more slowly through thick material, which supports the idea that light is made up of tiny particles.
Compton Effect:
When he was aiming for carbon in 1922, Arthur Compton noticed the scattering of X-rays from electrons. It was discovered that the wavelength of the x-rays that were scattered was longer than those that struck the target. Only in the case of wave-particle duality in light could the Compton effect be understood.
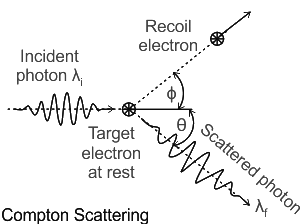
The outcomes of Compton’s experiment furthered the photoelectric effect, which Einstein proposed in 1905 and which decisively validated the validity of the quantum theory of light, as a first-hand demonstration of the unique character of light.
FAQs on Wave-Particle Duality - Physics Optional Notes for UPSC
| 1. What is the concept of wave-particle duality in quantum mechanics? |  |
| 2. Who were the key scientists involved in the development of wave-particle duality? |  |
| 3. How does wave-particle duality relate to the behavior of electrons? |  |
| 4. What experiments demonstrate the wave-particle duality of light? |  |
| 5. Why is wave-particle duality important in the field of quantum mechanics? |  |















