NEET Exam > NEET Notes > Chemistry Class 11 > Mind Map: Redox Reaction
Mind Map: Redox Reaction | Chemistry Class 11 - NEET PDF Download
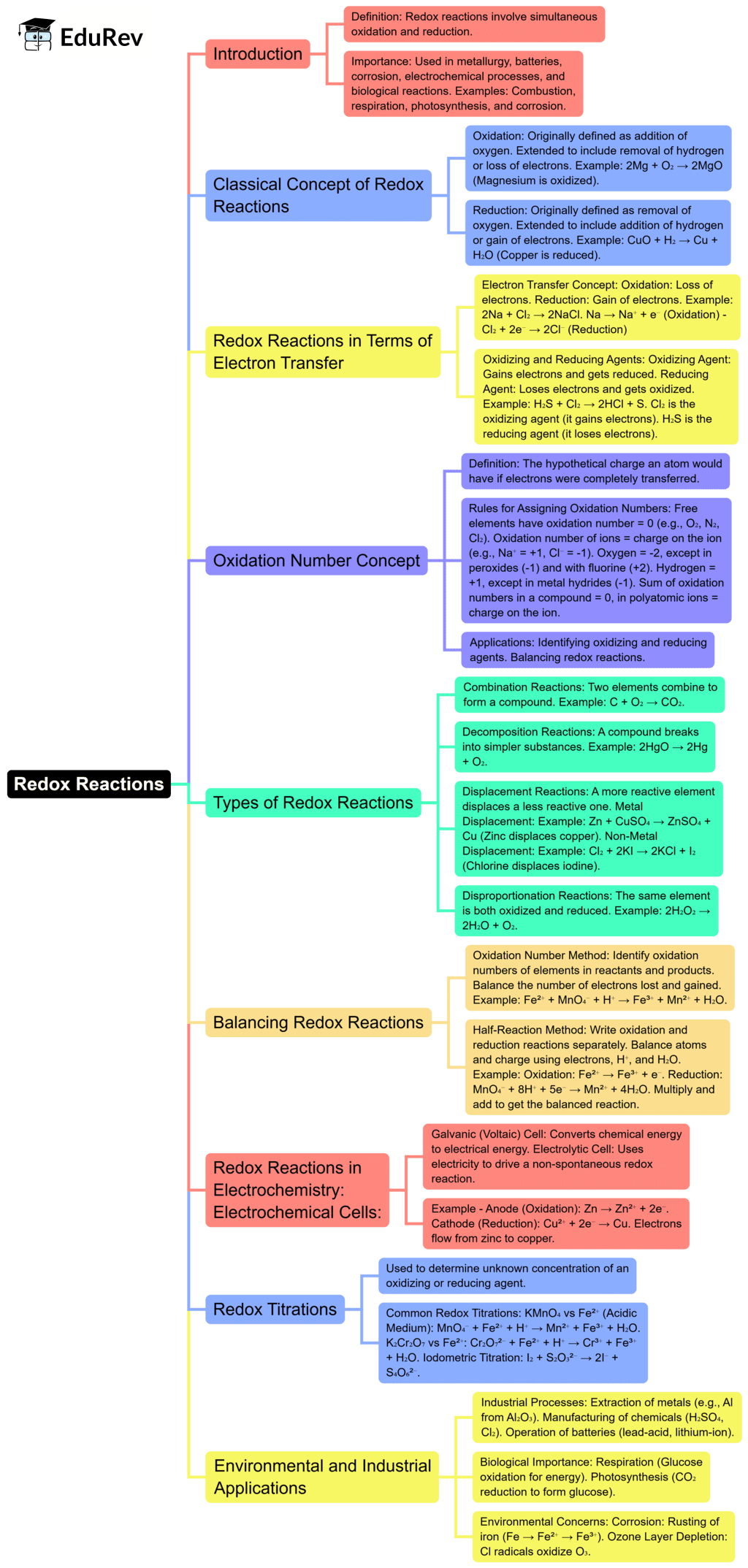
The document Mind Map: Redox Reaction | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
119 videos|338 docs|74 tests
|
FAQs on Mind Map: Redox Reaction - Chemistry Class 11 - NEET
| 1. What is a redox reaction? |  |
Ans. A redox reaction, short for reduction-oxidation reaction, is a chemical process in which the oxidation state of one or more substances changes. It involves the transfer of electrons between two species: oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. These reactions are fundamental in various biological, industrial, and environmental processes.
| 2. What are the key characteristics of redox reactions? |  |
Ans. The key characteristics of redox reactions include the presence of an oxidizing agent (which gets reduced) and a reducing agent (which gets oxidized). Additionally, these reactions often involve changes in oxidation states, the transfer of electrons, and can be accompanied by energy changes in the form of heat or light.
| 3. How can you identify oxidation and reduction in a reaction? |  |
Ans. To identify oxidation and reduction in a reaction, look at the changes in oxidation states of the elements involved. If an element's oxidation state increases, it is being oxidized. Conversely, if an element's oxidation state decreases, it is being reduced. Additionally, you can track the movement of electrons to see which species are donating and accepting electrons.
| 4. What are some common examples of redox reactions? |  |
Ans. Common examples of redox reactions include combustion (like burning of hydrocarbons), respiration (where glucose is oxidized), and corrosion (such as rusting of iron). Other examples can be found in batteries where chemical energy is converted to electrical energy through redox processes.
| 5. Why are redox reactions important in real life? |  |
Ans. Redox reactions are crucial in real life because they play a vital role in energy production (such as in batteries and fuel cells), metabolism in living organisms, and industrial processes (like metal extraction and waste treatment). Understanding these reactions helps in developing better energy solutions and managing environmental issues.
Related Searches

















