Class 10 Science Chapter 4 Previous Year Questions - Carbon and its compounds
Previous Year Questions 2025
Q1: A hydrocarbon which does not belong to the same homologous series of carbon compounds is: (1 Mark)
(a) C₄H₁₀
(b) C₆H₁₄
(c) C₇H₁₄
(d) C₁₀H₂₂
 View Answer
View Answer 
Ans: (c) C₇H₁₄
- C₄H₁₀, C₆H₁₄, and C₁₀H₂₂ are alkanes.
- C₇H₁₄ follows the general formula of alkenes, not alkanes. Hence, it does not belong to the alkane homologous series.
- Thus, C₇H₁₄ does not belong to the alkane homologous series.
Q2: Choose the incorrect statement about the common reaction used in hydrogenation of vegetable oils: (1 Mark)
(a) It is an addition reaction.
(b) It takes place in the presence of nickel or palladium catalyst.
(c) The product contains only single bonds between carbon atoms.
(d) It is an addition reaction which occurs in the presence of an acid catalyst.
 View Answer
View Answer 
Ans: (d) It is an addition reaction which occurs in the presence of an acid catalyst.
- Hydrogenation is an addition reaction where H₂ adds to double bonds in unsaturated oils, forming saturated fats (C).
- It uses nickel or palladium catalysts (B).
- No acid catalyst is used; thus, (D) is incorrect.
Q3: Select from the following a hydrocarbon having one C–C bond and one C ≡ C bond. (1 Mark)
(a) Benzene
(b) Cyclohexane
(c) Butyne
(d) Propyne
 View Answer
View Answer 
Ans: (d) Propyne
- Propyne (C₃H₄, HC≡C–CH₃) has one C–C single bond and one C≡C triple bond.
- Butyne (C₄H₆) has more carbons; benzene and cyclohexane lack triple bonds.
Q4: Two statements are given — one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct
answer to these questions from the codes (A), (B), (C) and (D) as given below. (1 Mark)
Assertion (A): Propanal and propanone are structural isomers.
Reason (R): Propanal and propanone both have the same molecular formula.
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
Ans: (A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
 View Answer
View Answer 
- Propanal (CH₃CH₂CHO) and propanone (CH₃COCH₃) are C₃H₆O, same molecular formula (R).
- Different structures (aldehyde vs. ketone) make them structural isomers (A).
Q5: Two statements are given — one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct
answer to these questions from the codes (A), (B), (C) and (D) as given below. (1 Mark)
Assertion (A): Carbon and its compounds are our major sources of fuels.
Reason (R): Most of the carbon compounds on burning release a large amount of heat and light.
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
 View Answer
View Answer 
Ans: (A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
- Carbon compounds (e.g., methane, petrol) are fuels (A).
- They release heat and light on combustion (R), explaining their fuel use.
Q6: Select from the following the members of same homologous series: (2 Marks)
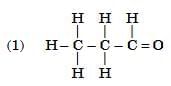
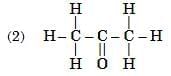
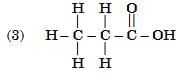
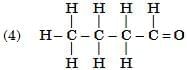
 View Answer
View Answer 
Ans: (3) and (6)
- (3) C3H8O (propanol, –OH) and (6) C4H10O (butanol, –OH) are alcohols.
- (1) C₃H₆O (propanal, –CHO) and (5) C₅H₁₀O (pentanal, –CHO) are aldehydes.
- (3) and (6) belong to the alcohol homologous series.
Q7: A saturated organic compound 'A' with two carbon atoms belongs to the homologous series of alcohols. On oxidation, it forms an organic acid 'B' with molecular mass 60 u. On heating 'A' with excess concentrated sulphuric acid at 443 K, an unsaturated hydrocarbon 'C' is formed.
(i) Name A, B, and C.
(ii) Calculate molecular mass of C.
(iii) What happens when a pinch of sodium carbonate is added to compound B? Write chemical equation for the reaction.
(iv) Draw electron dot structure of compound B. (5 Marks)
 View Answer
View Answer 
Ans:
(i) A: Ethanol (C₂H₅OH); B: Ethanoic acid (CH₃COOH); C: Ethene (C₂H₄).
(ii) Molecular Mass of C: C₂H₄ = (2×12) + (4×1) = 28 u.
(iii) Reaction: CO₂ gas evolves, turning lime water milky.
CH₃COOH + Na₂CO₃ → CH₃COONa + H₂O + CO₂
(iv) Electron Dot Structure of B (CH₃COOH):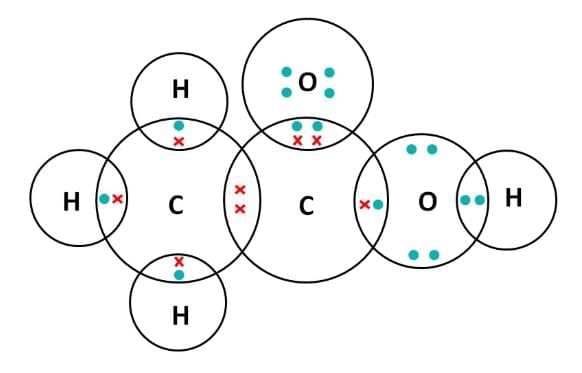 Explanation:
Explanation:
- Ethanol (C₂H₅OH) oxidizes to ethanoic acid (60 u: 2C + 4H + 2O).
- Dehydration forms ethene.
- Ethanoic acid reacts with Na₂CO₃, producing CO₂.
Q8: (i) Draw electron dot structure of chlorine molecule. (Atomic Number of Chlorine = 17)
(ii) What happens when chlorine reacts with methane in the presence of sunlight? Write the name of the reaction.
(iii) Name the two oxidising agents used for the conversion of alcohols to acids.
(iv) List four differences in properties between covalent compounds and ionic compounds. (5 Marks)
 View Answer
View Answer 
Ans:
(i) Cl₂ Structure: 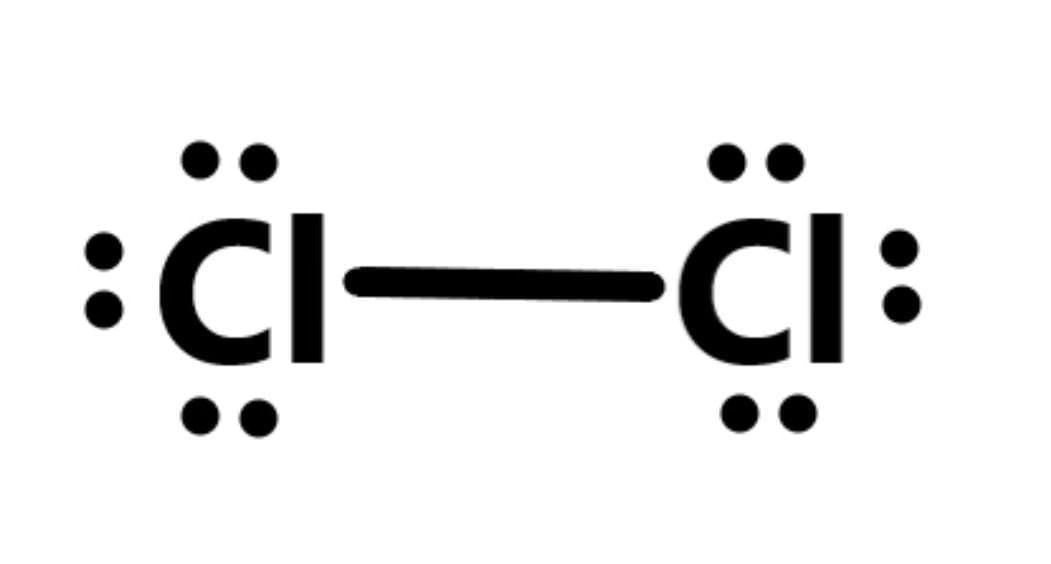
(iii) Oxidising Agents: KMnO₄, K₂Cr₂O₇.
(iv) Differences:
- Covalent: Low melting/boiling points; Ionic: High melting/boiling points.
- Covalent: Poor conductors; Ionic: Conduct in molten/aqueous state.
- Covalent: Soluble in organic solvents; Ionic: Soluble in water.
- Covalent: Molecular structure; Ionic: Lattice structure.
Explanation:
- Cl₂ forms a single covalent bond.
- Substitution replaces H with Cl in methane.
- KMnO₄/K₂Cr₂O₇ oxidize alcohols to acids.
- Covalent compounds lack ions, ionic compounds have strong electrostatic forces.
Q9: (i) A compound 'X' having two carbon atoms in its molecule turns blue litmus red and 5-8% solution of 'X' in water is widely used as a preservative. Identify the compound 'X' and write its structure.
(ii) Compare its pH nature with a mineral acid.
(iii) 'X' on reacting with alcohols produces sweet smelling compounds, used in making perfumes. Name the reaction and write its chemical equation.
(iv) When sodium carbonate is added to 'X', a colourless gas is produced which turns lime water milky. Write the chemical equation for the reaction giving the name of the salt produced. (5 marks)
 View Answer
View Answer 
Ans:
(i) X: Ethanoic acid (CH₃COOH).
Structure:
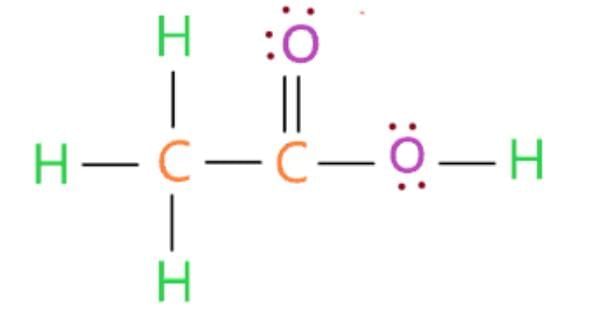
(ii) pH Comparison: Ethanoic acid (weak acid, pH ~3-4) is less acidic than mineral acids (e.g., HCl, pH ~1).
(iii) Reaction: Esterification; CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ + H₂O.
(iv) Equation: CH₃COOH + Na₂CO₃ → 2CH₃COONa (sodium ethanoate) + H₂O + CO₂.
Explanation:
- Ethanoic acid (vinegar) is a preservative, weakly acidic.
- Esterification forms sweet-smelling esters.
- CO₂ from Na₂CO₃ reaction turns lime water milky.
Q10: (a) Define the term "homologous series of carbon compounds". Write a homologous series of compounds having functional group -CHO.
(b) Design an experiment to distinguish between an alcohol and a carboxylic acid. Also write chemical equation for that case in which reaction occurs. (5 Marks)
 View Answer
View Answer 
Ans:
(a) Homologous Series: Compounds with the same functional group, differing by CH₂.
Aldehydes (-CHO): CH₃CHO, C₂H₅CHO, C₃H₇CHO.
(b) Experiment: Add NaHCO₃ to test tubes with alcohol (e.g., ethanol) and carboxylic acid (e.g., ethanoic acid).
Observation: Carboxylic acid produces CO₂ bubbles; alcohol does not.
Equation: CH₃COOH + NaHCO₃ → CH₃COONa + H₂O + CO₂
Explanation:
Carboxylic acids react with NaHCO₃ due to acidity; alcohols do not.
Q11: (a) Name two cyclic hydrocarbons and draw the structure of any one.
(b) Explain the process of micelle formation on adding soap in water. (5 marks)
 View Answer
View Answer 
Ans:
(a) Cyclic Hydrocarbons: Cyclohexane, Benzene.
Cyclohexane Structure:
C₆H₁₂ (six-carbon ring, single bonds)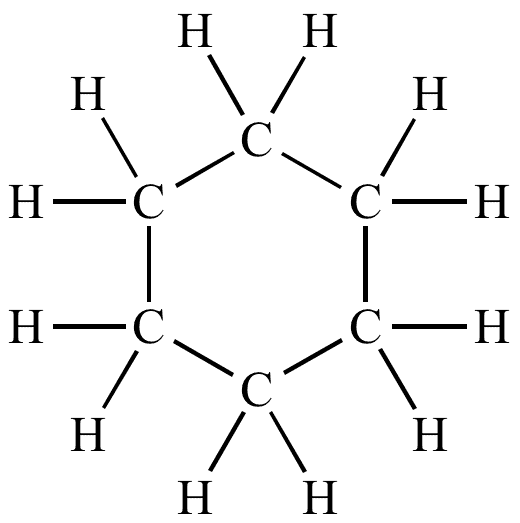
(b) Benzene Structure:
C₆H₆ (ring with alternating double bonds)
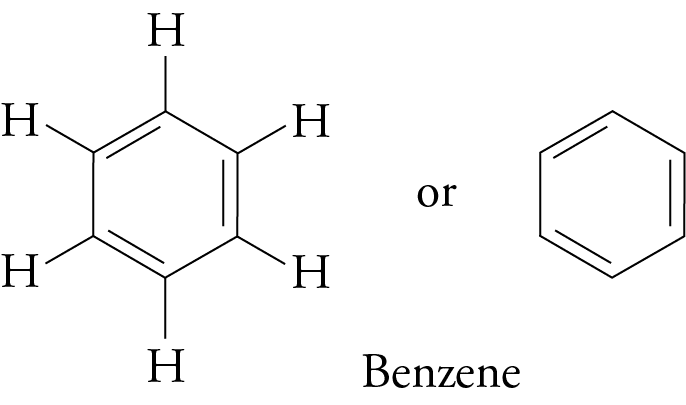
Explanation:
- Cyclic hydrocarbons have ring structures.
- Micelles form due to amphipathic nature of soap, aiding cleansing.
Q12: (A) Name an alcohol and a carboxylic acid having two carbon atoms in their structures. Draw their structures and state how this alcohol can be converted into a carboxylic acid. What happens when these two compounds react in the presence of an acid? Write chemical equations for the reactions involved in the two cases mentioned above. (5 Marks)
 View Answer
View Answer 
Ans: (A)
Alcohol: Ethanol (C₂H₅OH); Carboxylic Acid: Ethanoic acid (CH₃COOH).
Structures:
Ethanol: CH₃–CH₂–OH
Ethanoic acid: CH₃–COOH
Conversion: C₂H₅OH → CH₃COOH (oxidation, KMnO₄, heat).
Reaction: C₂H₅OH + CH₃COOH → CH₃COOC₂H₅ + H₂O (esterification).
Explanation:
Oxidation converts ethanol to ethanoic acid; esterification forms ethyl ethanoate.
Q13: The combining capacity of various elements depends on the number of valence electrons. Also the reactivity of elements is explained as their tendency to attain a completely filled outer shell, that is, to attain a noble gas configuration. This may be either through gain of electrons or loss of electrons or sharing of electrons.
(a) An element A has atomic number 16, how will it attain its nearest noble gas configuration?
(b) Write the number of (i) single and (ii) double covalent bonds in a molecule of butene (C₄H₈).
(c) (A) Explain the formation of a molecule of ammonia (NH₃), using electron dot structure. (Atomic number of nitrogen is 7)
OR
(c) (B) Why does carbon share its valence electrons with other atoms of carbon or with atoms of other elements? ( 4 Marks)
 View Answer
View Answer 
Ans:
(a) Element A (Sulfur): Gains 2 electrons to form S²⁻, achieving argon configuration (2,8,8).
(b) Butene (C₄H₈): (i) 9 single bonds; (ii) 1 double bond.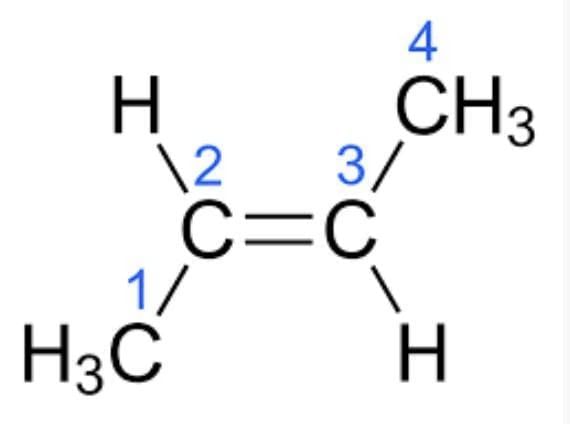 (c) (A) NH₃ Formation:
(c) (A) NH₃ Formation:
N (2,5) shares 3 electrons with 3 H atoms, forming three single bonds, one lone pair on N.

Explanation:
- Sulfur gains electrons to complete octet.
- Butene (e.g., CH₂=CH–CH₂–CH₃) has one C=C double bond.
- Nitrogen forms covalent bonds with hydrogen in NH₃.
Previous Year Questions 2024
Q1: Carbon compounds:(i) are good conductors of electricity.
(ii) are bad conductors of electricity.
(iii) have strong forces of attraction between their molecules.
(iv) have weak forces of attraction between their molecules.
The correct statements are: (1 Mark) (2024)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (i) and (iii)
 View Answer
View Answer 
Ans: (c)
The correct answer is (c) because carbon compounds generally do not conduct electricity well (bad conductors), and they have weak forces of attraction between their molecules, which means the molecules can move around easily. This explains why carbon compounds often exist as gases or liquids rather than solids.
Q2: (A) (i) Define a homologous series of carbon compounds.
(ii) Why is the melting and boiling points of C4H8 higher than that of C3H6 or C2H4?
(iii) Why do we NOT see any gradation in the chemical properties of homologous series compounds?
(iv) Write the name and structures of (i) aldehyde and (ii) ketone with molecular form C3H6O.
OR
(B) (i) Write the name and structure of an organic compound ‘X’ having two carbon atoms in its molecule and its name is suffixed with ‘-ol’.
(ii) What happens when ‘X’ is heated with excess concentrated sulphuric acid at 443 K? Write the chemical equation for the reaction stating the conditions for the reaction. Also, state the role played by concentrated sulphuric acid in the reaction.
(iii) Name and draw the electron dot structure of the hydrocarbon produced in the above reaction. (4 to 5 Marks) (2024)
 View Answer
View Answer 
Ans: (A) (i) A homologous series is a group of carbon compounds where each member differs from the next by a CH2 unit. They share the same functional group and exhibit similar chemical properties.
(ii) The melting and boiling points of C4H8 are higher than those of C3H6 or C2H4 because these points increase with molecular mass.
(iii) There is no gradation in the chemical properties of compounds in a homologous series because their properties are determined solely by their functional group, which remains constant.
(iv) (i) Aldehyde: Propanal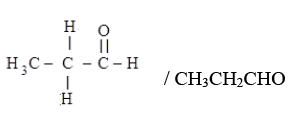
(ii) Ketone: Propanone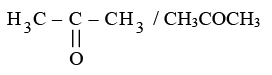
OR
(B) (i) The organic compound X with two carbon atoms and the suffix -ol is Ethanol.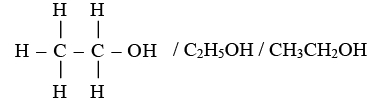
(ii) When X is heated with excess concentrated sulphuric acid at 443 K, it undergoes dehydration to form ethene. The reaction can be represented as: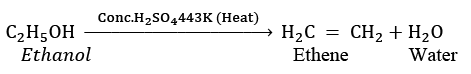
(iii)The hydrocarbon produced in the reaction is ethene.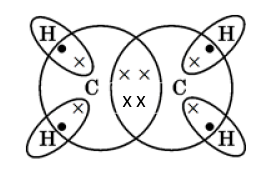
Q3: Consider the following statements about homologous series of carbon compounds :
(A) All succeeding members differ by — CH2 unit.
(B) Melting point and boiling point increases with increasing molecular mass.
(C) The difference in molecular masses between two successive members is 16 u.
(D) C2H2 and C3H4 are NOT the successive members of alkyne series.
The correct statements are — (1 Mark) (2024)
(a) (A) and (B)
(b) (B) and (C)
(c) (A) and (C)
(d) (C) and (D)
 View Answer
View Answer 
Ans: (a)
In a homologous series of carbon compounds, like alkanes or alkynes, each member differs from the next by a specific group of atoms (—CH2), which is explained in statement (A). As the size of the molecules increases (more carbon and hydrogen atoms), their melting and boiling points also generally increase, as stated in (B). Thus, both (A) and (B) are correct, making option (a) the right answer.
Q4: (a) (i) Give reason why carbon can neither form C4+ cations nor C4- anions but form covalent compounds.
(ii) What is homologous series of carbon compound? Write the molecular formula of any two consecutive members of homologous series of aldehydes.
(iii) Draw the structure of the molecule of cyclohexane (C6H12).
OR
(b) (i) Name a commercially important carbon compound having functional group —OH and write its molecular formula.
(ii) Write chemical equation to show its reaction with
(1) Sodium metal
(2) Excess cone, sulphuric acid
(3) Ethanoic acid in the presence of an acid catalyst
(4) Acidified potassium dichromate
Also write the name of the product formed in each case. (4 to 5 Marks) (2024)
 View Answer
View Answer 
Ans: (a) (i) Carbon cannot form C4+ cations because removal of 4 electrons from a carbon atom would require a large amount of energy and it cannot form C4- anion because it would be difficult for the nucleus with 6 protons to hold 10 electrons.
Thus it shares electrons to form covalent compounds.
(ii) A homologous series is a group of compounds that share the same functional group, with each member differing by a specific number of CH2 units. For example, the molecular formulas of two consecutive members of the aldehyde series are:
- CH3CHO (Ethanal)
- C2H5CHO (Butanal)
(iii)Structure of cyclohexane (C6H12)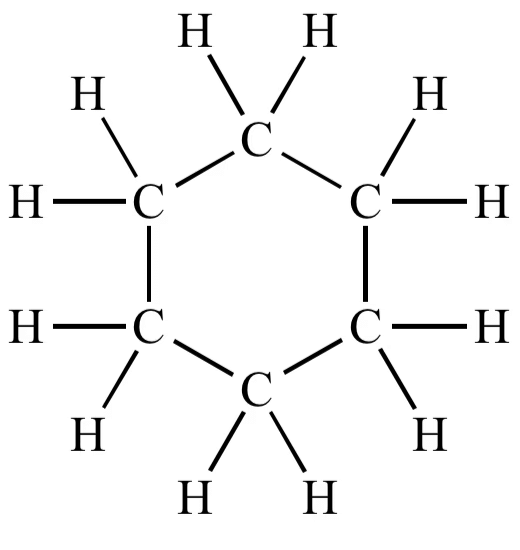 OR
OR
(b) (i) A commercially important carbon compound with the functional group —OH is Ethanol with the molecular formula C2H5OH.
(ii)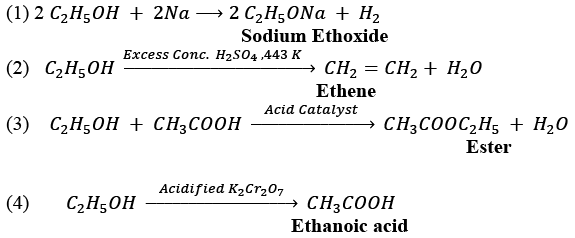
NOTE: Name of the product for each reaction is given in bold letters under the reaction.
Q5: The structural formula of Cyclohexane is (1 Mark) (CBSE 2024)
(a) 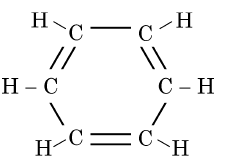
(b) 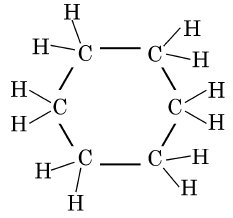
(c) 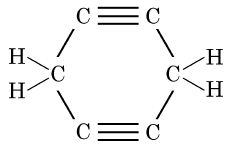
(d) 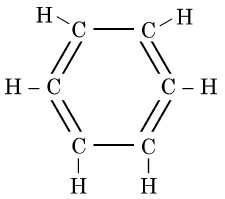
 View Answer
View Answer 
Ans: (b)
The correct structural formula for cyclohexane is shown in option (b). Cyclohexane, C6H12 , is a cyclic hydrocarbon with a six-membered ring structure, where each carbon atom is bonded to two other carbon atoms and two hydrogen atoms. This configuration ensures all carbon-carbon bonds are single, fitting the description and structure in option (b).
Q6: Which one of the following hydrocarbons is different from the others? (1 Mark) (2024)
(a) C4H10
(b) C7H14
(c) C5H12
(d) C2H6
 View Answer
View Answer 
Ans: (b)
- The hydrocarbons C4H10 (butane), C5H12 (pentane), and C2H6 (ethane) are all alkanes, which are saturated hydrocarbons containing only single bonds.
- However, C7H14 is an alkene, which has at least one double bond.
- Therefore, option (b) is different from the others.
Q7: More than three million carbon compounds have been discovered in the field of chemistry. The diversity of these compounds is due to the capacity of carbon atoms for bonding with one another as well as with other atoms. Most of the carbon compounds are poor conductors of electricity and have low melting and boiling points.
(a) Write the molecular formula of first two members of homologous series having functional group —Br.
(b) Given below are the formulae of some functional groups :
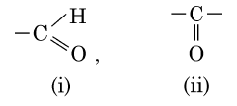
Write the name of these functional groups.
(c) What would be observed on adding a 5% alkaline potassium permanganate drop by drop to some warm ethanol taken in a test tube? State the role of KMn04 in the reaction and write the chemical equation for the reaction involved.
OR
(c) Write the name of the compound formed when ethanol is heated at 443 K temperature with excess of cone. H2S04. What is the role of cone. H2S04 in the reaction? Write the chemical equation for the reaction involved. (4 to 5 Marks) (2024)
 View Answer
View Answer 
Ans: (a) The first two members of homologous series having functional group —Br. are CH3Br, C2H5Br
(b) (i) Aldehyde (ii) Ketone
(c) When a 5% alkaline potassium permanganate solution is added drop by drop to warm ethanol in a test tube, the following is observed:
The purple colour of potassium permanganate fades, indicating a reaction.
The role of KMnO4 in this reaction is as an oxidising agent.
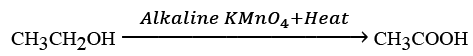
OR
(c) When ethanol is heated at 443 K with excess concentrated H2SO4, the compound formed is Ethene.
The role of concentrated H2SO4 is as a dehydrating agent.
The chemical equation for this reaction is:
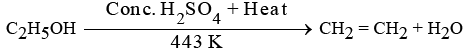
Q8: The number of single and double bonds present in a molecule of benzene (C6H6) respectively, are: (1 Mark) (2024)
(a) 6 and 6
(b) 9 and 3
(c) 3 and 9
(d) 3 and 3
 View Answer
View Answer 
Ans: (b)
Six single bonds between Carbons and Hydrogens, three single bonds between six Carbons and Hydrogens ( alternatively) . Three double bonds present between carbons ( alternatively in hexagonal ring). So a total of nine single bonds, three double bonds are present in benzene ring.
Q9: The melting and boiling points of carbon compounds are generally low and they are largely non-conductors of electricity. State two conclusions based on these two properties. (2 Marks) (2024)
 View Answer
View Answer 
Ans:
(i) Low melting points and boiling points - Weak intermolecular forces of attraction.
(ii) Non-conductors of electricity - Bonding in these compounds does not give rise to any ions. / Covalent bonds or sharing of electrons do not form any charged particles.
Q10: Case-based/data-based questions with 3 short sub-parts. Internal choice is provided in one of these sub-parts.
Carbon is a versatile element that forms the basis of all living organisms and many of the things we use. A large variety of compounds is formed because of its tetravalency. Compounds of carbon are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements.
Answer the following questions:
(a) What are hydrocarbons?
(b) List two properties by virtue of which carbon can form a large number of compounds.
(c) (i) Write the formula of the functional group present in
(1) aldehydes, and (2) ketones. Write chemical equation for the reaction that occurs between ethanoic acid and ethanol in the presence of a catalyst.
OR
(c) (ii) What are structural isomers ? Write the structures of two isomers of butane (C4H10). (4 to 5 Marks) (2024)
 View Answer
View Answer 
Ans: (a) Hydrocarbons are compounds made up of carbon and hydrogen only.
(b) Two key properties that allow carbon to form a vast number of compounds are:
- Tetravalency: Carbon can form four covalent bonds.
- Catenation: Carbon atoms can bond with each other to form long chains.
(c) (i)

(c) (ii) Structural isomers are compounds that have the same molecular formula but different structures. Two isomers of butane (C₄H₁₀) are: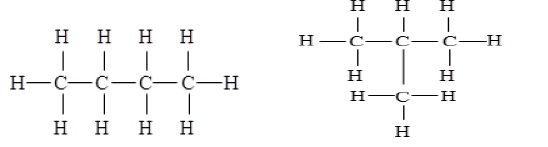
Q11: Distinguish between a saturated and an unsaturated hydrocarbon by flame test. List the products of combustion reaction of a saturated hydrocarbon. (2 Marks) (2024)
 View Answer
View Answer 
Ans:
The products of the combustion reaction of a saturated hydrocarbon are:
- Carbon dioxide (CO2)
- Water (H2O)
Q12: Why is the conversion of ethanol to ethanoic acid an oxidation reaction? Name the oxidising agent used in this conversion. Write chemical equation for this oxidation reaction. How is this reaction different from the reaction in which ethanol burns in the presence of oxygen? (3 Marks) (2024)
 View Answer
View Answer 
Ans:
(i) Oxygen is added to ethanol to produce ethanoic acid.
(ii) Alkaline potassium permanganate or Acidified potassium dichromate
(iii)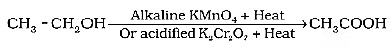
(iv) It is oxidation reaction while other is combustion reaction/ burning of ethanol is exothermic while other is endothermic.
Previous Year Questions 2023
Q1: Explain why carbon forms compounds mainly by covalent bonds. Explain in brief two main reasons for carbon forming a large number of compounds. Why does carbon form strong bonds with most other elements? (5 Marks) (2023) View Answer
View Answer 
Ans: As carbon has four valence electrons and it can neither loose nor gain four electrons thus, it attains noble gas configuration only by sharing of electrons. Thus, it forms covalent compounds. The existence of large number of compounds is due to some unique properties of carbon which are:
(i) Catenation: Carbon atoms possess an unique property to link together to form very long chains. This property is referred to as catenation.
A large number of carbon atoms can join together to form straight chains, branched chains and rings as shown below: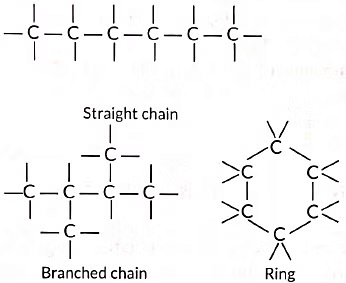
(ii) Tetravalency: Due to small size and presence of four valence electrons, a carbon atom can form multiple bonds with some other carbon atoms as well as with other atoms like oxygen, nitrogen etc., This increases the variety of compounds formed by it and hence the number of compounds is tremendously increased.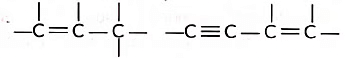 Due to small size, the nucleus of carbon atom can hold its shared pairs of electrons strongly. As a result, the bonds that carbon forms with most of the other elements such as hydrogen, oxygen, nitrogen, etc. are very strong there by making these compounds exceptionally stable.
Due to small size, the nucleus of carbon atom can hold its shared pairs of electrons strongly. As a result, the bonds that carbon forms with most of the other elements such as hydrogen, oxygen, nitrogen, etc. are very strong there by making these compounds exceptionally stable.
Q2: (i) Draw two structural isomers of butane.
(ii) Draw the structures of propanol and propanone.
(iii) Name the third homologue of:
(a) alcohols
(b) aldehydes
(iv) Name the following: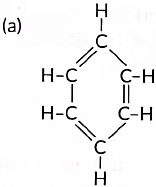
(b) CH3 - CH2CH = CH2
(v) Show the covalent bond formation in nitrogen molecule. (5 Marks) (2023)
 View Answer
View Answer 
Ans: (i) Structural isomers of butane are the following: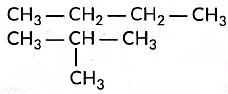
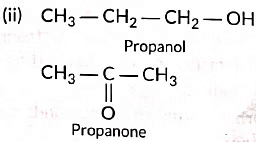
(iii) (a) Three homologue of alcohol are the following:CH3OH, CH3CH2OH, CH3CH2CH2OH
Third homologue of alcohol is CH3CH2CH2OH
(b) Three homologue of aldehyde are the following:
HCHO, CH3CHO, CH3CH,CHO
Third homologue of aldehyde is CH3CH2CHO.
(iv) (a) Benzene (b) But-1-ene
(v) Z = 7, 
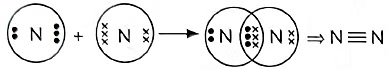
Q3: Write the chemical equation for the following:
(i) Combustion of methane
(ii) Oxidation of ethan
(iii) Hydrogenation of ethene
(iv) Esterification reaction
(v) Saponification reaction (5 Marks) (2023)
 View Answer
View Answer 
Ans: (i) The process in which compounds of carbon react with oxygen to give carbon dioxide, water, heat and light, is known as combustion. Alkanes burn in air and release large amount of heat, therefore can be used as excellent fuels.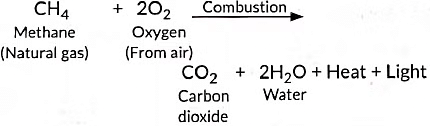
(ii) Oxidation is a process in which oxygen is added to a substance.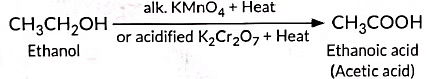
(iii) Hydrogenation means addition of hydrogen to an unsaturated compound.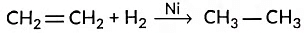
(iv) When alcohol is added to carboxylic acid in the presence of acid catalyst then, a fruity smelling ester is formed. This process is called esterification.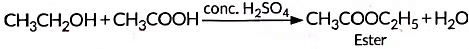
(v) Esters react in the presence of an acid or a base to give the alcohol and carboxylic acid. This reaction is known as saponification because it is used in the preparation of soap.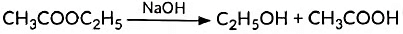
Q4: Consider the structures of the three cyclic carbon compounds (I), (II) and (III) given below and select the correct option from the following: 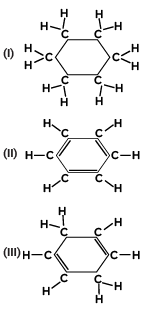
(a) (I) and (III) are isomers of hexane and (II) is benzene.
(b) (I) is an isomer of hexane, (II) is benzene and (III) is an isomer of hexene.
(c) (I) is a saturated cyclic hydrocarbon and (II) and (III) are unsaturated cyclic hydrocarbons.
(d) (I) is cyclohexane and (II) and (III) are the isomers of benzene. (1 Mark) (CBSE 2023)
 View Answer
View Answer 
Ans: (c)
Structure (I) is cyclohexane, a saturated cyclic hydrocarbon with single bonds between all the carbon atoms.
Structure (II) is benzene, an unsaturated cyclic hydrocarbon with alternating double bonds, giving it aromatic stability.
Structure (III) is cyclohexene, an unsaturated cyclic hydrocarbon with one double bond.
Thus, (I) is a saturated cyclic hydrocarbon, and (II) and (III) are unsaturated cyclic hydrocarbons, making (c) the correct answer.
Q5: A saturated organic compound ‘A’ belongs to the homologous series of alcohols. On heating ‘A’ with concentrated sulphuric acid at 443 K, it forms an unsaturated compound ‘B’ with molecular mass 28 u. The compound ‘B’ on addition of one mole of hydrogen in the presence of Nickel, changes to a saturated hydrocarbon ‘C’.
(A) Identify A, B and C.
(B) Write the chemical equations showing the conversion of A into B.
(C) What happens when compound C undergoes combustion?
(D) State one industrial application of hydrogenation reaction.
(E) Name the products formed when compound A reacts with sodium. (4 to 5 Marks) (CBSE 2023)
 View Answer
View Answer 
Ans: (A) Ethanol (C2H5OH) belongs to alcohol's homologous series.
A- Ethanol (C2H5OH)
B- Ethene (C2H4)
C- Ethane (C2H6)
(B) Dehydration occurs when ethanol is heated with concentrated sulphuric acid at 443 K, resulting in the formation of ethene Concentrated sulphuric acid serves as a dehydrating agent in this reaction.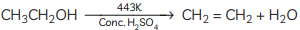 (C) Compound C is ethane. When it undergoes combustion, it forms CO2 and water.
(C) Compound C is ethane. When it undergoes combustion, it forms CO2 and water. (D) Hydrogenation reactions are used in the production of saturated vegetable ghee from unsaturated vegetable oils.
(D) Hydrogenation reactions are used in the production of saturated vegetable ghee from unsaturated vegetable oils.
(E) Sodium ethoxide is formed when ethanol (C2H5OH) reacts with sodium (Na). The reaction is as follows: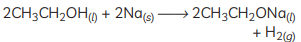
Previous Year Questions 2022
Q1: Carbon forms compounds mainly by covalent bonding. Why ? (Term II, 2021-22 C) View Answer
View Answer 
Ans: Carbon forms compounds mainly by covalent bonding.Carbon primarily forms covalent compounds due to its unique electronic structure:
- Carbon has four electrons in its outer shell, making it challenging to gain or lose electrons to achieve a stable octet.
- To attain a stable configuration, carbon shares its valence electrons with other atoms, either with other carbon atoms or different elements.
This ability to share electrons leads to the formation of a vast number of compounds, including:
- Catenation: Carbon can bond with itself, creating long chains, branched structures, or rings.
- Strong bonds: The small size of carbon allows for strong interactions with shared electrons, resulting in stable compounds.
Overall, carbon's unique properties enable it to form a diverse range of stable covalent compounds.
Q2: (a) Write the molecular formula of the following carbon compounds :
(i) Methane
(ii) Propane
(b) Carbon compounds have low melting and boiling points. Why? (Term II, 2021-22)
 View Answer
View Answer 
Ans: (a) (i) Methane (CH4)
(ii) Propane (CH3CH2CH3) or C3H8
(b) Due to weak intermolecular forces of attraction, covalent compounds generally have low melting and boiling points.
Q3: Covalent compounds have low melting and boiling points. Why? (2022)
 View Answer
View Answer 
Ans: Covalent compounds have low melting and boiling points due to the following reasons:
- The forces of attraction between the molecules are very weak.
- A small amount of heat can easily break these molecular forces.
- This results in low melting and boiling points compared to other types of compounds.
Previous Year Questions 2021
Q1: Write the name of an allotrope of carbon. (2021C) View Answer
View Answer 
Ans: Diamond is a well-known allotrope of carbon.
Q2: "Carbon prefers to share its valence electrons with other atoms of carbon or with atoms of other elements rather than gaining or losing the valence electrons in order to attain noble gas configuration.” Give reasons to justify this statement. (Term II, 2021)
 View Answer
View Answer 
Ans: Carbon prefers to share its valence electrons with other atoms of carbon or with atoms of other elements rather than gaining or losing the valence electrons in order to attain noble gas configuration. Carbon has an atomic number of 6, meaning it has four electrons in its outer shell. Here are the reasons why it prefers sharing electrons:
- If carbon were to gain four electrons, it would form a C4- anion. This would create strong repulsions among the eight electrons in the valence shell, making it hard for the nucleus to hold onto ten electrons.
- If it lost four electrons, it would create a C4+ cation. This process requires a significant amount of energy, as the nucleus would then only hold onto two electrons.
To overcome these challenges, carbon shares its valence electrons with other atoms. This sharing leads to the formation of a covalent bond, allowing both atoms to achieve a stable noble gas configuration.
Q3: Draw the electron dot structure of the molecules of (a) Oxygen, and (b) Nitrogen. The atomic numbers of oxygen and nitrogen are 8 and 7 respectively. (Term II, 2021-22 C)
 View Answer
View Answer 
Ans: (a) Formation of oxygen molecule: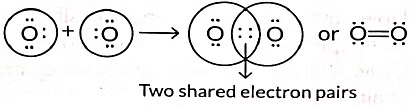
(b) Formation of nitrogen molecule: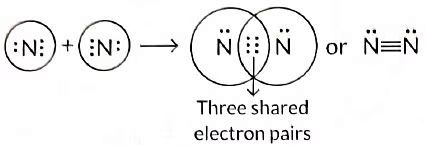
Q4: Carbon forms compounds mainly by covalent bonding. Why? (Term II, 2021-22 C)
 View Answer
View Answer 
Ans: Carbon can form only covalent compounds because carbon can neither gain nor lose four electrons to acquire stable octet. The only way by which it can acquire the nearest noble gas configuration is by sharing its valence electrons with other C-atoms or atoms of other elements. Hence, carbon forms compounds mainly by covalent bonding.
Q5: State the reason why
(i) carbon compounds have low melting and boiling points.
(ii) carbon compounds do not conduct electricity.
(iii) carbon can form only covalent compounds. (Term II, 2021-22)
 View Answer
View Answer 
Ans: (i) Carbon compounds typically have low melting and boiling points because:
- They are held together by weak intermolecular forces.
- This weak attraction results in lower energy requirements to change states.
(ii) Carbon compounds do not contain ions and hence, are generally poor conductors of electricity.
(iii) Carbon can form only covalent compounds because carbon can neither gain nor lose four electrons to acquire stable octet. The only way by which it can acquire the nearest noble gas configuration is by sharing its valence electrons with other C-atoms or atoms of other elements.
Q6: (a) Draw the electron dot structure for ethyne. (Term II, 2021-22)
(b) List two differences between the properties exhibited by covalent compounds and ionic compounds.
 View Answer
View Answer 
Ans: 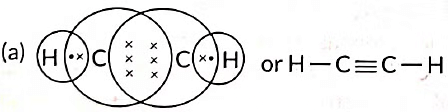
(b) 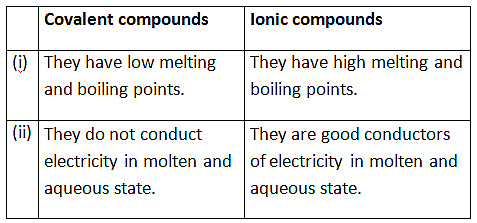
Q7: (a) Write the molecular formula of the following carbon compounds:
(i) Methane
(ii) Propane
(b) Carbon compounds have low melting and boiling points. Why? (Term II, 2021-22)
 View Answer
View Answer 
Ans: (a) (i) Methane (CH4)
(ii) Propane (CH3CH2CH3) or C3H8
(b) Due to weak intermolecular forces of attraction, covalent compounds generally have low melting and boiling points.
Q8: Consider the carbon compounds having following molecular formula:
(i) C3H6
(ii) C3H8
(iii) C4H6
(iv) C6H6
(v) C6H12
(a) State the number of double covalent bonds present in C3H6.
(b) Write the formula of first member of the homologous series to which the carbon compound C4H6 belongs.
(c) Which one of the above compounds forms ring structure of carbon atoms?
(d) Identify, which of the above compounds, is a member of alkane series. (Term II, 2021-22)
 View Answer
View Answer 
Ans: (a) C3H6(or CnH2n, n = 3) i.e, alkene series thus, has one double covalent bond.
(b) C4H6 (or CnH2n-2, n = 4) i.e., alkyne series.
The first member of alkyne series is ethyne (C2H2) HC ≡ CH.
(c) C6H12 can form ring structure of C-atoms.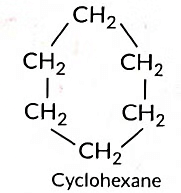
(d) Alkane series: Cn H2n+2
Only C3H8 is a member of alkane series CH3 — CH2 — CH3
Q9: The molecular formulae of two alkynes, A and B are CxH2 and C3Hy respectively.
(a) Find the values of x and y.
(b) Write the names of A and B. (Term II, 2021-22)
 View Answer
View Answer 
Ans: (a) General formula of alkyne CnH2n - 2
For CxH2, 2n - 2 = 2 ⇒ n = 2 ∴ x = 2.
For C3Hy, n = 3, y = 2n - 2 = 2 x 3 - 2 = 4
Hence, x = 2, y = 4
(b) A is ethyne (C2H2) and B is propyne (C3H4).
Q10: What is a homologous series? Find the difference in molecular mass between the two consecutive members of a homologous series. State how in a homologous series of carbon compounds the following properties vary with increase in molecular mass: (Term II, 2021-22)
(i) Melting and boiling points
(ii) Chemical properties
 View Answer
View Answer 
Ans: A homologous series is the family of organic compounds having the same functional group, similar chemical properties but the successive (adjacent members of the series differ by a —CH2 unit or 14 mass units.
(i) As the molecular mass increases in a homologous series, melting and boiling points also increases.
(ii) Chemical properties remains same for the members of homologous series because they all have same functional group.
Q11: Draw two different possible structures of a saturated hydrocarbon having four carbon atoms in its molecule. What are these two structures of the hydrocarbon having same molecular formula called? Write the molecular formula and the common name of this compound. Also write the molecular formula of its alkyne. (Term II, 2021-22)
 View Answer
View Answer 
Ans: 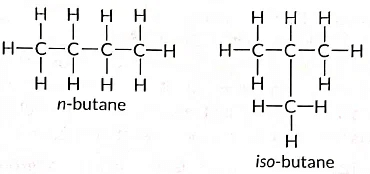
These are called structural isomers as they have the same molecular formula i.e., C4H10 but different structures. As the molecular formula is C4H10, common name of this compound is butane. The alkyne of four carbon atoms is butyne. Its structure i is as follows: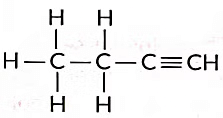
The molecular formula of butyne is C4H6.
Q12: (i) Write the molecular formula of benzene and draw its structure.
(ii) Write the number of single and double covalent bonds present in a molecule of benzene.
(iii) Which compounds are called alkynes? (Term II, 2021-22)
 View Answer
View Answer 
Ans: (i) Molecular formula of benzene is C6H6.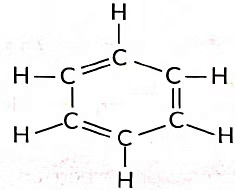
(ii) Number of single bonds = 9
Number of double bonds = 3
(iii) Alkynes are the unsaturated hydrocarbons that contain at least one triple bond between two carbon atoms (—C ≡ C—)
Q13: Consider the following organic compounds:
(i) 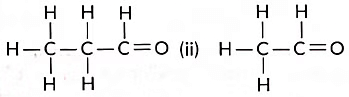
(a) Name the functional group present in these compounds.
(b) Write the general formula for the compounds of this functional group.
(c) State the relationship between these compounds and draw the structure of any other compound having similar functional group (Term II, 2021-22)
 View Answer
View Answer 
Ans: (a) Aldehyde (—CHO) group.
(b) CnH2nO
(c) Compound (i) is propanal, and compound (ii) is ethanal. They belong to the same homologous series where each successive compound differs from each other by a—CH2 unit.
Other member of same homologous series: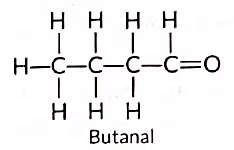
Previous Year Questions 2020
Q1: How are covalent bonds formed? (2020) View Answer
View Answer 
Ans: Covalent bonds are formed when two atoms share a pair of electrons. This sharing allows both atoms to achieve a stable electronic configuration, similar to that of noble gases.
- The shared electrons belong to the outermost shells of the atoms.
- Covalently bonded molecules have strong bonds within the molecule.
- However, the forces between these molecules (intermolecular forces) are generally weak.
Q2: Covalent compounds are generally poor conductors of electricity. Why? (2020)
 View Answer
View Answer 
Ans: Covalent compounds are generally poor conductors of electricity due to the following reasons:
- Covalent compounds do not contain ions.
- They consist of atoms that share electrons, rather than transferring them.
- This lack of charged particles means they cannot conduct electricity effectively.
Q3: Name a cyclic unsaturated carbon compound. (2020)
 View Answer
View Answer 
Ans: A cyclic unsaturated carbon compound is a compound that contains a carbon ring with one or more double or triple bonds between the carbon atoms. An example of a cyclic unsaturated carbon compound is benzene, which has a ring of six carbon atoms with alternating double bonds.
Q4: Carbon, a member of group 14, forms a large number of carbon compounds estimated to be about three million. Why is this property not exhibited by other elements of this group? Explain. (2020)
 View Answer
View Answer 
Ans: Carbon is unique among group 14 elements due to its ability to form a vast number of compounds, estimated at around three million. This is primarily because of two key properties:
- Catenation: Carbon can bond with itself, creating long chains, branched structures, or rings. This allows for the formation of large molecules.
- Bond Strength: Carbon forms strong bonds with other elements, including single, double, and triple bonds. This stability is due to its small size, which allows the nucleus to hold onto shared electrons effectively.
In contrast, other elements in this group, like silicon, can form fewer and less stable compounds. Their bond energies decrease as you move down the group, leading to limited catenation.
Q5: 3 mL of ethanol is taken in a test tube and warmed gently in a water bath. A 5% solution of alkaline potassium permanganate is added first drop by drop to this solution, then in excess.
(i) How is 5% solution of KMnO4 prepared?
(ii) State the role of alkaline potassium permanganate in this reaction. What happens on adding it in excess?
(iii) Write chemical equation of this reaction. (2020)
 View Answer
View Answer 
Ans: (i) 5% solution of KMnO4 is prepared by adding 5 g of KMnO4 in 95 g of water.
(ii) Here alkaline KMnO4 acts as an oxidising agent. It oxidises ethanol to ethanoic acid by donating nascent oxygen. If excess of KMnO4 is added the purple colour will persist indicating no more alcohol is left and reaction stops.
(iii) 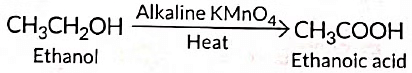
Q6: (a) Define isomerism. Draw all possible isomers of butane.
(b) "A compound ‘X’ on combustion gives a yellow flame with lots of smoke.” What inference would you draw from this statement ?
(c) State the role of alkaline KMnO4 in the reaction involving conversion of an alcohol to corresponding carboxylic acid. (2020)
 View Answer
View Answer 
Ans: (a) Isomers are those molecules which have the same molecular formula but different structural formulae i.e., show different properties and the phenomenon is called isomerism. The structures of possible isomers of butane (C4H10) are: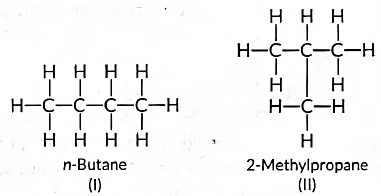 (b) The compound 'X' is likely an unsaturated compound (such as an alkene or alkyne) that burns in air, producing a yellow, sooty flame with significant smoke.
(b) The compound 'X' is likely an unsaturated compound (such as an alkene or alkyne) that burns in air, producing a yellow, sooty flame with significant smoke.
(c) Alkaline KMnO4 acts as an oxidising agent which oxidise alcohol (—OH) to corresponding carboxylic acid (—COOH).
Q7: (a) What is a homologous series? Explain with an example.
(b) Define the following terms giving one example of each.
(i) Esterification
(ii) Addition reaction (2020)
 View Answer
View Answer 
Ans: (a) A homologous series is a group of organic compounds that share the same functional group and have similar chemical properties. Each successive member of the series differs by a -CH2- unit or 14 mass units.
- The alkane series, for example, has the general formula CnH2n+2.
- The first member is methane (CH4).
- The second member is ethane (C2H6).
- The third member is propane (C3H8).
(b) (i) Carboxylic acids react with alcohols in the presence of a little concentrated sulphuric acid to form pleasant smelling esters. This reaction is called esterification reaction.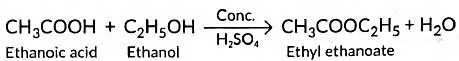
(ii) Those reactions in which atoms or group of atoms are simply added to a double or triple bond without the elimination of any atom or molecule, are known as addition reactions.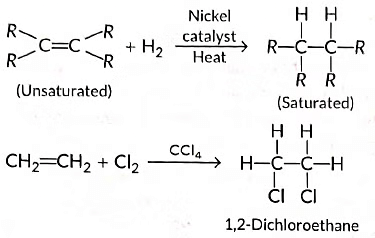
Q8: (a) Carry out following conversions:
(i) Ethanol to ethene
(ii) Ethanol to ethanoic acid
(b) Differentiate between addition reaction and substitution reaction. Give one example of each. (2020)
 View Answer
View Answer 
Ans: (a) (i) When ethanol is heated with cone. H2SO4 at 443 K, ethene is obtained due to dehydration of ethanol.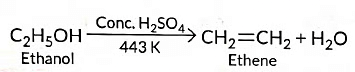
(ii) When 5 % alkaline KMn04 solution is added drop by drop to warm ethanol then it gets oxidised to ethanoic acid.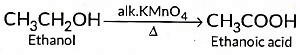
(b) Addition reactions: Those reactions in which atoms or group of atoms are simply added to a double or triple bond without the elimination of any atom or molecule, are known as addition reactions.
Substitution reactions: The reactions which involve the displacement or substitution of an atom or a group of atoms in an organic compound by another atom or group of atoms, are known as substitution reactions. Saturated hydrocarbons are fairly unreactive and inert in the presence of most of the reagents. However, in presence of sunlight, hydrocarbons undergo rapid substitution reactions. e.g.,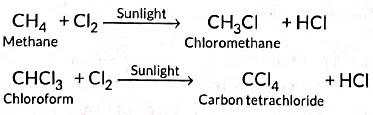
Q9: (a) A compound 'X' undergoes addition reaction with H2 to form a compound Y having molecular mass 30 g mol-1. ‘X’ decolorises bromine water and burns with a smoky flame. Identify ‘X’ and Y and write chemical equations of the reactions involved.
(b) Write the structural formulae of (i) Butanone, and (ii) Pentanoic acid.
(c) Would you be able to check if water is hard by using a detergent ? Give reason to justify your answer. (2020 C)
 View Answer
View Answer 
Ans: (a) As the molecular mass of Y is 30 g mol-1, it is ethane (C2H6 = 12 x 2 + 6 x 1 = 30).
‘X’ is ethene (CH2 = CH2) which decolourises Br2 water and burns with a smoky flame.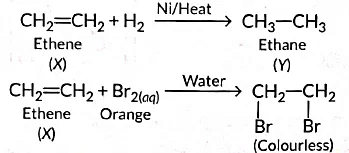
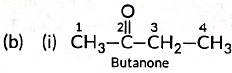
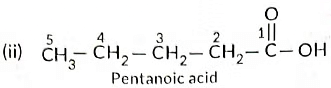
(c) No, you cannot determine if water is hard using a detergent. This is because detergents function effectively in both hard and soft water. The calcium and magnesium salts of detergents are soluble, allowing them to work well even in hard water.
Q10: Assertion(A): Esterification is a process in which a sweet smelling substance is produced.
Reason (R): When esters react with sodium hydroxide an alcohol and sodium salt of carboxylic acid are obtained.
(a) Both (A) and (R) are true, and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, and (R) is not the correct explanation of (A).
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true. (CBSE 2020)
 View Answer
View Answer 
Ans: (b)
Assertion (A): Esterification is a process in which a sweet-smelling substance is produced. This is true. Esterification is a chemical reaction between an acid (usually a carboxylic acid) and an alcohol, resulting in the formation of an ester, which typically has a fruity or sweet smell.
Reason (R): When esters react with sodium hydroxide, an alcohol and sodium salt of carboxylic acid are obtained. This is also true. This reaction is known as saponification, where an ester reacts with a strong base to produce an alcohol and the salt of a carboxylic acid.
Since both statements are true, but the reason does not explain the assertion (as esterification and saponification are different processes), the correct answer is (b) Both (A) and (R) are true, and (R) is not the correct explanation of (A).
Q11: Assertion(A): Carbon has a strong tendency to either lose or gain electrons to attain noble gas configuration.
Reason (R): Carbon has four electrons in its outermost shell and has the tendency to share electrons with carbon or other elements.
(a) Both (A) and (R) are true, and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, and (R) is not the correct explanation of (A).
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true. (CBSE 2020)
 View Answer
View Answer 
Ans: (d)
Assertion (A): Carbon has a strong tendency to either lose or gain electrons to attain noble gas configuration. This is false. Carbon does not lose or gain electrons easily because that would require a lot of energy. Instead, it tends to share electrons.
Reason (R): Carbon has four electrons in its outermost shell and has the tendency to share electrons with carbon or other elements. This is true. Carbon typically forms covalent bonds by sharing electrons to achieve a stable electron configuration.
Since the assertion is false but the reason is true, the correct answer is (d) (A) is false but (R) is true.
Q12: Which oils should be chosen for cooking to remain healthy? (CBSE 2020)
 View Answer
View Answer 
Ans: Oils that are best for cooking are those rich in unsaturated fatty acids. These oils are beneficial for health, while animal fats typically contain saturated fats, which can be harmful.
- Choose oils like olive oil, canola oil, and sunflower oil.
- Avoid animal fats such as butter and lard.
- Unsaturated fats can help reduce cholesterol levels.
Previous Year Questions 2019
Q1: What is a homologous series of carbon compounds? Give an example and list its three characteristics. (2019) View Answer
View Answer 
Ans: A homologous series is defined as a group of compounds having the same functional group, similar chemical properties in which the successive members differ by a -CH2 group or 14 mass unit.
For example, in alkane homologous series, the general formula is CnH2n+2 i.e.; first three members are CH4. C2H6 and C3H8 where two successive members differ by -CH2 group.
Characteristics of homologous series:
- All compounds in the series can be represented by a general formula, e.g., for alcohol it is CnH2n+1OH, for alkane CnH2n+2, for alkene CnH2n and for alkynes CnH2n-2, where, n = 1, 2, 3.....
- All compounds in the series have similar chemical properties.
- All members of the series, show a gradual change in their physical properties.
- Physical properties generally increase as the molecular mass increases.
Q2: What happens when 5% alkaline potassium permanganate solution is added drop by drop to warm propyl alcohol (propanol) taken in a test tube? Explain with the help of a chemical equation. (CBSE 2019)
 View Answer
View Answer 
Ans: When a 5% alkaline potassium permanganate solution is added drop by drop to warm propanol, a chemical reaction occurs:
- The propanol is oxidised to propanoic acid.
- In this process, the alkaline potassium permanganate acts as the oxidising agent.
The chemical equation for this reaction is:

Q3: The formulae of four organic compounds are given as following:
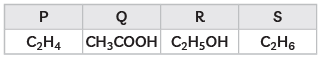
(A) Which one of these compounds P, Q, R or S is a saturated hydrocarbon?
(B) Identify the organic acid and give its structural formula. (CBSE 2019)
 View Answer
View Answer 
Ans: (A) S is a saturated hydrocarbon.
(B) Q is an organic acid.
Structural formula: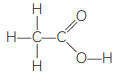
Previous Year Questions 2018
Q1: A compound 'X' on heating with excess conc. sulphuric acid at 443 K gives an unsaturated compound 'Y'. 'X' also reacts with sodium metal to evolve a colourless gas 'Z'. Identify 'X', 'Y and 'Z. Write the equation of the chemical reaction of formation of 'Y' and also write the role of sulphuric acid in the reaction. (2018) View Answer
View Answer 
Ans: As X reacts with conc. H2SO4 to give an alkene so it should be an alcohol as cone. H2SO4 acts as a dehydrating agent. The reaction of X with Na also confirms that it is an alcohol because alcohols react with Na metal to evolve colourless hydrogen gas.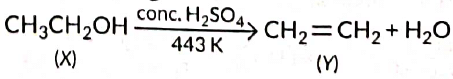
Here, cone H2SO4 acts as a dehydrating agent i.e., helps in the removal of water.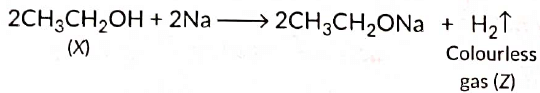
Q2: (a) Why are most carbon compounds poor conductors of electricity?
(b) Write the name and structure of a saturated compound in which the carbon atoms are arranged in a ring. Give the number of single bonds present in this compound. (2018)
 View Answer
View Answer 
Ans: (a) Most carbon compounds are poor conductors of electricity because:
- Carbon forms covalent bonds with other elements.
- There are no mobile electrons in these compounds.
- Carbon compounds do not dissociate into ions.
(b) Structure: 
Name: Cyclopentane
Number of single bonds: 15
Structure: C5H10
Q3: (a) Compare soaps and detergents on the basis of their composition and cleansing action in hard water.
(b) What happens when ethanol is treated with sodium metal? State about the behaviour of ethanol in this reaction.
(c) Draw the structure of cyclohexane.
(d) Name the following compound. (2018)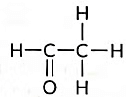
 View Answer
View Answer 
Ans: (a)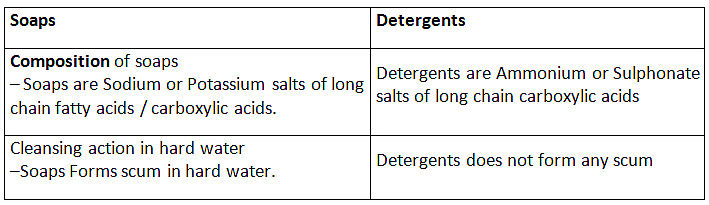
(b) Reaction of Ethanol with Sodium Metal:
- When ethanol reacts with sodium metal, hydrogen gas is released.
- Ethanol behaves like an acid in this reaction.
(c) structure of cyclohexane is below-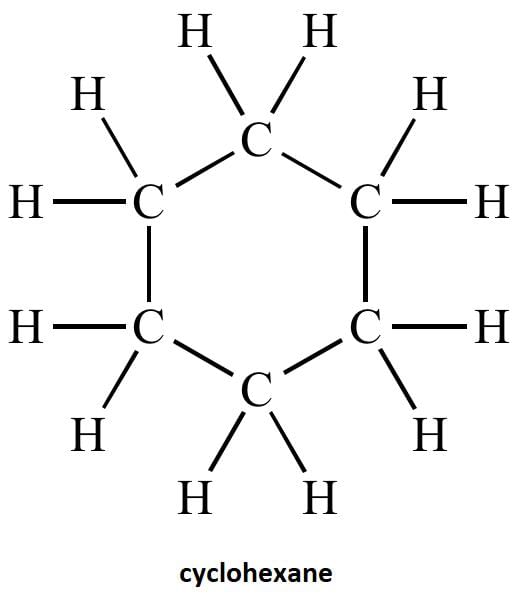
(d) Name of the compound is Ethanal and it is an Acetaldehyde
Q4: A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved. (CBSE 2018, 16)
 View Answer
View Answer 
Ans: When ethanol reacts with sodium metal, it forms sodium ethoxide (C2H5O– Na+) and hydrogen gas is released. The reaction is as follows: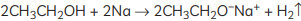 So, the gas is hydrogen that is evolved during the reaction.
So, the gas is hydrogen that is evolved during the reaction.
Previous Year Questions 2017
Q1: Write the molecular formula of first two members of homologous series having functional group -CI. (Delhi 2017)
 View Answer
View Answer 
Ans: The molecular formula of first two members of homologous series having - Cl functional group are CH3CI and CH3CH2CI.
Q2: Write the molecular formula of first two members of homologous series having functional group -OH. (Delhi 2017)
 View Answer
View Answer 
Ans: The molecular formula of first two members of homologous series having - OH functional group are CH3OH and CH3CH2OH.
Q3: Write the molecular formula of the 2nd and 3rd member of the homologous series whose first member is ethene. (AI 2017)
 View Answer
View Answer 
Ans: Homologous series of alkenes have general formula, CnH2n whose first member is ethene.
2nd member of homologous series of alkenes is C3H6 i.e., propene.
3rd member of homologous series of alkenes is C4H8 i.e., butene.
Q4: Write the molecular formula of the 2nd and 3rd member of the homologous series whose first member is methane. (AI 2017)
 View Answer
View Answer 
Ans: Methane, CH4 is an alkane. Alkanes have general formula, CnH2n+2.
2nd member of homologous series of alkanes is C2H6 i.e., ethane.
3rd member of homologous series of alkanes is C3H8 i.e., propane.
Q5: Two carbon compounds X and Y have the molecular formula C4H8 and C5H12 respectively. Which one of these is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case. (Delhi 2017)
 View Answer
View Answer 
Ans: All unsaturated hydrocarbons (containing double or triple bonds) have tendency to get converted to saturated hydrocarbons (single bonds) by adding small molecules such as hydrogen (H2), halogens (X2), etc. Such reactions are called addition reactions. Compound with the molecular formula C4H8 belongs to alkene series (CnH2n). Hence, it will undergo addition reaction.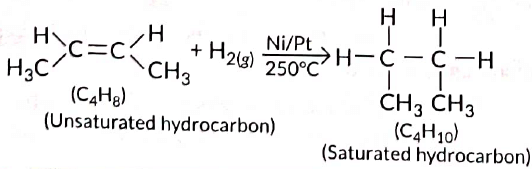
Q6: Soaps and detergents are both types of salts. State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to the use of detergents instead of soaps. (Delhi 2017, Al 2015)
 View Answer
View Answer 
Ans: The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. Detergents are generally ammonium or sulphonate salts of long chain carboxylic acids.
Difference between Soap and Detergent
Soaps:
(i) Soaps are sodium salts of long chain carboxylic acids.
(ii) The ionic group in soap is -COO-Na+.
(iii) Soaps are not useful when water is hard.
Detergents
(i) Detergents are the odium salt of long chain benzene sulphonic acids.
(ii) The ionic groups in detergents is SO3-Na+ or SO4-Na+.
(iii) Detergent can be used for washing purpose even when water is hard
Cleansing action of soap can be described as follows:
The soap molecule is generally represented as RCOONa. In solution, it ionises to form RCOO- and Na+. Each soap molecule has a polar head group (carboxylate ion, COO- group) and a long nonpolar hydrocarbon tail (R group from long chain fatty acid). The polar head attracts the polar water molecule and is called hydrophilic end and the nonpolar tail attracts the water-insoluble oily or greasy dirt particles.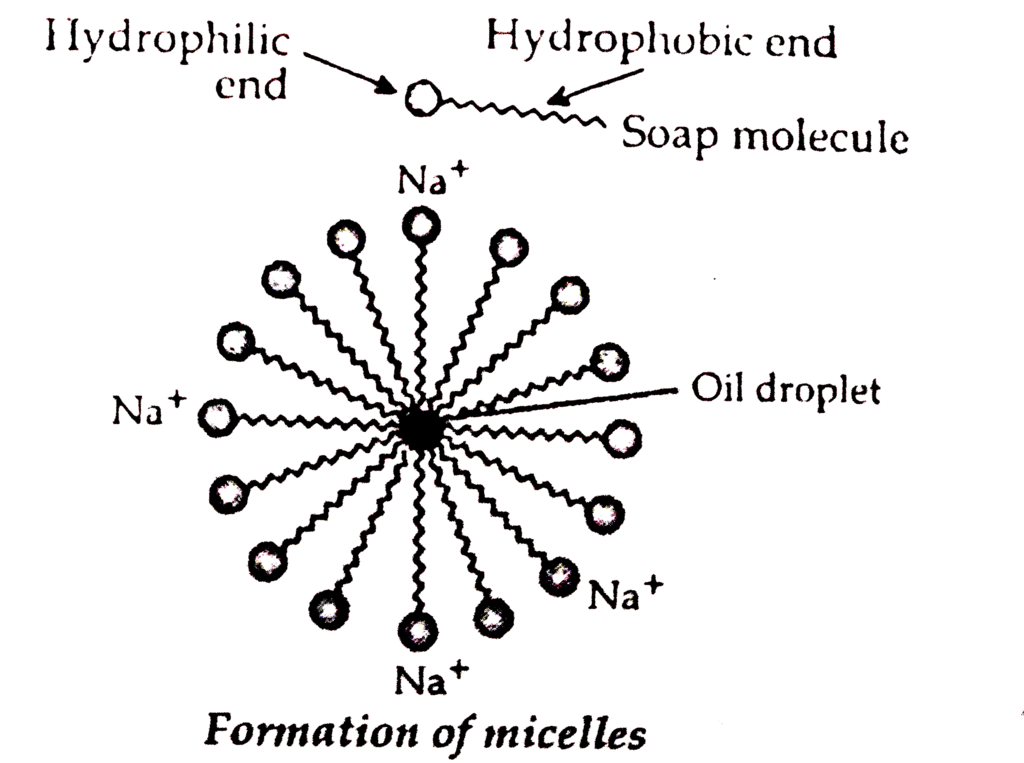
Q7: Why are certain compounds called hydrocarbons? Write the general formula for homologous series of alkanes, alkenes and alkynes and also draw the structure of the first member of each series. Write the name of the reaction that converts alkenes into alkanes and also write a chemical equation to show the necessary conditions for the reaction to occur. (AI 2017)
 View Answer
View Answer 
Ans: Compounds consisting of carbon and hydrogen are known as hydrocarbons.
(a) Saturated hydrocarbons: Alkanes (CnH2n+2) are the compounds of carbon that have a single bond.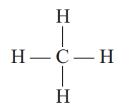
(b) Unsaturated hydrocarbons: The compounds of carbon having double bonds are alkene (CnH2n) and having triple bonds are alkyne (CnH2n-2). The reaction which converts unsaturated hydrocarbons to saturated hydrocarbons i.e. alkenes to alkane is known as hydrogenation reaction. It is used to obtain ghee from oil.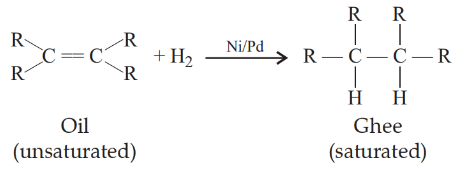
Q8: A student took four test tubes P, Q, R and S and filled about 8 mL of distilled water in each. After that he dissolved an equal amount of Na2SO4 in P, K2SO4 in Q, CaSO4 in R and MgSO4 in S. On adding an equal amount of soap solution and shaking each test tube well, a good amount of lather will be obtained in the test tubes:
(a) P and Q
(b) P and R
(c) P, Q and S
(d) Q, R and S
 View Answer
View Answer 
Ans: (a)
Soap forms lather easily in soft water, while hard water (which contains calcium and magnesium ions) reacts with soap to form scum, preventing the formation of lather.
In this case:
(i) Test tube P contains Na₂SO₄ (sodium sulfate), and Test tube Q contains K₂SO₄ (potassium sulfate). Both sodium and potassium salts do not contribute to water hardness, so they will allow soap to form lather.
(ii) Test tube R contains CaSO₄ (calcium sulfate), and Test tube S contains MgSO₄ (magnesium sulfate). Both calcium and magnesium contribute to water hardness, which prevents lather formation.
Therefore, a good amount of lather will be obtained in test tubes P and Q.
Q9: A student is given equal amount of three samples of water with temporary hardness labelled as ‘A’, ‘B’ and ‘C’. He keeps the three samples at different temperatures – A at room temperature, B at 50 ºC and C at 95 ºC. Which sample will give maximum amount of lather when 10 mL of soap solution is added to each sample and shaken for equal time?
(a) A only
(b) Both A and B
(c) Both B and C
(d) C only (CBSE 2017)
 View Answer
View Answer 
Ans: (d)
In this scenario:
- Sample C is kept at 95 ºC, which is close to boiling temperature. At this high temperature, the temporary hardness will be significantly reduced due to the decomposition of bicarbonates, allowing more lather to form when soap is added.
- Samples A (at room temperature) and B (at 50 ºC) will still retain more temporary hardness than sample C, and therefore will produce less lather.
Thus, sample C will give the maximum amount of lather, making the correct answer (d) C only.
Q10: What is a saturated hydrocarbon? Write the formula of any one saturated hydrocarbon. (CBSE 2017)
 View Answer
View Answer 
Ans: A saturated hydrocarbon is a type of hydrocarbon where the carbon atoms are connected by only single covalent bonds. This means that each carbon atom is bonded to as many hydrogen atoms as possible.The formula for one example of a saturated hydrocarbon is CH4, which is known as methane.
Q11: Ethanol reacts with sodium and forms two products. These are:
(a) sodium ethanoate and hydrogen
(b) sodium ethanoate and oxygen
(c) sodium ethoxide and hydrogen
(d) sodium ethoxide and oxygen (CBSE 2017, 16, 14, 11)
 View Answer
View Answer 
Ans: (c)
When ethanol (C₂H₅OH) reacts with sodium (Na), it forms sodium ethoxide (C₂H₅ONa) and hydrogen gas (H₂) as products. The reaction is as follows: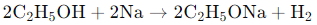 In this reaction, sodium displaces hydrogen from ethanol, resulting in the formation of sodium ethoxide and the release of hydrogen gas.
In this reaction, sodium displaces hydrogen from ethanol, resulting in the formation of sodium ethoxide and the release of hydrogen gas.
Thus, the correct answer is (c) sodium ethoxide and hydrogen.
Previous Year Questions 2016
Q1: Write the next homologue of each of the following: (Delhi 2016)
(i) C2H4
(ii) C4H6
 View Answer
View Answer 
Ans. (i) C2H4 belongs to alkene series having general formula, CnH2n.
Thus, next homologue will be C3H2x3 = C3H6
(ii) C4H6 belongs to alkyne series having general formula, CnH2n-2.
Thus, next homologue will be C5H2x5-2 = C5H8
Q2: Name the following compounds: (Delhi 2016)
(a) CH3—CH2—OH
(b) 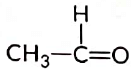
 View Answer
View Answer 
Ans: (a) CH3 - CH2 - OH: Ethanol (Alcohol)
(b) 
Q3: Select saturated hydrocarbons from the following: (Delhi 2016)
C3H6; C5H10; C4H10; C6H14; C2H4
 View Answer
View Answer 
Ans: Saturated hydrocarbons (alkanes) follow the general formula CnH2n+2. From the given compounds, the following satisfy this formula:
- C4H10
- C6H14
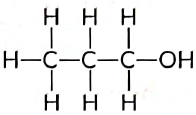
Q4: Write the name and structure of an alcohol with three carbon atoms in its molecule. (Al 2016)
 View Answer
View Answer 
Ans: An alcohol with three carbon atoms is called propanol. Its structure can be represented as follows:
- Carbon chain: C-C-C
- Hydroxyl group: -OH attached to one of the carbons
In summary, propanol consists of:
- Three carbon atoms
- One hydroxyl group
Q5: Write the name and structure of an alcohol with four carbon atoms in its molecule. (AI 2016)
 View Answer
View Answer 
Ans: An alcohol with four carbon atoms is butanol and its structure is: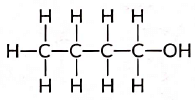
Q6: Write the name and structure of an aldehyde with four carbon atoms in its molecule. (AI 2016)
 View Answer
View Answer 
Ans: An aldehyde with four carbon atoms is butanal and its structure is: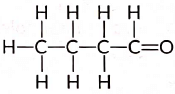
Q7: Which element exhibits the property of catenation to maximum extent and why? (Foreign 2016)
 View Answer
View Answer 
Ans: Carbon exhibits the property of catenation to the maximum extent due to several key factors:
- Carbon can form strong bonds with other carbon atoms, creating large and complex molecules.
- This ability allows for various structures, including long chains, branched chains, and rings.
- Carbon's small size contributes to the strength of the carbon-carbon bonds, making them stable.
- With a valency of four, carbon can bond with up to four other atoms, enhancing its versatility.
Other elements, like silicon, show limited catenation, forming fewer and more reactive compounds.
Q8: Write the name and molecular formula of the fourth member of alkane series. (Foreign 2016)
 View Answer
View Answer 
Ans: The general formula of the alkane series is CnH2n+2. For fourth member of alkane series, n = 4
∴ C4H2 x 4 + 2 = C4 H10 i.e., butane.
Q9: What is homologous series of carbon compounds? (Foreign 2016)
 View Answer
View Answer 
Ans: A homologous series is a group of organic compounds that share the same functional group and exhibit similar chemical properties. The key characteristics include:
- Each member differs from the next by a -CH2- unit.
- They have a consistent pattern in their physical properties, such as boiling points and solubility.
- The chemical properties remain similar due to the presence of the same functional group.
For example, compounds like alcohols and alkenes form homologous series where each successive compound has one more carbon atom than the previous one.
Q10: When ethanol reacts with ethanoic acid in the presence of cone. H2SO4, a substance with fruity smell is produced. Answer the following:
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of cone. H2SO4 in this reaction. (Delhi 2016)
 View Answer
View Answer 
Ans: (i) When ethanol reacts with ethanoic acid in the presence of concentrated H₂SO₄, the product formed is ethyl ethanoate, which belongs to the class of ester compounds known for their fruity smell.
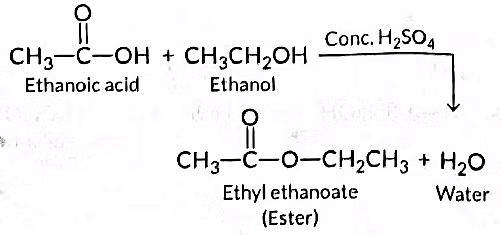
(ii) The role of concentrated H₂SO₄ in this reaction is two fold:
- It acts as a dehydrating agent, helping to remove water.
- It serves as a catalyst to speed up the reaction.
Q11: Write chemical equation of the reaction of ethanoic acid with the following:
(a) Sodium;
(b) Sodium hydroxide;
(c) Ethanol
Write the name of one main product of each reaction. (Al 2016)
 View Answer
View Answer 
Ans: Ethanoic acid reacts with sodium as well as sodium hydroxide to form sodium ethanoate.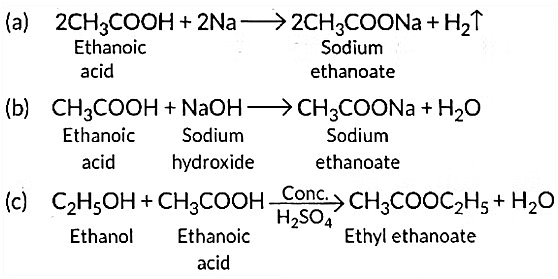
Q12: Write three different chemical reactions showing the conversion of ethanoic acid to sodium ethanoate. Write balanced chemical equation in each case. Write the name of the reactants and the products other than ethanoic acid and sodium ethanoate in each case. (Al 2016)
 View Answer
View Answer 
Ans: Ethanoic acid reacts with Na2CO3 to form sodium ethanoate and CO2 gas is liberated.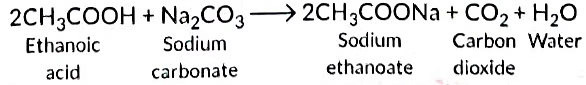
With sodium hydrogen carbonate it forms sodium ethanoate.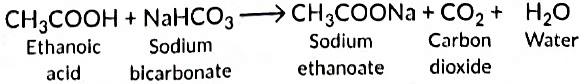
With NaOH It forms sodium ethanoate.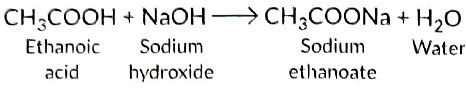
Q13: List two tests for experimentally distinguishing between an alcohol and a carboxylic acid and describe how these tests are performed. (Al 2016)
 View Answer
View Answer 
Ans: Tests for distinguishing between an alcohol and a carboxylic acid are:
(i) Litmus test: When we place a drop of carboxylic acid on blue litmus paper it turns red while alcohol will not change the colour of blue litmus paper.
(ii) Sodium hydrogen carbonate test/sodium carbonate
test: If a pinch of NaHCO3 or Na2CO3 is added to two test tubes containing alcohol and carboxylic acid respectively, then test tube containing carboxylic acid will show the evolution of colourless gas with brisk effervescence while test tube containing alcohol does not show any reaction.
Q14: Why is reaction between when soap is added to water? Will a micelle be formed in other solvents such as ethanol also? State briefly how the formation of micelles help to clean the clothes having oily spots. (Foreign 2016)
 View Answer
View Answer 
Ans: A micelle is a tiny cluster of molecules with non-polar groups inside and hydrophilic groups outside. When soap is added to water:
- The hydrophobic tails of soap molecules avoid water, forming a cluster.
- The hydrophilic heads remain in contact with water, stabilising the structure.
If ethanol is used instead of water, micelles will not form because ethanol lacks the necessary polar characteristics.The formation of micelles helps clean oily spots on clothes by:
- Trapping oily dirt in the centre of the micelle.
- Allowing the dirt to be easily rinsed away due to the colloidal nature of micelles.
Thus, soap effectively removes grease and dirt from fabrics.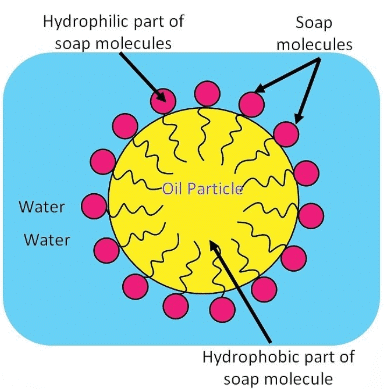
Q15: Which of the following are correct structural isomers of C4H10?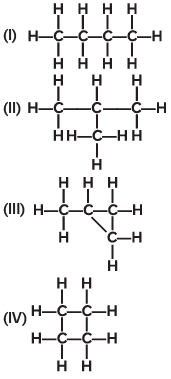
(a) (I) and (III)
(b) (II) and (IV )
(c) (I) and (II)
(d) (III) and (IV ) (CBSE 2016, 15, 14, 11)
 View Answer
View Answer 
Ans: (c)
The molecular formula C₄H₁₀ represents butane and its isomer isobutane (also called 2-methylpropane).
Structures (I) and (II)in the image represent these two structural isomers of C₄H₁₀:
(I) shows a straight chain structure (n-butane).
(II) shows a branched structure (isobutane or 2-methylpropane).
Thus, the correct answer is (c) (I) and (II).
Q15: Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of the catenation of the two elements. Give reasons. (CBSE 2016, 11, 10)
 View Answer
View Answer 
Ans: Catenation is the ability of an atom to bond with other atoms of the same element. This property is notably exhibited by both carbon and silicon, but carbon demonstrates it to a far greater extent.
- Carbon can form long chains, branched structures, or rings due to its strong carbon-carbon bonds.
- Carbon has a valency of four, allowing it to bond with up to four other atoms, including other carbon atoms.
- This results in a vast number of stable compounds, as the bonds formed are strong and do not easily break.
- In contrast, silicon can form chains of only seven or eight atoms with hydrogen, but these compounds are highly reactive.
Overall, the unique ability of carbon to catenate leads to an extensive variety of compounds, making it essential for life and numerous applications.

Previous Year Questions 2015
Q1: Write the name and formula of the 2nd member of homologous series having general formula CnH2n. (Delhi 2015)
 View Answer
View Answer 
Ans: Homologous series of alkenes have general formula, CnH2n whose first member is ethene.
2nd member of homologous series of alkenes is C3H6 i.e., propene.
Q2: Write the name and formula of the 2nd member of homologous series having general formula CnH2n+2. (Delhi 2015)
 View Answer
View Answer 
Ans: Alkanes have general formula, CnH2n + 2.
2nd member of homologous series of alkanes is C2H6 i.e., ethane.
Q3: Write the name and formula of the 2nd member of homologous series having general formula CnH2n-2. (Delhi 2015)
 View Answer
View Answer 
Ans: General formula, CnH2n-2 belongs to alkyne series. The second member of this series is propyne i.e., (C3H4) or CH3—C≡CH.
Q4: Write the number of covalent bonds in the molecule of ethane. (Al 2015, Delhi 2014)
 View Answer
View Answer 
Ans: The structural formula of ethane (C2H6) is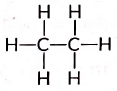 There are total 7 covalent bonds. Six C — H covalent bonds and one C — C covalent bond.
There are total 7 covalent bonds. Six C — H covalent bonds and one C — C covalent bond.
Q5: Write the number of covalent bonds in the molecule of butane, C4H10. (AI 2015)
 View Answer
View Answer 
Ans: Butane (C4H10) has the following structural formula as: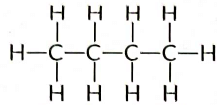
Butane (C4H10) contains a total of 13 covalent bonds. This includes:
- 10 C — H bonds
- 3 C — C bonds
Q6: With the help of an example, explain the process of hydrogenation. Mention the essential conditions for the reaction and state the change in physical property with the formation of the product. (Delhi 2015)
 View Answer
View Answer 
Ans: The process of adding hydrogen to an unsaturated compound is called hydrogenation. For example, the hydrogenation of ethene leads to the formation of ethane.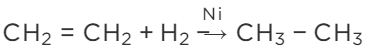
Conditions for Hydrogenation
(i) Presence of an unsaturated compound (i.e. an unsaturated hydrocarbon)
(ii) Presence of a catalyst such as finely divided palladium or nickel
In case of the hydrogenation of vegetable oils, liquid unsaturated fatty acids are converted into solid saturated fatty acids.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science Chapter 4 Previous Year Questions - Carbon and its compounds
| 1. What are the main characteristics of carbon and its compounds? |  |
| 2. How does the structure of carbon compounds affect their properties? |  |
| 3. What are some common reactions of carbon compounds? |  |
| 4. What is the significance of carbon compounds in everyday life? |  |
| 5. How are carbon compounds classified and what are their uses? |  |

















