Biomolecules Chapter Notes | Biology Class 11 - NEET PDF Download
| Table of contents |

|
| What is Biomolecule? |

|
| How to Analyse Chemical Composition? |

|
| Primary and Secondary Metabolites |

|
| Biomacromolecules |

|
| Proteins |

|
| Structure of Proteins |

|
| Polysaccharides |

|
| Nucleic Acids |

|
| Enzymes |

|
In our world, there are many different kinds of living things. But do they all have the same building blocks? When we look at plants, animals, or tiny organisms like microbes, and compare them to particles from the ground, like soil or rocks, we find they're made of similar particles. They all have elements like carbon, hydrogen, and oxygen. However, if we look closer, we see that living things have more carbon and hydrogen compared to other elements than non-living things like soil or rocks.
What is Biomolecule?
Biomolecules are chemical compounds present in living beings, necessary for their functioning and existence. They serve as the fundamental components of life and constitute various types such as proteins, carbohydrates, lipids, enzymes, and nucleic acids. These biomolecules come in diverse sizes and shapes, contributing to the complexity and diversity of living organisms.
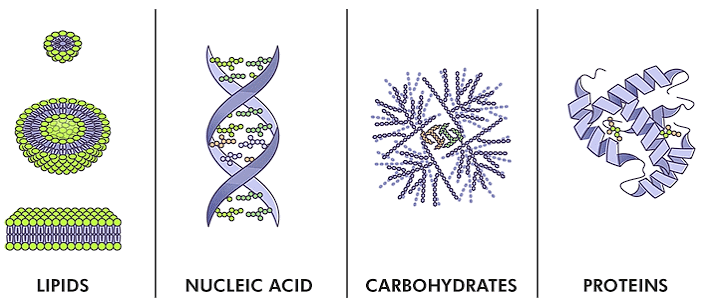 Types of Biomolecules
Types of Biomolecules
Biomolecules serve as the foundational elements of life, contributing to the maintenance and operation of numerous biological processes. Ranging from tiny molecules like micromolecules (primary metabolites) to important macromolecules such as hormones, proteins, and lipids, they exhibit a wide range of sizes and functions within living systems.
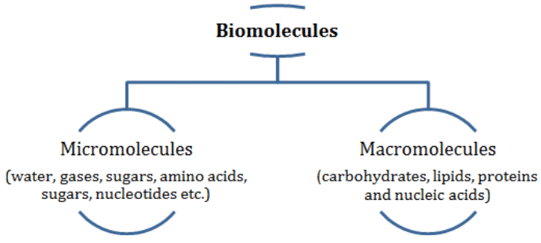 Biomolecules
Biomolecules
How to Analyse Chemical Composition?
To understand what substances are present in living organisms, we need to analyze their chemical composition. This involves breaking down samples of living tissue and separating the different compounds they contain.
Separation Process:
- Initial Extraction: Living tissue is ground up in trichloroacetic acid to create a thick slurry.
- Filtration: The slurry is filtered to obtain two fractions: the acid-soluble pool (filtrate) and the acid-insoluble fraction (retentate).
- Compound Isolation: Scientists use various techniques to separate and purify individual compounds from the acid-soluble pool.
Analytical Techniques:
- Identification: Analytical methods are applied to isolated compounds to determine their molecular formula and probable structure.
- Functional Group Analysis: Functional groups such as aldehydes, ketones, and aromatic compounds are identified from a chemical standpoint, while from a biological perspective, compounds are classified into amino acids, nucleotide bases, and fatty acids.
Key Biomolecules:
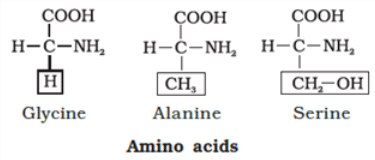
- Amino Acids: Amino acids are organic compounds with an amino group and an acidic group on the same carbon, known as the α-carbon. This is why they're called α-amino acids. They have four substituent groups:hydrogen, carboxyl, amino, and a variable R group. There are 20 types of amino acids found in proteins, each with a different R group. Amino acids can be acidic, basic, or neutral, depending on the number of amino and carboxyl groups. They also include aromatic amino acids like tyrosine and phenylalanine. A unique property of amino acids is their ability to ionize in different pH levels, altering their structure.
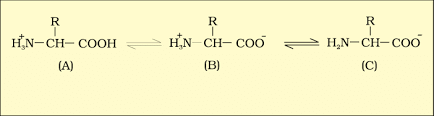 B is called zwitterionic form
B is called zwitterionic form
- Lipids: Lipids are usually not soluble in water. They can be simple fatty acids, which consist of a carboxyl group attached to an R group, which can vary from a methyl to longer chains of carbon atoms. For instance, palmitic acid has 16 carbons, while arachidonic acid has 20. Fatty acids can be saturated (no double bonds) or unsaturated (with double bonds). Glycerol, a trihydroxy propane, is another basic lipid. Many lipids contain both glycerol and fatty acids, forming monoglycerides, diglycerides, and triglycerides, commonly known as fats and oils based on their melting points. Phospholipids, containing phosphorus, are found in cell membranes, with lecithin being a familiar example. Some tissues, like neural tissues, have more complex lipid structures.
 Carbohydrates, Amino acids, Fats and oils (lipids)
Carbohydrates, Amino acids, Fats and oils (lipids)
- Nucleic Acids: Living organisms contain various carbon compounds with heterocyclic rings, including nitrogen bases such as adenine, guanine, cytosine, uracil, and thymine. When these bases are attached to a sugar, they're called nucleosides. If a phosphate group is also attached to the sugar, they're called nucleotides. Examples of nucleosides include adenosine, guanosine, thymidine, uridine, and cytidine, while nucleotides include adenylic acid, thymidylic acid, guanylic acid, uridylic acid, and cytidylic acid. Nucleic acids like DNA and RNA are made up of nucleotides and serve as genetic material.
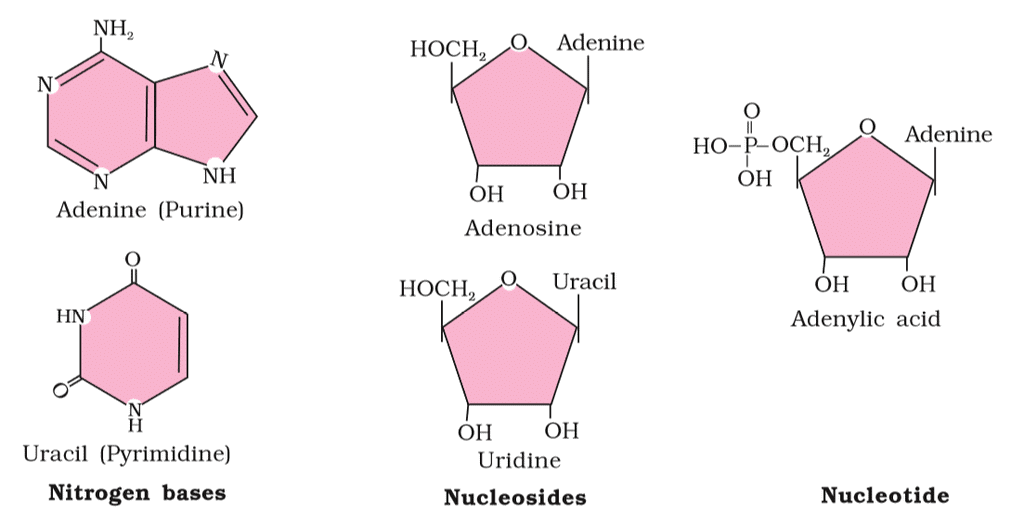 Nucleic Acids
Nucleic Acids
Primary and Secondary Metabolites
- The most interesting part of chemistry involves isolating and understanding thousands of compounds found in living organisms, both small and large, and possibly creating them artificially.
- These compounds, including amino acids and sugars, are collectively referred to as "metabolites" due to their role in metabolism.
- Animal tissues contain a wide range of these compounds known as primary metabolites.
- However, plant, fungal, and microbial cells contain many additional compounds known as secondary metabolites, such as alkaloids, flavonoids, and essential oils.
- While primary metabolites have known functions in normal physiological processes, the roles of secondary metabolites are not fully understood yet.
- However, many secondary metabolites, like rubber, drugs, and spices, are beneficial to humans, while others have ecological significance.
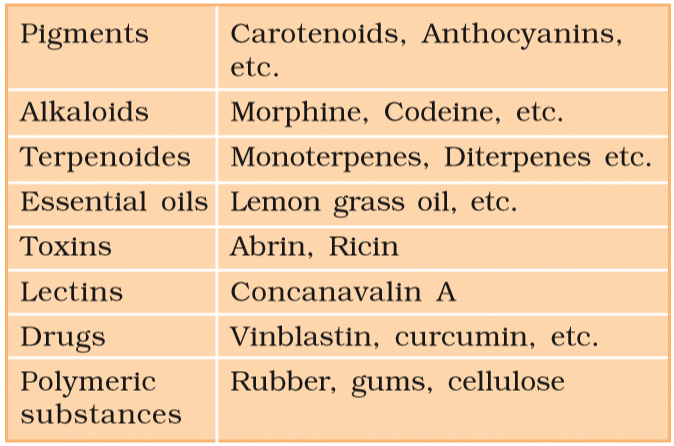 Some Secondary Metabolites
Some Secondary Metabolites
Biomacromolecules
- Compounds in the acid-soluble pool have molecular weights ranging from 18 to around 800 daltons (Da).
- The acid-insoluble fraction contains proteins, nucleic acids, polysaccharides, and lipids, all with molecular weights exceeding ten thousand daltons.
- Biomolecules are categorized into micromolecules (molecular weights less than one thousand daltons) and macromolecules (found in the acid-insoluble fraction).
- Lipids, despite their small molecular weight, are classified as macromolecules due to their presence in cell membranes and other structures disrupted during tissue grinding.
- The acid-soluble pool reflects cytoplasmic composition, while macromolecules from cytoplasm and organelles constitute the acid-insoluble fraction.
- Water is the most abundant chemical in living organisms when considering chemical composition.
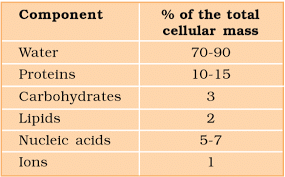 Average Composition of Cells
Average Composition of Cells
Proteins
They are polypeptide chains made up of amino acids. There are 20 types of amino acids joined together by peptide bond between amino and carboxylic group. There are two kinds of amino acids-
- Essential amino acids are obtained by living organism along with food.
- Non-essential amino acids can be prepared by our body from raw materials.
The main functions of protein in living cell are
- Transport of nutrient across the membrane.
- Fight infectious organisms.
- Produce enzyme and proteins.
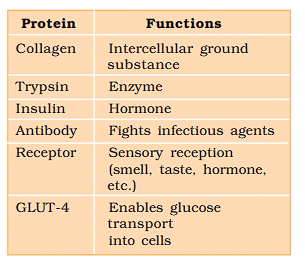 Some Proteins and their Functions
Some Proteins and their Functions
Collagen is the most abundant protein in animal world.
Structure of Proteins
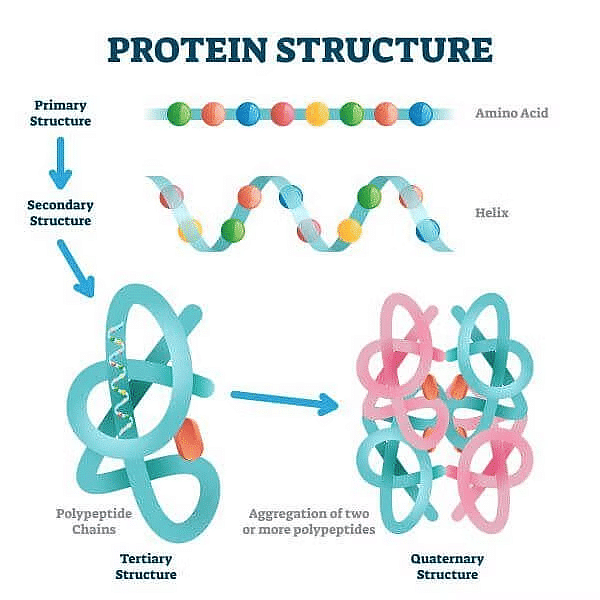
(a) Primary Structure of Protein
It is the basic structure of protein in which a number of polypeptides are involved having sequence of amino acids. The first amino acid of sequence is called N-terminal amino acid and last amino acid of peptide chain is called C-terminal amino acid.
(b) Secondary Structure of Protein
Its threads forms helix. There are three types of secondary structure- α helix, β pleated and collagen. In α helix, the polypeptide chain is coiled spirally in right handed manner. In β pleated secondary proteins two or more polypeptide chains are interconnected by hydrogen bonds. In collagen there are three strands or polypeptides coiled around one another by hydrogen bonds.
(c) Tertiary Structure of Protein
In this, long protein chain is folded upon itself like a hollow woollen ball to give three dimensional view of protein.(d) Quaternary Structure of Protein
In this, each polypeptide develops its own tertiary structure and function as subunit of protein. Eg. Hemoglobin. In adult human hemoglobin 4 sub-units are involved. The two subunits are of α type and two subunits of β types.
Polysaccharides
- The acid-insoluble pellet also contains polysaccharides, which are long chains of sugars.
- Polysaccharides are made up of different monosaccharides and resemble threads, with glucose being a common building block.
- Cellulose, a homopolymer of glucose, is a primary component of plant cell walls.
- Starch and glycogen are variants of cellulose, serving as energy stores in plants and animals, respectively.
- Inulin is another polysaccharide made of fructose.
- Polysaccharide chains, like glycogen, have a reducing end and a non-reducing end, with branching.
- Starch forms helical structures capable of holding I2 molecules, resulting in a blue coloration, while cellulose lacks such complex helices and cannot hold I2.
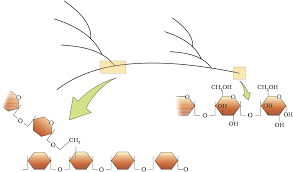 Diagrammatic representation of a portion of glycogen
Diagrammatic representation of a portion of glycogen
Nucleic Acids
Nucleic acids are polynucleotides. A nucleic acid has three chemically distinct components- heterocyclic compound ( nitrogenous base), polysaccharides ( ribose/ deoxy-ribose sugar) and phosphate or phosphoric acid.
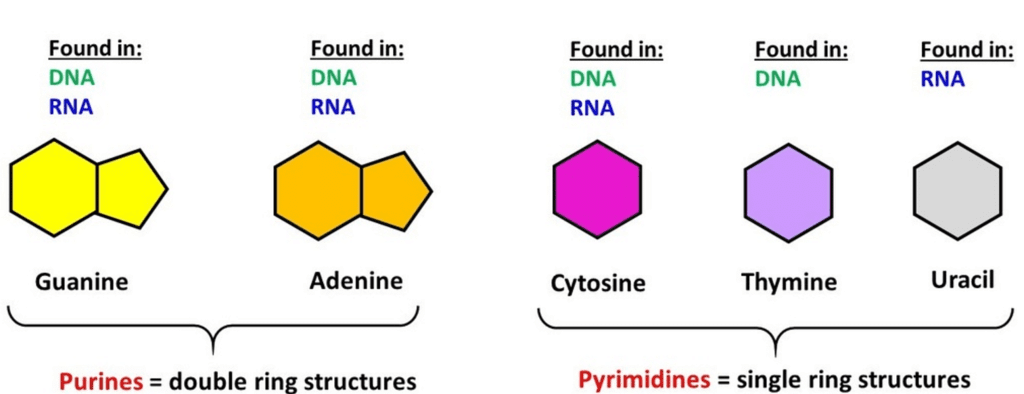
- The sugar found in nucleic acid is either ribose or deoxyribose. Nucleic acid containing deoxyribose sugar is called DNA (Deoxyribonucleic Acid) and those containing ribose sugars are called RNA (Ribonucleic acid).
- Biomolecules are constantly being changed into some other biomolecules and also made from other biomolecules. This breaking and making is through chemical process called metabolism.
- In living organism, all the metabolic reactions are enzyme catalyzed. Catalysts are those substances that alter the rate of reaction. The protein with catalytic power is called enzyme.
Metabolic Basis for Living Organism
- The metabolic pathways that lead to more complex structure from simpler structure are called biosynthetic or anabolic pathways and those pathways that lead to simpler structure from complex structure are called catabolic pathways.
- Photosynthesis and protein synthesis are example of anabolic pathway. Respiration and digestion are examples of catabolic pathway.
- ATP (adenosine triphosphate) is the most important form of energy currency in living world.
- All living organism exist in steady state characterized by concentration of each of the metabolites. The living state is a non-equilibrium steady state to be able to perform work.
Enzymes
Enzymes and Their Structure
- Most enzymes are proteins, but some nucleic acids called ribozymes can also act as enzymes.
- An enzyme can be represented by a line diagram, and like any protein, it has a primary structure(amino acid sequence), secondary structure, and tertiary structure.
- In the tertiary structure, the protein chain folds and crisscrosses, creating crevices or pockets, one of which is the active site.
- The active site is where the substrate fits, allowing enzymes to catalyze reactions at a high rate.
Differences between Enzyme Catalysts and Inorganic Catalysts
- Enzyme catalysts differ from inorganic catalysts in several ways. One major difference is that inorganic catalysts work efficiently at high temperatures and pressures, while enzymes can be damaged at high temperatures (usually above 40°C).
- However, enzymes isolated from organisms that thrive in extreme conditions, such as hot vents and sulfur springs, can remain stable and retain their catalytic abilities at high temperatures (up to 80°-90°C).
- This thermal stability is an important characteristic of enzymes from thermophilic organisms.
Chemical Reactions
To grasp the concept of enzymes, we first need to understand what a chemical reaction is. Chemical compounds can undergo two types of changes: physical and chemical.
Physical Changes
- A physical change involves a change in shape without the breaking of bonds. For example, when ice melts into water or when water evaporates into vapor, these are physical processes.
Chemical Changes
- A chemical change occurs when bonds are broken and new bonds are formed during the transformation of substances. For instance, the reaction between barium hydroxide and sulfuric acid to form barium sulfate and water is a chemical reaction. Similarly, the hydrolysis of starch into glucose is an example of an organic chemical reaction.
Ba(OH)2 + H2SO4 → BaSO4 + 2H2O
Rate of Reactions
- The rate of a physical or chemical process refers to the amount of product formed per unit time. It can be expressed as:
rate = δP/ δ t - Rate can also be called velocity if the direction is specified. The rates of physical and chemical processes are influenced by factors such as temperature. A general rule is that the rate doubles or halves for every 10°C change in either direction.
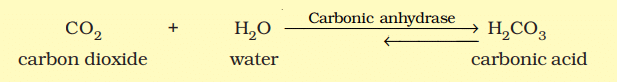
Catalysis by Enzymes
- Catalysed reactions occur at rates significantly higher than uncatalysed ones. Enzyme-catalysed reactions, for example, are much faster than the same reactions without enzymes.
- For instance, the reaction between carbon dioxide and water to form carbonic acid is very slow without the enzyme carbonic anhydrase. Without the enzyme, about 200 molecules of carbonic acid are formed in an hour. However, with carbonic anhydrase, the reaction speeds up dramatically, producing about 600,000 molecules per second. The enzyme accelerates the reaction rate by approximately 10 million times, showcasing the incredible power of enzymes.
Metabolic Pathways
- There are thousands of types of enzymes, each catalysing a unique chemical or metabolic reaction. A series of chemical reactions, each catalysed by the same enzyme complex or different enzymes, is called a metabolic pathway.
- For example, the conversion of glucose to pyruvic acid involves ten different enzyme-catalysed metabolic reactions. Depending on the conditions, different end products can be formed from this pathway. In skeletal muscle under anaerobic conditions, lactic acid is produced. Under aerobic conditions, pyruvic acid is formed. In yeast, during fermentation, the same pathway leads to the production of ethanol (alcohol).
Glucose → 2 Pyruvic acid
How do Enzymes bring about such High Rates of Chemical Conversions?
Enzymes and Substrates
- Enzymes are proteins with a special area called the "active site." This is where the enzyme does its work.
- A chemical reaction happens when an enzyme converts a substance called a "substrate" into a product.
- Symbolically, this process is shown as:S → P, where S is the substrate, and P is the product.
Formation of the ES Complex
- For the reaction to occur, the substrate (S) must fit into the enzyme's active site, creating a temporary complex called the "ES complex." This is a crucial step in the process.
- The formation of this complex is a quick and temporary event.
- When the substrate binds to the enzyme's active site, it undergoes a change and forms a new structure called the "transition state." This is a critical stage in the reaction.
Transition State and Product Formation
- After the transition state is reached, the substrate is transformed into the product through bond breaking and making.
- Once the transformation is complete, the product is released from the enzyme's active site.
- In simpler terms, the substrate changes into the product, passing through the transition state and possibly other unstable intermediate structures along the way.
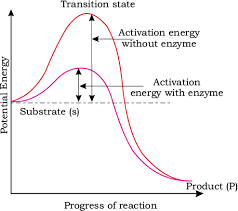 Concept of activation energy
Concept of activation energy
Energy and Stability
- The stability of these intermediate structures is related to their energy levels.
- When we look at this process on a graph, the y-axis represents potential energy, while the x-axis shows the progression of the structural transformation through the transition state.
- The difference in energy between the substrate (S) and the product (P) is important:
- If the product (P) is at a lower energy level than the substrate (S), the reaction is called "exothermic." This means that energy is released during the reaction.
- Whether the reaction is exothermic or endothermic (requiring energy), the substrate must first reach a higher energy state, known as the transition state.
- The energy difference between the substrate (S) and the transition state is called "activation energy." Enzymes help lower this energy barrier, making it easier for the substrate to transform into the product.
Nature of Enzyme Action
- Enzyme-Substrate Binding: The process begins with the binding of the substrate (S) to the enzyme (E) at the active site. This forms a highly reactive enzyme-substrate complex (ES).
- Induced Fit: Upon binding, the enzyme undergoes a conformational change, fitting more snugly around the substrate. This change is crucial for the catalytic process.
- Formation of Enzyme-Product Complex: The close proximity of the substrate in the active site facilitates the breaking of chemical bonds within the substrate, leading to the formation of the enzyme-product complex (EP).
- Product Release: The enzyme then releases the products (P) of the reaction, regenerating the free enzyme (E) in the process. The free enzyme is now ready to bind to another substrate molecule and repeat the catalytic cycle.
- The overall reaction can be summarized as: E + S ⇌ ES → EP → E + P
Factors Affecting Enzyme Activity
- Enzyme activity can be influenced by various factors that alter the tertiary structure of the protein.
- These factors include temperature,pH,substrate concentration, and the binding of specific chemicals that regulate enzyme activity.
Temperature and pH
- Enzymes typically operate within a narrow range of temperature and pH.
- Each enzyme has an optimum temperature and optimum pH at which it exhibits maximum activity.
- Activity declines when conditions are below or above these optimum levels.
- Low temperatures preserve enzymes in a temporarily inactive state, while high temperatures can denature proteins and destroy enzymatic activity.
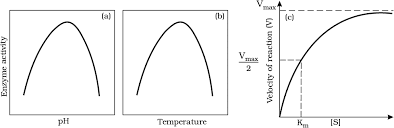 Effect of change in : (a) pH (b) Temperature and (c) Concentration of substrate on enzyme activity
Effect of change in : (a) pH (b) Temperature and (c) Concentration of substrate on enzyme activity
Concentration of Substrate
- As substrate concentration increases, the rate of the enzymatic reaction initially increases.
- However, it eventually reaches a maximum velocity (Vmax) that cannot be exceeded, regardless of further increases in substrate concentration.
- This limitation occurs because there are fewer enzyme molecules than substrate molecules.
- Once all enzyme molecules are saturated, there are no free enzymes available to bind with additional substrate molecules.
Enzyme Inhibition
- The activity of an enzyme can be sensitive to the presence of specific chemicals that bind to the enzyme.
- Inhibition occurs when a chemical binds to the enzyme and shuts off its activity, making the chemical an inhibitor.
- Competitive inhibitors are a type of inhibitor that closely resembles the substrate in molecular structure.
- These inhibitors compete with the substrate for the enzyme's substrate-binding site.
- As a result, the substrate cannot bind, leading to a decline in enzyme activity.
- For example, malonate inhibits succinic dehydrogenase by resembling the substrate succinate in structure.
- Competitive inhibitors are often used in the control of bacterial pathogens.
Classification and Nomenclature of Enzymes
Enzymes are biological catalysts that speed up chemical reactions in living organisms. They can be classified into different groups based on the type of reactions they facilitate. There are six main classes of enzymes, each with specific subclasses. Here’s a detailed look at each class:
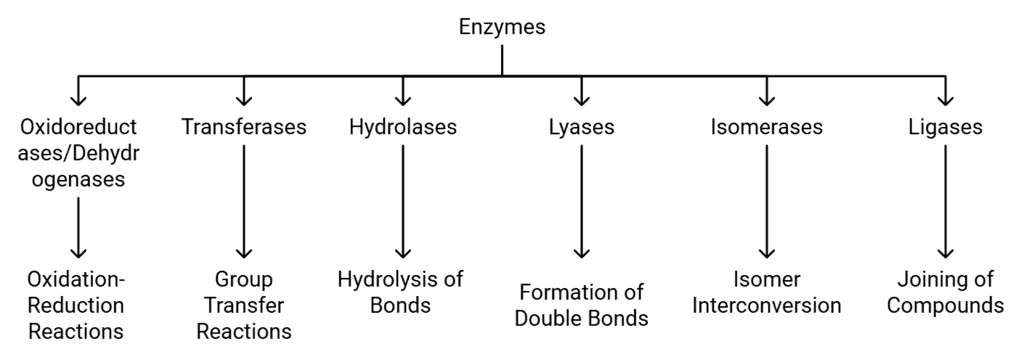 Classification of Enzymes
Classification of Enzymes
1. Oxidoreductases/Dehydrogenases
These enzymes are involved in oxidation-reduction reactions between two substrates, S and S’. In these reactions, one substrate is reduced while the other is oxidized. For example:
- S reduced + S’ oxidised→S oxidised + S’ reduced
2. Transferases
Transferases catalyze the transfer of a specific group (other than hydrogen) from one substrate to another. For instance:
- S - G + S’→S + S’ - G
3. Hydrolases
Hydrolases are responsible for the hydrolysis of various types of bonds, including:
- Ester bonds
- Ether bonds
- Peptide bonds
- Glycosidic bonds
- C-C bonds
- C-halide bonds
- P-N bonds
4. Lyases
Lyases catalyze the removal of groups from substrates through mechanisms other than hydrolysis, resulting in the formation of double bonds.
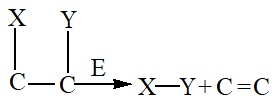
5. Isomerases
Isomerases facilitate the interconversion of optical, geometric, or positional isomers within a substrate.
6. Ligases
Ligases are involved in joining two compounds together, forming bonds such as C-O, C-S, C-N, or P-O bonds. For example, they may catalyze the joining of:
- C-O bonds
- C-S bonds
- C-N bonds
- P-O bonds
Co- Factors
Enzymes are made up of one or more polypeptide chains. However, there are instances where non-protein components called cofactors are necessary for the enzyme to be catalytically active. In such cases, the protein part of the enzyme is referred to as the apoenzyme.
Cofactors can be categorized into three types: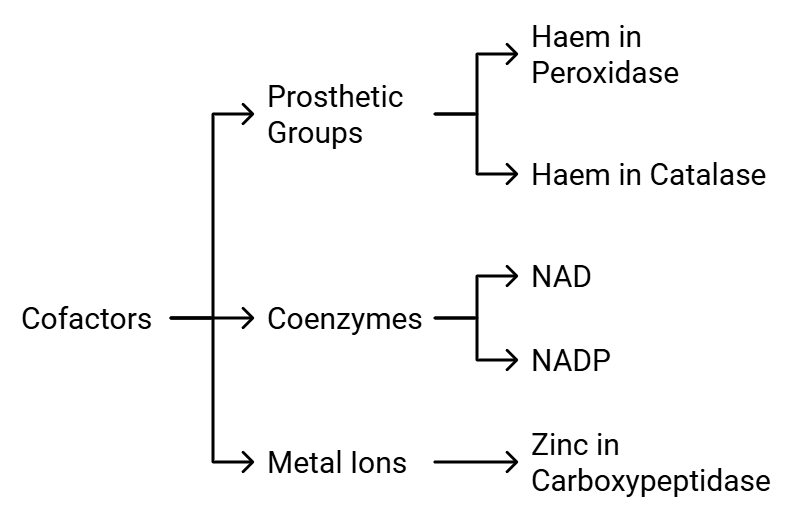 Types of Cofactors
Types of Cofactors
- Prosthetic Groups: These are organic compounds that are tightly bound to the apoenzyme. For instance, in enzymes like peroxidase and catalase, which break down hydrogen peroxide into water and oxygen, the prosthetic group is haem, which is an integral part of the enzyme's active site.
- Coenzymes: Coenzymes are also organic compounds, but their association with the apoenzyme is temporary, usually occurring during the catalytic process. Coenzymes often serve as cofactors in various enzyme-catalyzed reactions. Many coenzymes are derived from vitamins; for example, coenzymes like nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP) contain the vitamin niacin.
- Metal Ions: Some enzymes require metal ions for their activity. These metal ions form coordination bonds with side chains at the active site and also establish coordination bonds with the substrate. For instance, zinc acts as a cofactor for the proteolytic enzyme carboxypeptidase.
The catalytic activity of an enzyme is compromised when the cofactor is removed, highlighting the crucial role that cofactors play in the enzyme's catalytic function.
|
150 videos|399 docs|136 tests
|
FAQs on Biomolecules Chapter Notes - Biology Class 11 - NEET
| 1. What are biomolecules and why are they important? |  |
| 2. How can the chemical composition of biomolecules be analyzed? |  |
| 3. What are the differences between primary and secondary metabolites? |  |
| 4. What are biomacromolecules and their significance? |  |
| 5. How do enzymes function as proteins in biological systems? |  |





















