Class 10 Science: CBSE Sample Question Paper- Term I (2019-20) - 1 | Science Class 10 PDF Download
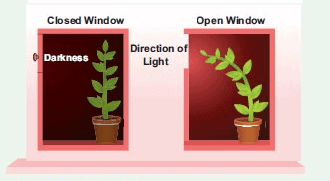
Ques 1: Explain in brief the cause of shoots of the plant bending towards light.
Ans: Stems are positive phototropic and bend towards the direction of light. The movement is due to occurrence of more auxin on the darker side and lesser auxin on the illuminated side. As a result, there is more growth on the darker side which causes the stem to bend towards light.
Ques 2: Write balanced equation for the reaction between magnesium and hydrochloric acid. Name the product obtained and also identify the type of reaction.
Ans: The balanced equation for the reaction between magnesium and hydrochloric acid is
Mg(s) + 2HCl(dil.) → MgCl2 (aq) + H2(g)
(1) The product formed is magnesium chloride and hydrogen gas. The type of reaction is displacement reaction.
Ques 3: A DC motor is rotating in clockwise direction. How can the direction of rotation be reversed?
Ans: The direction of rotation of the DC motor can be reversed by reversing the direction of current through the coil. This can be achieved by interchanging the terminals of the battery connected to the brushes of the motor.
Ques 4: A student added dilute HCl to Zn granules taken in a test tube. Identify the correct observation.
Ans: H2 gas is evolved.
Zn(s) + 2HCl(dil.) → ZnCl2(aq) + H2(g)
Ques 5: A young green plant receives sunlight from one direction only. What will happen to its shoots and roots?
Ans: Shoots of that plant will move to the direction of light and roots will move to the opposite direction of light.
Ques 6: Due to injury, flow of blood from vessels reduces the efficiency of pumping system. How is the leakage prevented?
Ans: Blood contains blood platelets. In the region of injury, the platelets burst. It helps in coagulation of blood and seal the place of leakage or injury.
Ques 7: Why is the zig-zag copper tube painted black in a solar water heater?
Ans: Black bodies are good absorbers of heat energy and copper is a good conductor of heat. Blackend copper pipes rapidly transfer the solar energy to the water and help in rapid heating. That's why copper tube is painted black in solar water heater.
Ques 8: Show how would you connect three resistors, each of resistance 6Ω, so that the combination has a resistance of (a)9Ω (b)4Ω
Ans: (a) When two 6Ω resistances are in parallel and the third is in series combination to this, the equivalent resistance will be 9Ω.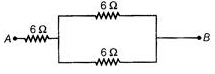
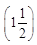
(b) When two are in series and the third is in parallel to them, then it will be 4Ω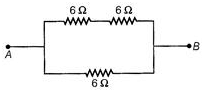
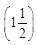
Ques 9: Several electric bulbs designed to be used on a 220 V electric supply line are rated 10 W. How many lamps can be connected in parallel with each other across the two wires of 220 V line if the maximum allowed current is 5 A?
Ans: In parallel, equivalent power is p' = p1 + p2 + p3 + ...n times = p + p + p ...n times = np
Potential difference (V) = 220
∴ Current drawn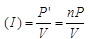
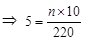
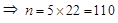
Thus, 110 bulbs can be connected in parallel with each other across the two wires of 220 V.
Ques 10: A current-carrying straight conductor is placed in the East-West direction. What will be the direction of the force experienced by this conductor due to the earth's magnetic field? How will this force get affected on (a) reversing the direction of flow of current? (b) doubling the magnitude of current?
Ans: The direction of the earth's magnetic field is from geographic South to North. Let the direction of current in the conductor be from West to East. Applying Fleming's left-hand rule, we find that the direction of the force acting on the conductor will be vertically upwards.
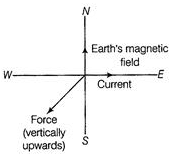
(a) By reversing the direction of current the direction of force experienced by the conductor will be reversed i. e., it acts vertically downwards.
(b) The magnitude of the force is doubled i.e., F ∝ I.
Ques 11: Under what conditions permanent electromagnet is obtained if a current-carrying solenoid is used? Support your answer with the help of a labelled circuit diagram.
Ans: Under following conditions permanent electromagnet is obtained if current-carrying solenoid is used
(i) The magnitude of direct current through the solenoid should be large.
(ii) The number of turns in the solenoid should be large and closely packed, so that a strong uniform magnetic field inside it is produced.
(iii) The rod kept inside is made of a magnetic materials such as steel.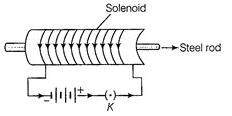
Ques 12: Crystals of copper sulphate are heated in a test tube for sometime.
(a) What is the colour of copper sulphate crystals (i) before heating and (ii) after heating?
(b) What is the source of liquid droplets seen on the inner upperside of the test tube during the heating process?
Ans: (a) (i) The colour before heating is blue.
(ii) The colour after heating is white.
(b) During the heating process the liquid droplets are the water of crystallisation present in the compound.
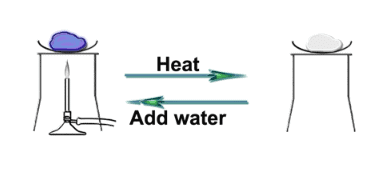 Ques 13: A compound X of sodium is commonly used in the kitchen for making crispy pakoras. It is also used for curing acidity in the stomach. Identify X. Write its chemical formulae. State the reaction which takes place when it is heated during cooking.
Ques 13: A compound X of sodium is commonly used in the kitchen for making crispy pakoras. It is also used for curing acidity in the stomach. Identify X. Write its chemical formulae. State the reaction which takes place when it is heated during cooking.
Ans: X is baking soda. It formula is NaKCO3. When sodium hydrocarbonate is heated during cooking, it releases carbon dioxide. The reaction involved is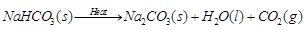
Ques 14: An ore on heating in air produces sulphur dioxide. Which process would you suggest for its concentration? Describe briefly any two steps involved in the conversion of this concentrated ore in the related metal.
Ans: When an ore on heating in air produces SO2, then the ore is a sulphide. Sulphide ores are concentrated by Froth floatation process. A concentrated sulphide ore is converted into the metal by (i) Roasting
(ii) Reduction of oxide Roasting.
The sulphide ore is heated in the presence of excess of air such that it changes to a metallic oxide. e.g.,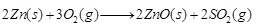
Reduction of oxide: The roasted ore is reduced to metal by heating with an appropriate reducing agent such as carbon, carbon monoxide or hydrogen. e.g.,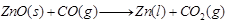
Ques 15: Write the balanced chemical equations for the following reactions
(a) Sodium carbonate on reaction with hydrochloric acid in equal molar concentrations gives sodium chloride and sodium hydrogen carbonate.
(b) Sodium hydrogen carbonate on reaction with hydrochloric acid gives sodium chloride, water and liberates carbon dioxide.
(c) Copper sulphate on treatment with potassium iodide precipitates cuprous iodide (Cu2I2), liberates iodine gas and also forms potassium sulphate.
Ans: (a)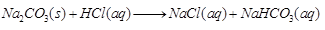
(b)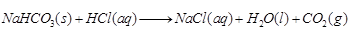
(c)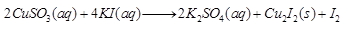
Ques 16: In the given diagram, it shows the milliammeter reading in the circuit. 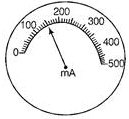
Write the value of current in the circuit.
Ans: Least count = 500/50 = 10 mA
Number of divisions = 16 Reading = 10 x 16 = 160 mA
Ques 17: What is the right procedure to remove chlorophyll from a destarched leaf?
Ans: The correct procedure to remove chlorophyll from a destarched leaf is to boil the destarched leaf in alcohol.
Ques 18: In the experimental set up to show that carbon dioxide (CO2) is given out during respiration, why potassium hydroxide (KOH) is kept in the small test tube inside the flask?
Ans: Potassium hydroxide (KOH) is kept in the small test tube inside the flask because it absorbs CO2 and thereby creates partial vacuum inside the flask.
Ques 19: In an experiment on studying the dependence of the current (I) flowing through a given resistor, on the potential difference (V) applied across it, a student is to change the value of the current. While doing so, what can he change?
Ans: Student can change the number of cells used or by setting the battery eliminator.
Ques 20: The following circuit diagram shows the experimental set up for the study of dependence of current on potential difference. Which two circuit components are connected in series?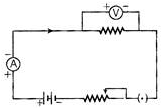
Ans: Ammeter and rheostat are connected in series because one common point exists between ammeter, cell and rheostat.
Ques 21: To prepare a good temporary mount of the petunia leaf peel showing many stomata, from which part the student should take the peel?
Ans: The student should take the peel from lower surface of the leaf.
Ques 22: The solar cooker is painted black from inside. Why?
Ans: The black surface of the solar cooker absorbs more heat as compared to the white surface. That's why it is painted black from inside.
Ques 23: A green layer is gradually formed on a copper plate left exposed to air for a week in a bathroom. What could this green substance be?
Ans: This green substance is due to the formation of basic copper carbonate [CuCO3 .Cu(OH)].
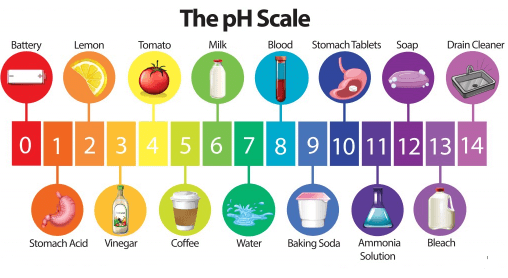
Ques 24: Four students measured the pH values of water, lemon juice and sodium bicarbonate solution. Write the correct sequence of solutions in decreasing order of pH value.
Ans: The pH values of water is 7, sodium bicarbonate is 8.4 and lemon juice is 2 to 6. Therefore, the correct decreasing sequence is Sodium bicarbonate > Water > Lemon juice
Ques 25: Suggest two ways to check the rancidity of food articles.
Ans: The two ways to check rancidity of food articles are
(i) Keep the food articles in airtight containers.
(ii) Keep the food articles in refrigerator.
Ques 26: You are provided with samples of bitter and sour food extracts. How can you test the presence of acids and bases among these solutions without tasting them?
Ans: Add beetroot extract in each of them separately. Observe the colour change. The colour of beetroot in sour substances and bitter substances will be different which shows the presence of acid in sour substance and base in bitter substances.
Ques 27: (a) Give an example of a combination reaction which is also exothermic reaction. (b) Complete the following chemical reaction and balance it. C6H12O6 + O2 → (c) Which two gases are evolved on heating ferrous sulphate?
Ans: (a)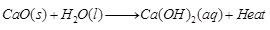
The above reaction is combination reaction as well as exothermic reaction.
(b) 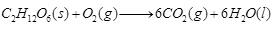
(c) SO2 and SO3 gases are evolved on heating 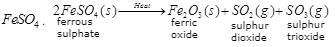
Ques 28: (a) Justify that brain is a highly protected organ. (b) Which part of the brain controls all the voluntary actions and the involuntary action of the body?
Ans: (a) The brain is a delicate organ which sits inside a bony box in our body. Inside the box, the brain is contained in a fluid-filled balloon which provides shock absorption. Thus, it is justified that brain is a highly protected organ.
(b) Voluntary actions are controlled by cerebrum and involuntary actions are controlled by medulla.
Ques 29: Write any three functions of the nervous system.
Ans: The three functions of the nervous system are
(i) It regulates involuntary actions and it controls and coordinates voluntary muscular activities.
(ii) It controls all the reflex actions in our body, thus protects us from harm.
(iii) It keeps us informed about the outside world through the sense organs.
Ques 30: Sujeet was making an electric bell. For that he was looking for an appropriate material. Sumit suggested him to use soft iron for this purpose and helped him in making electromagnet.
(a) What values are associated with Sumit?
(b) Why Sumit suggested for soft iron?
Ans: (a) Sumit is friendly, has scientific temperament and of helping nature.
(b) Any material having high permeability and low recentivity is suitable for making electromagnets. So, in electric bell, we use soft iron for making electromagnet.
Ques 31: (a) A current of 1 A flows in a series circuit containing an electric lamp and a conductor of when connected to a 10 V battery. Calculate the resistance of the electric lamp.
(b) Now if a resistance of is connected in parallel with this series combination, what change (if any) in current flowing through conductor and potential difference across the lamp will take place? Give reason.
Or
Three bulbs each having power P are connected in series in an electric circuit. In another circuit, another set of three bulbs of same power are connected in parallel to the same source.
(a) Will the bulbs in both the circuits glow with the same brightness? Justify your answer.
(b) Now let one bulb in each circuit get fused. Will the rest of the bulbs continue to glow in each circuit? Give reason.
(c) Representing each bulb by a resistor, draw circuit diagram for each case.
Ans: (a) Given, Current (I) = 1 A, Potential difference V = 10V, Rlamp = ? Rc = 5Ω
Total resistance in the circuit 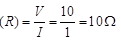
∴ Conductor of 5Ω and lamp in series, R = Rc = Rlamp ⇒ Rlamp R - Rc = 10 - 5 = 5Ω Potential difference across lamp 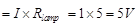 (b) When 10Ω resistance is connected in parallel with R, then total resistance R' in the circuit is given by
(b) When 10Ω resistance is connected in parallel with R, then total resistance R' in the circuit is given by 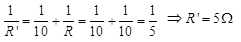
Current through the circuit 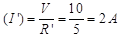 Since, 10Ω and R are in parallel,
Since, 10Ω and R are in parallel,
∴ Current through, 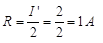 Thus, current through lamp and conductor of 5Ω in series is 1 A i.e., there is no change in current through conductor of 5Ω.
Thus, current through lamp and conductor of 5Ω in series is 1 A i.e., there is no change in current through conductor of 5Ω.
Potential difference across lamp = I'/2 (Rlamp) 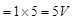 i.e., there is no change in potential difference across the lamp.
i.e., there is no change in potential difference across the lamp.
Or (a) Bulbs in parallel provide more illumination because of the following
(i) Each bulb gets same voltage and is equal to the applied voltage.
(ii) Each bulb draws required current from the mains. Hence, they work properly.
(b) When one bulb in each circuit get fused, In series Rest of the bulbs will not glow. This is because in series arrangement, there is only a single path for the flow of current. In parallel Rest of the bulbs will continue to glow as in parallel.
(i) Individual branch in the circuit completes its own circuit.
(ii) Different paths are available for the flow of current.
(c) Circuit diagram
For series 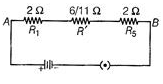
For parallel 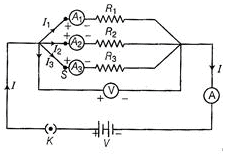
Ques 32: Draw a labelled diagram of an electric motor. Explain its principle and working. What is the function of split rings in an electric motor? Or A coil of insulated copper wire is connected to a galvanometer. What will happen if a bar magnet is (i) pushed into the coil withdrawn from inside the coil (iii) held stationary inside the coil?
Ans: An electric motor converts electrical energy into mechanical energy. The diagram of electric motor is shown below
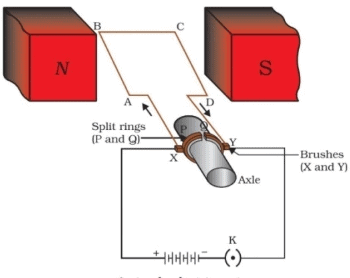 Principle
Principle
It works on the principle of magnetic effect of current. When a current-carrying conductor is placed perpendicular to the magnetic field, it experiences a force.
Working
Consider at the beginning, the arms AB and CD are perpendicular to the direction of applied magnetic field.
(a) Initially, let the current in the coil flows along the path ABCD. Then, according to the Fleming's left-hand rule, a downward force acts on the arm AB pushes it downwards and an upward force on arm CD pushes it upwards. These forces cause the coil to rotate in anti-clockwise direction about its own axis.
(b) After completing half rotation, the position of split rings interchanges due to which direction of current in the coil gets reversed and current flows along the path DCBA. Again according to Fleming's left-hand rule, the force acting on the arms AB and CD also get reversed. This makes the coil to rotate and complete the next half cycle of rotation in the same direction. Thus, the interchanging of split rings at each half turn, makes the coil to rotate continuously in the same direction as long as the current is passing through it. Function of split ring It reverses the direction of current in the armature coil.
Or
A current induces in a solenoid if a bar magnet is moved relative to it. This is the principle of electromagnetic induction.
(i) When a bar magnet is pushed into a coil of insulated copper wire, a current is induced momentarily in the coil. As a result, the needle of the galvanometer deflects momentarily in a particular direction.
(ii) When the bar magnet is withdrawn from inside the coil of the insulated copper wire, a current is again induced momentarily in the coil in the opposite direction. As a result, the needle of the galvanometer deflects momentarily in the opposite direction.
(iii) When a bar magnet is held stationary inside the coil no current will be induced in the coil. Hence, galvanometer will show no deflection.
Ques 33: (a) Draw a diagram of an excretory unit of human kidney and label the following Bowman's capsule, glomerulus, collecting duct and renal artery. (b) Write the important function of structural and functional unit of kidney. (c) Write any one function of an artificial kidney.
Or
(a) Draw a schematic representation of transport and exchange of oxygen and carbon dioxide during transportation of blood in human beings and label on it. Lung capillaries; Pulmonary artery to lungs Aorta to body; Pulmonary veins from lungs.
(b) What is the advantage of separate channels in mammals and birds for oxygenated and deoxygenated blood?
Ans: (a) The excretory unit of human kidney is nephron. Its diagram is given below
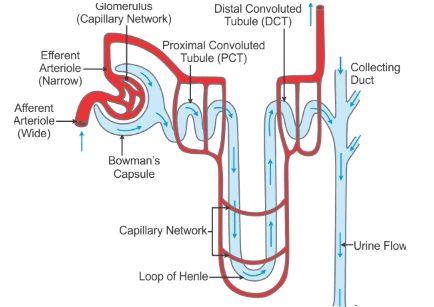
(b) Nephron takes part in filtration, reabsorption and selective secretion to form urine.
(c) Artificial kidneys helps in removal of toxins, relieve uraemia and remove wastes in patients with damaged kidneys.
OR
(a)
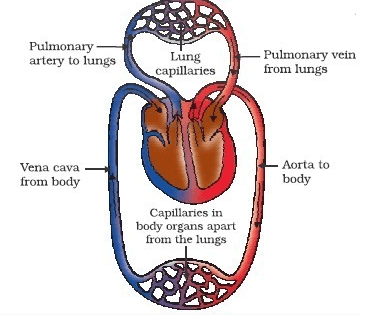 (b) It is necessary to separate oxygenated and deoxygenated blood in mammals and birds, because they need high energy and large amount of oxygen. The separation of oxygenated and deoxygenated blood provides high oxygen supply to the organs.
(b) It is necessary to separate oxygenated and deoxygenated blood in mammals and birds, because they need high energy and large amount of oxygen. The separation of oxygenated and deoxygenated blood provides high oxygen supply to the organs.
Ques 34: A sulphate salt of group 2 element of the Periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left in open for sometimes, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt. Why does it show such behaviour? Give the reaction involved.
Or
(a) What is meant by metallurgy?
(b) State two steps associated with extraction of copper from its ore.
(c) How is impure copper purified by electrolytic refining? Draw a labeled diagram to illustrate it.
Ans: Salt is 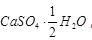 Plaster of Paris, white soft substance. It can be dough, moulded into different shapes, as 2 molecules of CaSO4 share 1 molecule of H2O molecule.
Plaster of Paris, white soft substance. It can be dough, moulded into different shapes, as 2 molecules of CaSO4 share 1 molecule of H2O molecule. 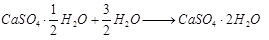
When it is left in open, it becomes solid mass CaSO4 . 2H2O (gypsum) which cannot be used for moulding purposes as it is hard solid mass.
Or
(a) The collection of all the processes involved in extracting metal from its ore is called metallurgy.
(b) (i) Froth-floatation process (ii) Roasting
(c) Impure copper is taken as anode whereas pure copper is taken as cathode. Copper sulphate solution (CuSO4) is taken as electrolyte. When electric current is passed, impure copper changes to ions which gain electrons at cathode and change into pure copper. Impurities are left behind as anode mud. At anode At cathode 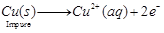
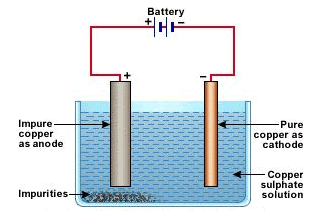 Fig: Electrolytic Refining of Copper
Fig: Electrolytic Refining of Copper
Ques 35: Draw the labelled diagram of dome type bio-gas plant and explain its construction and working. Or Energy from various sources is considered to have been derived from the sun. Do you agree? Justify your answer.
Ans: The diagram of dome type bio-gas plant is shown as
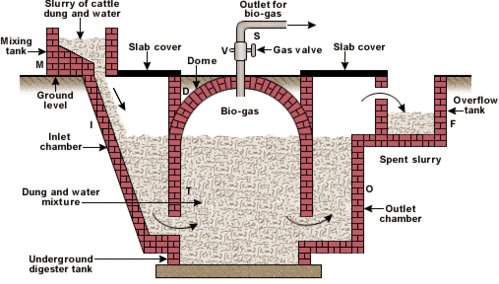
Construction
The bio-gas plant is a. brick and cement structure having the following five sections
- Mixing tank: It is present above the ground level.
- Inlet chamber: The mixing tank opens underground into a slopping inlet chamber.
- Digester: The inlet chamber opens from below into the digester which is a huge tank with a dome like ceiling. The ceiling of the digester has an outlet with a valve for the supply of bio-gas.
- Outlet chamber: The digester opens from below into an outlet chamber.
- Overflow tank: The outlet chamber opens from the top into a small over flow tank.
Working
- The various forms of bio-mass are mixed with an equal quantity of water in the mixing tank. This forms the slurry.
- The slurry is fed into the digester through the inlet chamber.
- When the digester is partially filled with the slurry, the introduction of slurry is stopped and the plant is left unused for about two months.
- During these two months, an anaerobic bacterium present in the slurry decomposes or ferments the bio-mass in the presence of water.
- As a result of anaerobic fermentation, bio-gas is formed, which starts collecting in the dome of the digester.
- As more and more bio-gas starts collecting, the pressure exerted by the bio-gas forces the spent slurry into the outlet chamber.
- From the outlet chamber, the spent slurry overflows into the overflow tank.
- The spent slurry is manually removed from the overflow tank and used as manure for plants. The gas valve connected to a system of pipelines is opened when a supply of bio-gas is required. To obtain a continuous supply of bio-gas, a functioning plant can be fed continuously with the prepared slurry.
OR
Yes, sun is the ultimate source of energy.
Directly or indirectly, all the forms of energy are derived from solar energy. Non-renewable Sources of Energy Fossil fuels like coal petroleum and natural gas are formed due to burial of large plants and ancient creatures whose ultimate source of energy is sun. Renewable Sources of Energy
They are indirectly derived from solar energy such as
(i) Energy from Flowing Water: Clouds are formed when water in lakes, rivers, seas, etc evaporates due ro solar energy. They bring rainfall and snowfall. The rain and melting snow feed rivers, streams, etc. This flowing water can be used for getting hydroelectricity.
(ii) Wind Energy: It arises due to uneven heating of the earth's surface by the sun rays at two different adjoining places. Due to this, wind possesses kinetic energy.
(iii) Bio Energy: Plants in the process of photosynthesis converts the solar energy into food (chemical energy). This food is consumed by animals. This, the animal wastes and remains of the plants constitute bio-mass which can be utilised as a source of energy.
(iv) Wave Energy: The waves are generated by strong winds (due to solar energy) blowing across the sea.
(v) Ocean Thermal Energy: Sun is responsible for the temperature difference between the water at the surface and water at depth in seas and oceans. Solar heating devices derive their energy directly from solar energy and convert it into other usable forms of energy. Thus, the energy from various sources are considered to have been derived from the sun.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science: CBSE Sample Question Paper- Term I (2019-20) - 1 - Science Class 10
| 1. What is the format of CBSE Sample Question Paper for Science Term I (2019-20)? |  |
| 2. How many marks are allotted to Part A and Part B in the CBSE Sample Question Paper for Science Term I (2019-20)? |  |
| 3. What is the time duration of the CBSE Sample Question Paper for Science Term I (2019-20)? |  |
| 4. Are the questions in the CBSE Sample Question Paper for Science Term I (2019-20) based on the latest syllabus? |  |
| 5. What is the difficulty level of the CBSE Sample Question Paper for Science Term I (2019-20)? |  |

















