Electronic Configuration of Elements & Periodic Table | Chemistry Class 11 - NEET PDF Download
Introduction: What are Electron Configurations?
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.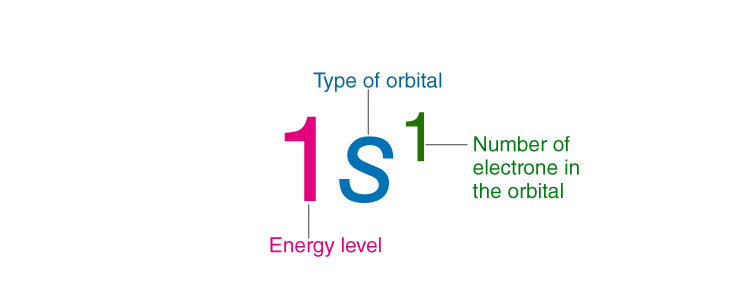
However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number). In such cases, an abbreviated or condensed notation may be used instead of the standard notation. In the abbreviated notation, the sequence of completely filled subshells that correspond to the electronic configuration of a noble gas is replaced with the symbol of that noble gas in square brackets. Therefore, the abbreviated electron configuration of sodium is [Ne]3s1 (the electron configuration of neon is 1s22s22p6, which can be abbreviated to [He]2s22p6).
Electron Configurations are useful for:
- Determining the valency of an element.
- Predicting the properties of a group of elements (elements with similar electron configurations tend to exhibit similar properties).
- Interpreting atomic spectra.
This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
Writing Electron Configurations
Shells
The maximum number of electrons that can be accommodated in a shell is based on the principal quantum number (n). It is represented by the formula 2n2, where ‘n’ is the shell number. The shells, values of n, and the total number of electrons that can be accommodated are tabulated below.
Shell and ‘n’ value | Max. Electrons in the Electron Configuration |
K shell, n = 1 | 2*12 = 2 |
L shell, n = 2 | 2*22 = 8 |
M shell, n = 3 | 2*32 = 18 |
N shell, n = 4 | 2*42 = 32 |
Subshells
- The subshells into which electrons are distributed are based on the azimuthal quantum number (denoted by ‘l’).
- This quantum number is dependent on the value of the principal quantum number, n. Therefore, when n has a value of 4, four different subshells are possible.
- When n = 4. The subshells correspond to l = 0, l = 1, l = 2, and l = 3 and are named the s, p, d, and f subshells, respectively.
- The maximum number of electrons that can be accommodated by a subshell is given by the formula 2*(2l + 1).
- Therefore, the s, p, d, and f subshells can accommodate a maximum of 2, 6, 10, and 14 electrons, respectively.
All the possible subshells for values of n up to 4 are tabulated below.
Principle Quantum Number Value | Value of Azimuthal Quantum Number | Resulting Subshell in the Electron Configuration |
n = 1 | l = 0 | 1s |
n = 2 | l = 0 | 2s |
l = 1 | 2p | |
n = 3 | l = 0 | 3s |
l = 1 | 3p | |
l = 2 | 3d | |
n = 4 | l = 0 | 4s |
l = 1 | 4p | |
l = 2 | 4d | |
l = 3 | 4f |
Thus, it can be understood that the 1p, 2d, and 3f orbitals do not exist because the value of the azimuthal quantum number is always less than that of the principal quantum number.
Notation
- The electron configuration of an atom is written with the help of subshell labels.
- These labels contain the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number) and the total number of electrons in the subshell in superscript.
- For example, if two electrons are filled in the ‘s’ subshell of the first shell, the resulting notation is ‘1s2’.
- With the help of these subshell labels, the electron configuration of magnesium (atomic number 12) can be written as 1s2 2s2 2p6 3s2.
Filling of Atomic Orbitals
Aufbau Principle
- This principle is named after the German word ‘Aufbeen’ which means ‘build up’.
- The Aufbau principle dictates that electrons will occupy the orbitals having lower energies before occupying higher energy orbitals.
- The energy of an orbital is calculated by the sum of the principal and the azimuthal quantum numbers.
- According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
The order in which electrons are filled in atomic orbitals as per the Aufbau principle is illustrated below.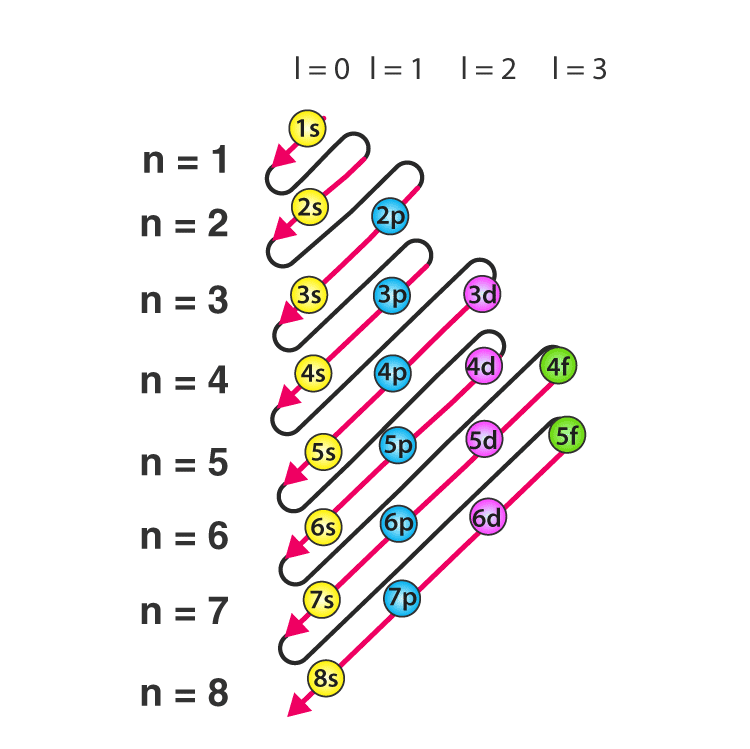
It is important to note that there exist many exceptions to the Aufbau principle such as chromium and copper. These exceptions can sometimes be explained by the stability provided by half-filled or completely filled subshells.
Pauli Exclusion Principle
- The Pauli exclusion principle states that a maximum of two electrons, each having opposite spins, can fit in an orbital.
- This principle can also be stated as “no two electrons in the same atom have the same values for all four quantum numbers”.
- Therefore, if the principal, azimuthal, and magnetic numbers are the same for two electrons, they must have opposite spins.
Hund’s Rule
- This rule describes the order in which electrons are filled in all the orbitals belonging to a subshell.
- It states that every orbital in a given subshell is singly occupied by electrons before a second electron is filled in an orbital.
- In order to maximize the total spin, the electrons in the orbitals that only contain one electron all have the same spin (or the same values of the spin quantum number).
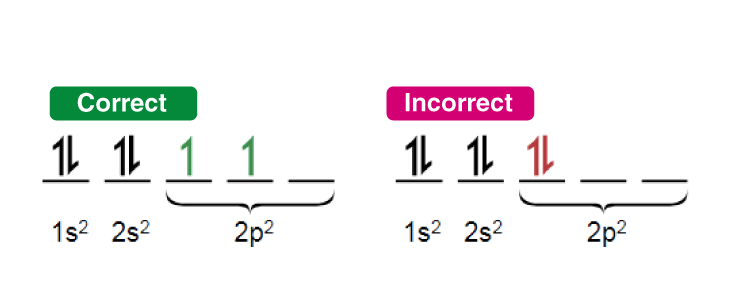
An illustration detailing the manner in which electrons are filled in compliance with Hund’s rule of maximum multiplicity is provided above.
Examples
The electron configurations of a few elements are provided with illustrations in this subsection.
Electron Configuration of Hydrogen
The atomic number of hydrogen is 1. Therefore, a hydrogen atom contains 1 electron, which will be placed in the s subshell of the first shell/orbit. The electron configuration of hydrogen is 1s1, as illustrated below. Electron Configuration of Hydrogen
Electron Configuration of Hydrogen
Electron Configuration of Oxygen
The atomic number of oxygen is 8, implying that an oxygen atom holds 8 electrons. Its electrons are filled in the following order:
- K shell – 2 electrons
- L shell – 6 electrons
Therefore, the electron configuration of oxygen is 1s2 2s2 2p4, as shown in the illustration provided below.
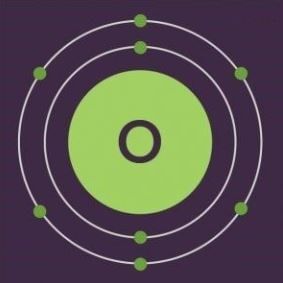 Electron Configuration of Oxygen
Electron Configuration of Oxygen
Chlorine Electronic Configuration
Chlorine has an atomic number of 17. Therefore, its 17 electrons are distributed in the following manner:
- K shell – 2 electrons
- L shell – 8 electrons
- M shell – 7 electrons
The electron configuration of chlorine is illustrated below. It can be written as 1s22s22p63s23p5 or as [Ne]3s23p5
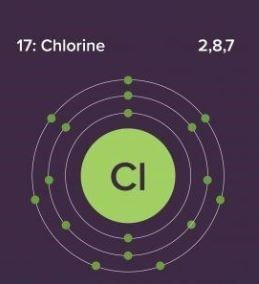 Electron Configuration of Chlorine
Electron Configuration of Chlorine
Electronic Configuration in Periods and Groups
The electronic configuration of an atom is the numerical representation of the arrangement of electrons distributed in the orbitals of the atom. This determines the position of an element in the periodic table and in turn its chemical behavior. It explains how the atoms are held together by the chemical bonds, and the peculiar trends which are observed in the rows and columns of the periodic table. In this article, we will discuss the electronic configuration of elements in the same periods and groups of the periodic table.
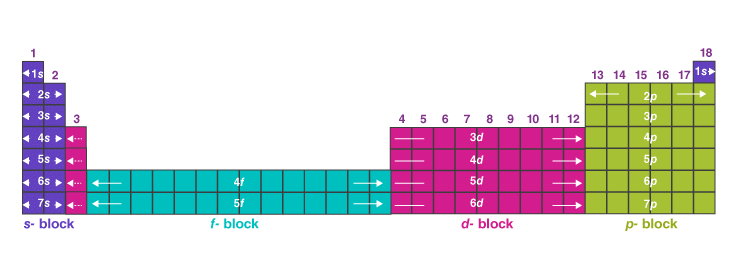
Electronic Configuration in Periods
- The value of n, the principal quantum number, for the valence shell is the period of the element.
- Different energy levels can accommodate different number of electrons.
- The maximum number of electrons that a given energy level can accommodate is given by 2n2, where n is the energy level. So the first energy level (K shell) can hold up to 2 electrons, second level (L shell) can hold up to 8 electrons, and third level (M shell) can also hold 18 electrons and so on.
- Thus, the first period with n = 1 can hold up to 2 elements by filling the lowest level 1s. The elements are Hydrogen and Helium with electronic configuration 1s1 and 1s2 This marks the complete filling of K shell.
- The second period starts with Lithium and Beryllium which have 3 and 4 electrons and hence the last electrons enter the level 2s and they have an electronic configuration of 1s22s1 and 1s22s2 This is followed by the start of the 2p orbital filling. It starts with Boron (1s22s22p1) and ends with Neon (1s22s22p6) which marks the completion of L shell. Thus, 8 elements are present in the second period.
- The third period starts with Sodium and ends at Argon while successively filling 3s and 3p orbitals. 8 elements are present in this period too.
- The fourth period with n = 4 starts by filling the level 4s. It begins with Potassium. However, we know that 3d orbital is to be filled before filling of 4p orbital starts. This marks the beginning of the 3d transition elements with Scandium (electronic configuration- [Ar]3d14s2). At zinc (electronic configuration- [Ar]3d104s2), the 3d orbital is filled.
- The fifth period with n = 5 starts by filling the level 5s. This period consists of the 4d transition series which starts with Yttrium. Xenon ends the period by completely filling the 5p orbital.
- The sixth period with n = 6 holds 32 elements with electrons filling 6s, 4f, 5d and 6p orbitals. Cerium marks the electrons entering 4f orbital giving rise to the 4f-inner transition elements, called the lanthanide series.
- The seventh period with n = 7 includes the man-made radioactive elements with electrons filling 7s, 5f, 6d and 7p orbitals. Similar to period 6, this period also leads to the filling of electrons to 5f orbital, giving rise to the 5f-inner transition elements known as the actinide series.
Electronic Configuration in Groups
Elements in the same group have the same number of electrons in their outermost shell leading to similar valence shell electronic configuration. Thus, we observe a similar trend in the properties and chemistry of the elements in the same group.
|
114 videos|263 docs|74 tests
|
FAQs on Electronic Configuration of Elements & Periodic Table - Chemistry Class 11 - NEET
| 1. What is an electron configuration? |  |
| 2. How does electron configuration vary across periods in the periodic table? |  |
| 3. How does electron configuration vary within groups in the periodic table? |  |
| 4. How can electron configuration be determined using the periodic table? |  |
| 5. What role does electron configuration play in determining the chemical properties of elements? |  |
















