Electronic Configurations & Types of Elements: s, p, d & f-block Elements | Chemistry Class 11 - NEET PDF Download
Classification Of The Elements
- It is based on the type of orbitals which receives the differentiating electron (i.e., last electron).
- Based on the electronic configuration, elements can be classified into four types in the periodic table, viz s-block, p-block, f-block, and d-block elements.
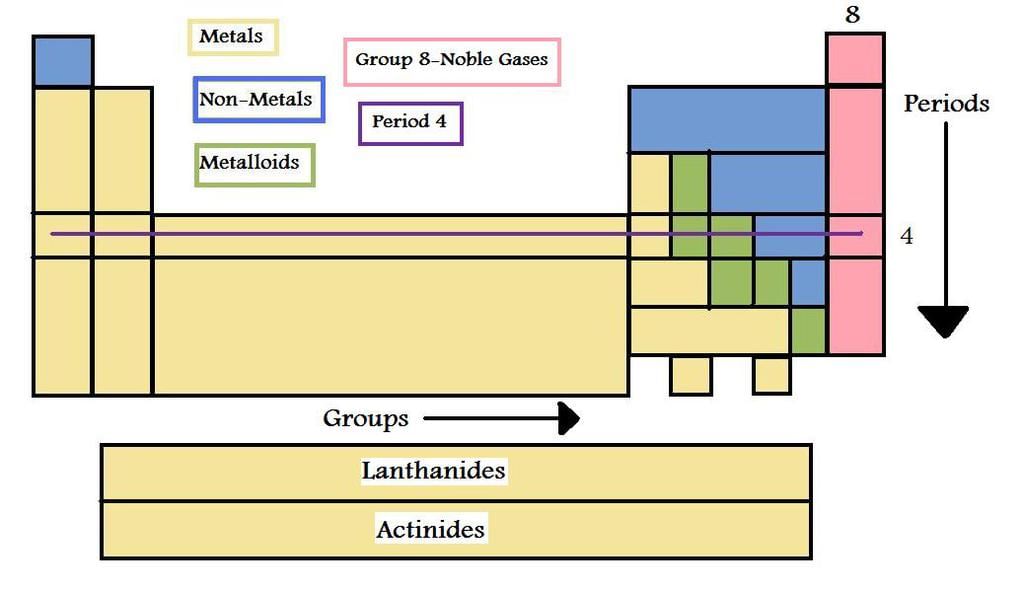
- More than 70-80% of the known elements are metals.
- Elements located at the top of the periodic table are nonmetals and are less than 20. In a group, as the atomic number increases, the metallic character increases.
- In a period, there is a decrease in the metallic character from left to right.
- Also, the chemical, as well as the physical properties, vary with their atomic number.
- Elements of the same group exhibit similar chemical properties because of the similar valence shell electronic configuration
1. s-block elements
- When shells upto (n - 1) are completely filled and the last electron enters the s-orbital of the outermost (nth) shell, the elements of this class are called s-block elements.
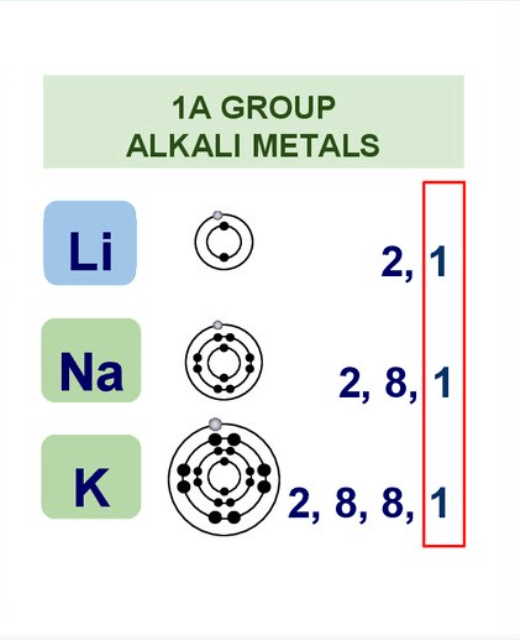
- Group 1 & 2 elements constitute the s-block.
- General electronic configuration is [inert gas] ns1-2
- s-block elements lie on the extreme left of the periodic table.
- This block includes metals.
Group - IA or 1 Alkali metals
Group - II A or 2 , Alkaline earth metals
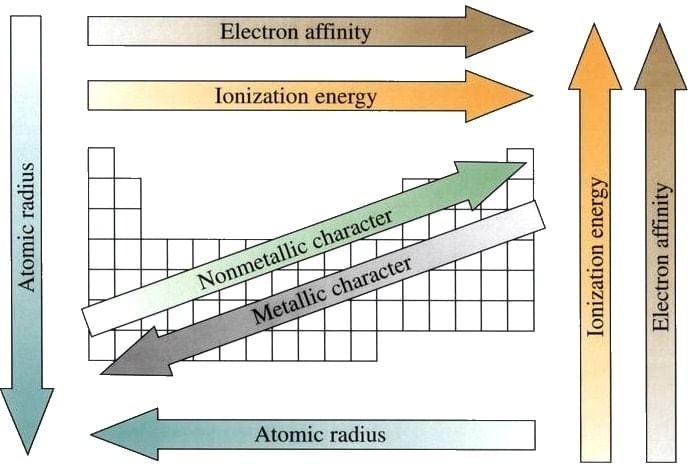 Summary of various trends in Periodic Table.
Summary of various trends in Periodic Table.
2. p-block elements
When shells upto (n -1) are completely filled and differentiating electron enters the p-orbital of the nth orbit, elements of this class are called p-block elements.
- Group 13 to 18 elements constitute the p-block.
- General electronic configuration is [inert gas] ns2np1-6
- p-block elements lie on the extreme right of the periodic table.
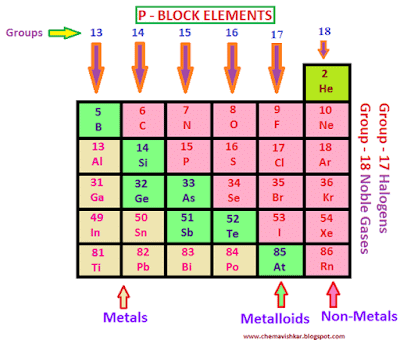
- This block includes some metals, all non-metals and metalloids.
- s-block and p-block elements are collectively called normal or representative elements.
(i) Group - III A or 13, boron family :
(ii) Group - IV A or 14, carbon family :
(iii) Group - V A or 15, nitrogen family :
(iv) Group - VI A or 16 , Oxygen family :
3. d-block elements
When outermost (nth) and penultimate shells (n - 1)th shells are incompletely filled and differentiating electon enters the (n -1) d orbitals (i.e., d-orbital of penultimate shell) then elements of this class are called d-block elements.
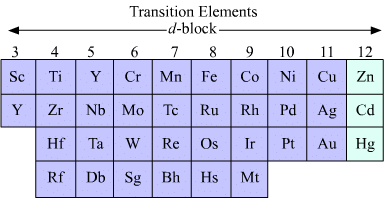
- Group 3 to 12 elements constitute the d-block.
- General electronic configuration is [inert gas] (n - 1)d1-10 ns0-2
- d-block elements are classified into four series
- Series Elements (n-1)d being filled
- Those elements which have partially filled d-orbits in neutral state or in any stable oxidation state are called s transition elements.
4. f-block elements
- When n, (n-1) and (n -2) shells are incompletely filled and last electron enters into f-orbital of antepenultimate i.e., (n-2)th shell, elements of this class are called f-block elements., General electronic configuration is (n - 2)f1-14 (n - 1)d0-1 ns2
- All f-block elements belong to 3rd group.
- They are metals
- Within each series, the properties of the elements are quite similar.
- They are also called as inner-transition elements as they contain three outer most shell incomplete and were also referred to as rare earth elements since their oxides were rare in earlier days.
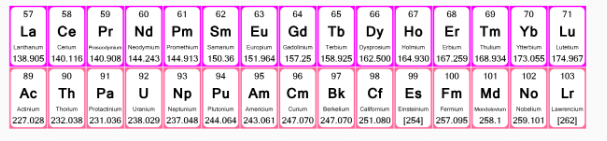
- The elements of f-block have been classified into two series:
- The actinides and lanthanides have been placed at the bottom of the periodic table to avoid the undue expansion of the periodic table.
1. Ist inner transition or 4 f-series, contains 14 elements 58Ce to 70Lu. Filling of electrons takes place in 4f subshell.
2. IInd inner transition or 5 f-series, contains 14 elements 90Th to 103Lr. Filling of electrons takes placed in 5f subshell.
Prediction of Period, Group and Block
- The block of an element corresponds to the type of subshell which receives the last electron.
- The group is predicted from the number of electrons in the valence shell or/and penultimate shell as follows.
(a) For s-block elements Group number = the no. of valence electrons.
(b) For p-block elements Group number = 10+ no. of valence electrons .
(c) For d-block elements Group number = no. of electrons in (n - 1) d sub shell + no. of electrons in valence shell.
Classification of elements
We can classify these elements on the basis of their physical and chemical properties as metals, non-metals and metalloids
Metals
- The metals are characterised by their nature of readily giving up the electron and from shinning lusture.
- Metals comprise more than 75% of all known elements and appear on the left-hand side of the periodic table.
- Metals are usually solids at room temperature (except mercury).
- They have high melting and boiling points and are good conductors of heat and electricity.

- Oxides of metals are basic in nature. (Some metals in their higher oxidation state form acid oxides e.g. CrO3)
Non-Metals
- Non-metals do not lose electrons but take up electrons to form corresponding anions. Non-metals are located at the top right-hand side of the periodic table. Non-metals are usually solids or gases at room temperature with low melting and boiling points. They are poor conductors of heat and electricity.
- Oxides of non-metals are acidic in nature.
Metalloids
- It is very much clear from the periodic table that non-metallic character increases as we move from left to right across a row. It has been found that some elements lying at the border of metallic and non-metallic behaviour, possess the properties that are characteristic of both metals and non-metals.
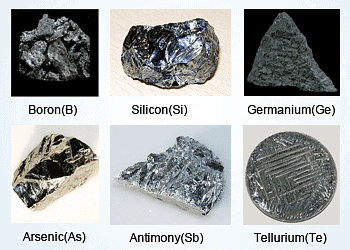
- These elements are called semimetals or metalloids.
- The metalloids comprise of the elements B, Si, Ge, As, Sb and Te.
- Oxides of metalloids are generally amphoteric in nature.
Typical Elements
- Third-period elements are called typical elements. These include Na, Mg, AI, Si, P, S, CI.
- The properties of all the elements belonging to a particular group resemble the properties of the corresponding typical element of that group. For example, the general properties of alkali metals (IA) can be predicted from the properties of Na, not Li, the first member of the group.
- The properties of the elements of the second period differ in the many respect belonging to the same group due to the smaller atomic size and absence of vacant d-orbitals.
Diagonal relationship
Some elements of certain groups of 2nd period resemble much in properties with the elements of third period of next group i.e. elements of second and third period are diagonally related in properties. This phenomenon is known as diagonal relationship.
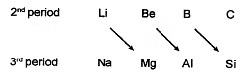
Diagonal relationship arises because of
(i) similar size of atom and ions
Li = 1.23 Å & Mg = 1.36 Å; Li+ = 0.60 Å & Mg2+ = 0.65 Å
(ii) similar electropositive characters
(iii) similar polarising powers (charge to radius ratio)
(iv) similarity in electronegativity values
(Li = 1.0 & Mg = 1.2; Be = 1.5 &AI = 1.5)
Similarities between properties of Li and Mg:
(a) Li and Mg both reacts directly with nitrogen to form lithium nitride (Li3N) and magnesium nitride (Mg3N2) whereas other alkali metals of I A group does not form nitride.
(b) Fluoride, carbonate and phosphate of Li and Mg are insoluble in water whereas these compounds of other alkali metals are soluble.
(c) Li and Mg both are hard metals, whereas other metals of I A group are soft.
(d) LiOH and Mg(OH)2 both are weak bases, whereas hydroxides of other elements of IA group are strong base.
(f) Metallic bond in Li and Mg both are strong compare to other alkali metals.
(g) Their melting and boiling points are high.
(h) By thermal disintegration of LiNO3 and Mg (NO3)2 ; Li2O and MgO is obtained respectively.
(I) Thermal stability of Li2CO3 and Mg CO3 is very less compare to other alkali metals and they liberates CO2 gas easily.
Similarly, Be shows similarity to Al of IIIA group compare to other elements of IIA group which are as follows:
(a) These both elements do not provide colour to Bunsen burner.
(b) They both are comparatively stable in air.
(c) Both are insoluble in NH3, therefore, do not form blue coloured solution.
(d) There is no tendency of making peroxide and superoxide in them.
(e) Reducing power is very less due to low value of standard electrode potential in the form of oxidation potential.
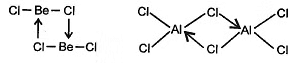
(f) Be and AI both forms halogen bridge halides.
|
119 videos|346 docs|74 tests
|
FAQs on Electronic Configurations & Types of Elements: s, p, d & f-block Elements - Chemistry Class 11 - NEET
| 1. What are the different classifications of elements based on their electronic configurations? |  |
| 2. How do metals, non-metals, and metalloids differ in terms of their properties? |  |
| 3. Can you predict the period, group, and block of an element based on its classification? |  |
| 4. What are the electronic configurations of s, p, d, and f-block elements? |  |
| 5. What are some examples of typical elements found in each type of block? |  |






















