Class 10 Science Chapter 4 Previous Year Questions - Carbon and its compounds
| Table of contents |

|
| Previous Year Questions 2020 |

|
| Previous Year Questions 2019 |

|
| Previous Year Questions 2018 |

|
| Previous Year Questions 2017 |

|
| Previous Year Questions 2016 |

|
| Previous Year Questions 2015 |

|
Previous Year Questions 2020
Q1: Several factories were pouring their wastes in rivers A and B. Water samples were collected from these two rivers. It was observed that sample collected from river A was acidic while that of river B was basic. The factories located near A and B are (2020)(a) Soaps and detergents factories near A and alcohol distillery near B.
(b) Soaps and detergents factories near B and alcohol distillery near A.
(c) Lead storage battery manufacturing factories near A and soaps and detergents factories near B.
(d) Lead storage battery manufacturing factories near B and soaps and detergents factories near A.
 View Answer
View Answer 
Ans: (c)
Q2: (a) Carry out following conversions:
(i) Ethanol to ethene
(ii) Ethanol to ethanoic acid
(b) Differentiate between addition reaction and substitution reaction. Give one example of each. (2020)
 View Answer
View Answer 
Ans: (a) (i) When ethanol is heated with cone. H2SO4 at 443 K, ethene is obtained due to dehydration of ethanol.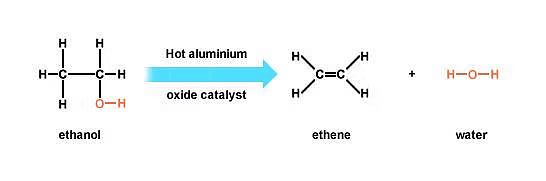
(a) (ii) When 5 % alkaline KMnO4 solution is added drop by drop to warm ethanol then it gets oxidized to ethanoic acid.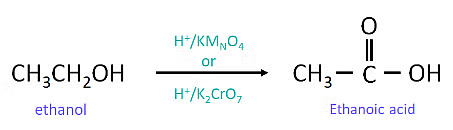
(b) Addition reactions : Those reactions in which atoms or group of atoms are simply added to a double or triple bond without the elimination of any atom or molecule, are known as addition reactions.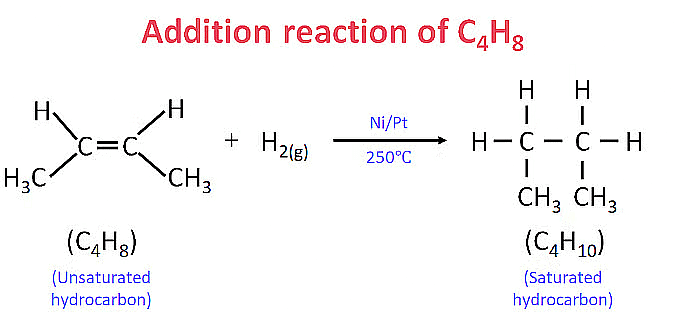
Substitution reactions: The reactions which involve the displacement or substitution of an atom or a group of atoms in an organic compound by another atom or group of atoms, are known as substitution reactions.
Saturated hydrocarbons are fairly unreactive and inert in the presence of most of the reagents. However, in presence of sunlight, hydrocarbons undergo rapid substitution reactions, e.g.,
Q3: (a) What is a homologous series? Explain with an example.
(b) Define the following terms giving one example of each.
(i) Esterification
(ii) Addition reaction (2020)
 View Answer
View Answer 
Ans: (a) A homologous series is the family of organic compounds having the same functional group, similar chemical properties but the successive (adjacent) members of the series differ by a -CH2 unit or 14 mass units.
For example, alkane series has general formula CnH2n + 2.
First member of homologous series of alkane is .methane, i.e., CH4.
Second member of homologous series of alkane is ethane, i.e., C2H6.
Third member of homologous series of alkane is propane i.e., C3H8.
(b) (i) Carboxylic acids react with alcohols in the presence of a little concentrated sulphuric acid to form pleasant smelling esters. This reaction is called esterification reaction.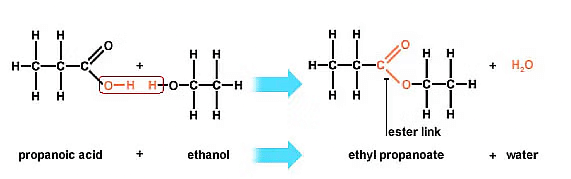
(ii) Addition reactions : Those reactions in which atoms or group of atoms are simply added to a double or triple bond without the elimination of any atom or molecule, are known as addition reactions.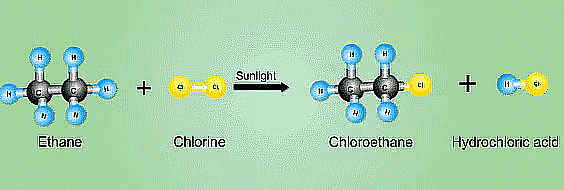
Q4: Assertion (A) : Ethanoic acid is also known as glacial acetic acid.
Reason (R) : The melting point of pure ethanoic acid is 290 K and hence it often freezes during winters in cold climates. (2020)
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
 View Answer
View Answer 
Ans: (a)
Pure ethanoic acid or acetic acid freezes below room temperature into white crystals that resemble glaciers.
Q5: Assertion (A) : Esterification is a process in which a sweet smelling substance is produced.
Reason (R): When esters react with sodium hydroxide, an alcohol and sodium salt of carboxylic acid are obtained. (2020)
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
 View Answer
View Answer 
Ans: (b)
When an ester reacts with the base saponification reaction occurs.
Q6: 3 mL of ethanol is taken in a test tube and warmed gently in a water bath. A 5% solution of alkaline potassium permanganate is added first drop by drop to this solution, then in excess.
(i) How is 5% solution of KMnO4 prepared?
(ii) State the role of alkaline potassium permanganate in this reaction. What happens when one adds it in excess?
(iii) Write chemical equation of this reaction. (2020)
 View Answer
View Answer 
Ans: (i) 5% solution of KMnO4 is prepared by adding 5 g of KMnO4 in 95 g of water.
(ii) Here alkaline KMnO4 acts as an oxidising agent. It oxidises ethanol to ethanoic acid by donating nascent oxygen. If excess of KMnO4 is added the purple colour will persist indicating no more alcohol is left and there is no reaction.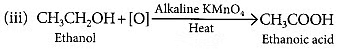
Q7: Carbon, a member of group 14, forms a large number of carbon compounds estimated to be about three million. Why is this property not exhibited by other elements of this group? Explain. (2020)
 View Answer
View Answer 
Ans: Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation. Carbon shows catenation due to its small size and Stronger carbon-carbon bond strength. As we move down the group, the element-element bond energies decrease rapidly. For this reason other elements of this group show little or no catenation property.
Q8: Assertion (A) : Following are the members of a homologous series
CH3OH, CH3CH2OH, CH3CH2CH2OH
Reason (R) : A series of compounds with same functional group but differing by -CH2 unit is called homologous series. (2020)
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
 View Answer
View Answer 
Ans: (a)
The given compounds are members of homologous series of alcohol.
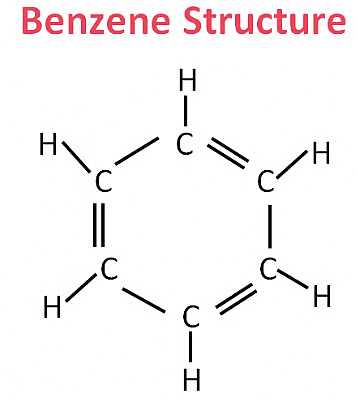
Q9: Name a cyclic unsaturated carbon compound. (2020)
 View Answer
View Answer 
Ans: Benzene is a cyclic unsaturated carbon compound.
Q10: Covalent compounds have low melting and boiling point. Why? [2020]
 View Answer
View Answer 
Ans: Covalent compounds have low melting and boiling points because the forces of attraction between molecules of covalent compounds are very weak. On applying a small amount of heat these molecular forces break.
Previous Year Questions 2019
Q11: (a) Define the term isomer.(b) Two compounds have same molecular formula C3H6O. Write the name of these compounds and their structural formula.
(c) How would you bring the following conversions:
(i) Ethanol to ethene
(ii) Propanol to propanoic acid? (2019)
 View Answer
View Answer 
Ans: (a) Isomers are those molecules which have the same molecular formula but different structural formula i.e., show different properties.
(b) 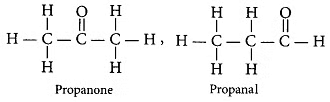
(c) (i) When ethanol is heated with conc. H2SO4 at 443 K, ethene is obtained due to dehydration of ethanol.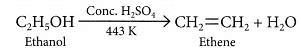
(c) (ii) Propanol to propanoic acid
Q12: Write the chemical formula and name of the compound which is the active ingredient of all alcoholic drinks. List its two uses. Write chemical equation and name of the product formed when this compound reacts with
(i) sodium metal
(ii) hot concentrated sulphuric acid. (2019)
 View Answer
View Answer 
Ans:
Ethanol having chemical formula C2H5OH is the active ingredient of all alcoholic drinks.
Uses of ethanol:
1. Ethanol is widely used in industry as a solvent.
2. Ethanol is used as an antiseptic for wounds in the form of rectified spirit.
Chemical equations:
(i) 2C2H5OH + 2Na → 2C2H5O–Na+ + H2 ↑
(ii)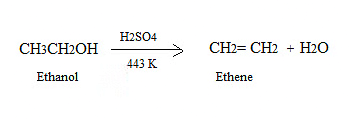
Q13: (a) State the reason why carbon can neither form C4+ cations nor C4- anions, but forms covalent bonds. Also state reasons to explain why covalent compounds
(i) are bad conductors of electricity.
(ii) have low melting and boiling points.
(b) Write the structural formula of benzene, C6H6 (2019)
 View Answer
View Answer 
Ans:
(a) Ionic compounds are formed either by gaining or losing electrons from the outermost shells, but carbon which has four electrons in its outermost shell cannot form ionic bonds because
1. If carbon forms ionic bonds by gaining four electrons to attain a noble gas configuration then it would be difficult for six protons in the nucleus to hold ten electrons.
2. If carbon forms ionic bonds by loss of four electrons then it would require a lot of energy to remove these electrons from outermost shell.
Due to these reasons carbon forms covalent bonds by sharing the valence electrons.
Type of bonds formed in ionic compounds are called electrovalent bonds and the type of bonds formed in carbon compounds are called covalent bonds.
(i) Covalent bonds are those bonds which are formed by sharing of the valence electrons between two atoms. 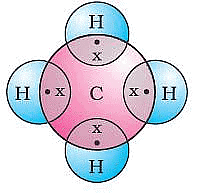
Electron dot structure of Methane
Covalent compounds are generally poor conductors of electricity because they do not have tree electrons or ions.
(ii) Covalent compounds have low melting and boiling points because the forces of attraction between molecules of covalent compounds are very weak. On applying a small amount of heat these molecular forces break.
(b) Structural formula of Benzene, C6H6 is shown below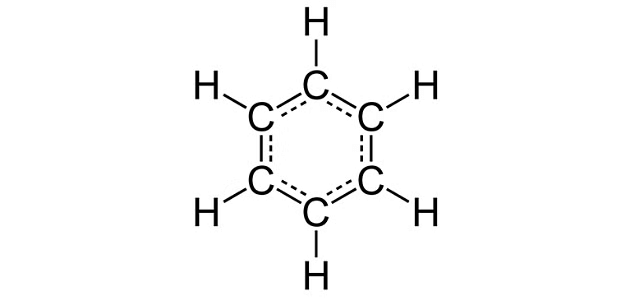
Q14: What happens when this compound burns with oxygen? (2019)
 View Answer
View Answer 
Ans:
(i) Methane is the first member of alkane series having formula CH4.
Covalent bonds are those bonds which are formed by sharing of the valence electrons between two atoms. Electron dot structure of methane is shown in the figure.
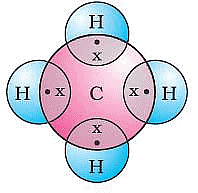 Electron dot structure of Methane
Electron dot structure of Methane
Covalent compounds are generally poor conductors of electricity because they do not have tree electrons or ions.
(ii) When methane is burnt in presence of oxygen then carbon dioxide will be produced.
CH4 + O2 → CO2 + H2O + heat + light
Covalent compounds have low melting and boiling points because the forces of attraction between molecules of covalent compounds are very weak. On applying a small amount of heat these molecular forces break.
Q15: What is methane? Draw its electron dot structure. Name the type of bonds formed in this compound. Why are such compounds: (Delhi 2019)
(i) poor conductors of electricity? and
(ii) have low melting and boiling points? What happens when this compound in oxygen?
 View Answer
View Answer 
Ans: (i) Methane is the simplest hydrocarbon which is produced when carbon atom shares its 4 valence electrons with 4 hydrogen atoms.
(ii) Methane achieves noble gas electronic configuration by sharing its four electrons with other elements, i.e. it forms covalent compounds.
Previous Year Questions 2018
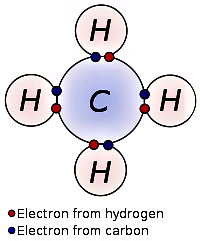
Q16: Which type of compounds can be formed by carbon? (CBSE 2018)
 View Answer
View Answer 
Ans: Covalent compounds can be formed by carbon.
Q17: What happens when hydrogen is added to a vegetable oil in the presence of nickel? Name the reaction and write one difference between the physical property of the vegetable oil and the product obtained in this reaction. Write the role of nickel in this reaction. (CBSE 2018C)
 View Answer
View Answer 
Ans:
- Vegetable oil undergoes hydrogenation to form saturated product known as vegetable ghee or vanaspati ghee. This reaction is known as hydrogenation of oils.
- Vegetable oil is liquid at room temperature whereas vegetable ghee is solid at room temperature.
- Nickel acts as catalyst and forms saturated hydrocarbons.
Q18: (a) Why are most carbon compounds poor conductors of electricity?
(b) Write the name and structure of a saturated compound in which the carbon atoms are arranged in a ring. Give the number of single bonds present in this compound. (CBSE 2018)
 View Answer
View Answer 
Ans:
(a) Carbon compounds are covalent in nature and they do not dissociate to form ions because of which they are poor conductors of electricity.
(b) Cyclohexane is the saturated compound in which the carbon atoms are arranged in a ring.
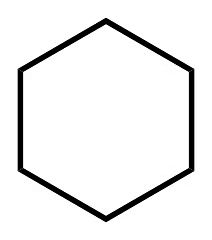 Cyclohexane
Cyclohexane
It has 18 single bonds.
Q19: A compound A (C2H4O2) reacts with Na metal to form a compound B and evolves a gas which burns with a pop sound. Compound A on treatment with an alcohol C in the presence of an acid forms a sweet smelling compound, D (C4H8O2). On addition of NaOH to D gives back B and C. Identify A, B, C and D. Write the reactions involved. (CBSE Sample Questions Paper 2018)
 View Answer
View Answer 
Ans: The compounds A, B, C and D are given as under:
A = CH3COOH
B = CH3COONa
C = C2H5OH
D = CH3COOC2H5
The reactions are explained as under:
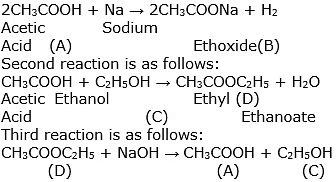
Previous Year Questions 2017
Q20: Two carbon compounds X and Y have the molecular formula C4H8 and C5H12 respectively. Which one of these is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case. (Delhi 2017)
 View Answer
View Answer 
Ans: ‘X’ (C4H8) will show addition reaction because it is unsaturated, i.e. it has a double bond e.g.
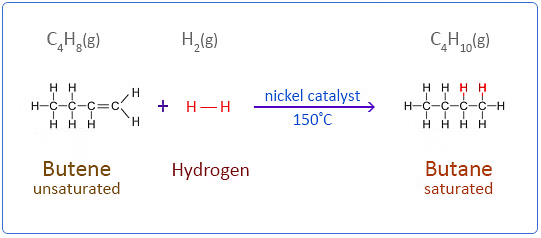
When a molecule or a compound is added to unsaturated compound to form a saturated compound is called addition reaction.
Q21: Complete the following chemical equations: (Delhi 2017)
(i) CH3COOC2H5 + NaOH →
(ii) CH3COOH + NaOH →
(iii) 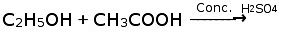
 View Answer
View Answer 
Ans: (i) CH3COOC2H5 + NaOH → CH3COONa + C2H5OH
(ii) CH3COOH + NaOH → CH3COONa + H2O
(iii) C2H5OH + CH3COOH → CH3COOC2H5 + H2O
Q22: Write the structural formula of ethanol and list its two physical properties. What happens when it is heated with excess of cone. H2SO4 at 443 K? State the role of conc. H2SO4 in this reaction. (DoE, AI 2017, Foreign 2013)
 View Answer
View Answer 
Ans: CH3—CH2OH is structural formula of ethanol
Physical properties:
(i) It is liquid and has sweet smell.
(ii) It is soluble in water in all proportions.
When ethanol is heated with cone. H2SO4 at 443 K, ethene is formed.
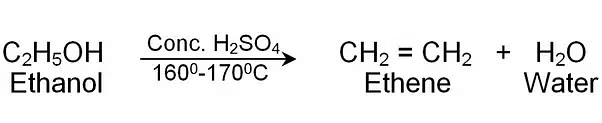
Conc. H2SO4 acts as dehydrating agent.
Q23: Ethanoic acid reacts with absolute ethanol in the presence of Conc. H2SO4 to form a compound: [AI 2017 C]
(i) Write the smell and class of compounds to which this compound belongs.
(ii) Write the chemical equation for the reaction and state the role of Conc. H2SO4 in the reaction.
(iii) Write one use of the product of this reaction.
 View Answer
View Answer 
Ans:
(i) The compound formed is ‘Esters’ It has a pleasant fruity smell.
(ii) 
Conc. H2SO4 acts as dehydrating agent.
(iii) It is used as a solvent in glass, nail polish removers and decaffeinating tea and coffee. It is used is solvent in printing to modify its drying rate.
Uses of esters:
- Esters are used as flavouring agents in ice creams, cold drinks etc.
- They are used in perfumes.
Q24: Distinguish between esterification and saponification reactions of organic compounds with the help of the chemical equation for each. What is the use of (i) esters and (ii) saponification process? (AI 2017, Foreign 2012)
 View Answer
View Answer 
Ans:
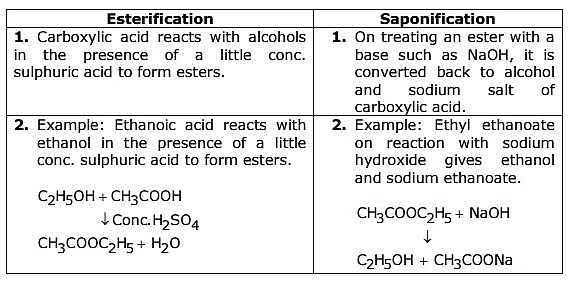
Uses:
(i) Esters are used in cold drinks, ice creams, perfumes and as artificial flavouring agents.
(ii) Saponification process is used in manufacture of soaps.
Q25: Name two oxidising agents that are used for the conversion of alcohols to acids. Distinguish between ethanol and ethanoic acid on the basis of (a) litmus test, and (b) reaction with sodium hydrogen carbonate. (Delhi 2013; AI 2012; Foreign 2017)
 View Answer
View Answer 
Ans: Alkaline KMnO4/NaOH (Potassium permanganate) and Acidified K2Cr2O7 / H2SO4 (Potassium dichromate).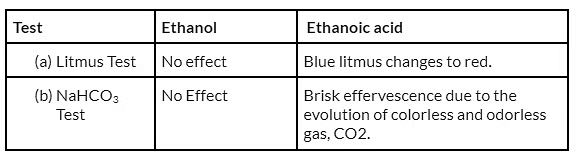
Q26: What is the homologous series of carbon compounds? List any two characteristics. Write the name and formula of next higher homologue of HCOOH. (CBSE 2017)
 View Answer
View Answer 
Ans: A series of compounds whose two successive members differ by - CH2 in the molecule formula and which show similar chemical properties are called homologous series.
Characteristics:
1. Melting and boiling points increase regularly with increase in molecular mass.
2. Solubility in a particular solvent shows a regular gradation.
3. Chemical properties remain the same in the homologous series.
Next higher homologue of HCOOH is CH3COOH. Its name is ethanoic acid or acetic acid.
Q27: When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following: (CBSE 2016)
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of cone. H2SO4 in this reaction.
 View Answer
View Answer 
Ans:
(i) Fruity smelling compounds belong to the class of esters.
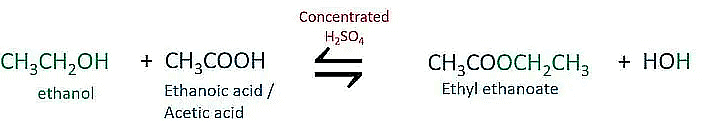
(ii) Conc. H2SO4 acts as a dehydrating agent and removes the water formed in the reaction.
Q28: Soaps and detergents are both types of salts. State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to the use of detergents instead of soaps. (Delhi 2017)
 View Answer
View Answer 
Ans: Soaps are sodium or potassium salts of fatty acids having —COONa group. Detergents are sodium or potassium salts of sulphonic acids having — SO3Na and —SO4Na group.
Cleansing Action of Soap. Soaps consist of a large hydrocarbon tail which is hydrophobic (water-hating or water repelling) with a negatively charged head which is hydrophilic (water-loving) as shown in figure.
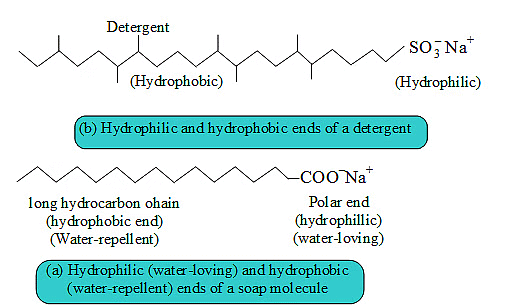
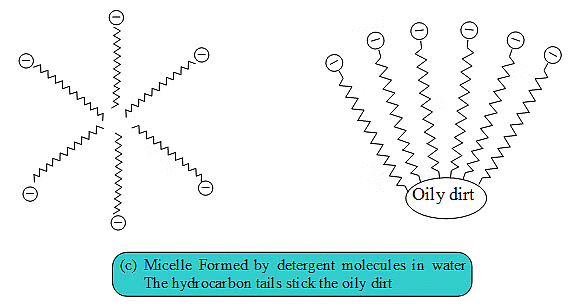
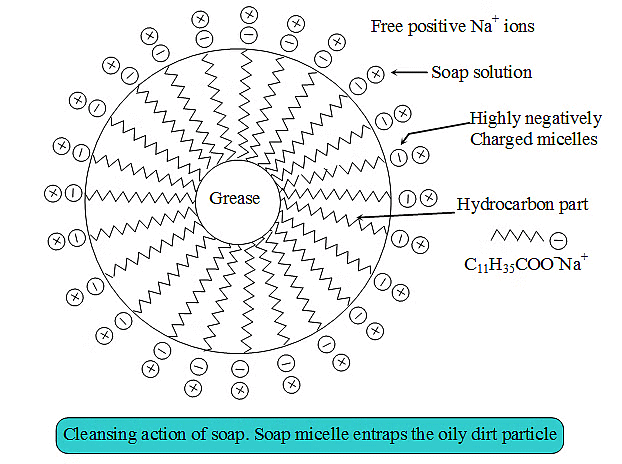 When a soap is dissolved in water, the molecules associate together as clusters called micelles in which, water molecules being polar in nature, surround the ions and the hydrocarbon part of the molecule attracts grease, oil and dirt.Hard water has Ca2+ and Mg2+ ions when they react with soap to form insoluble compound and soap goes waste.
When a soap is dissolved in water, the molecules associate together as clusters called micelles in which, water molecules being polar in nature, surround the ions and the hydrocarbon part of the molecule attracts grease, oil and dirt.Hard water has Ca2+ and Mg2+ ions when they react with soap to form insoluble compound and soap goes waste.
Disadvantages of Detergents
(i) Detergents are expensive.
(ii) Many detergents are branched chain hydrocarbon which are not biodegradable and create water pollution.
Previous Year Questions 2016
Q29: What are covalent compounds? Why are they different from ionic compounds? List their three characteristic properties. (CBSE 2016)
 View Answer
View Answer 
Ans:
Covalent compounds are the compounds that contain covalent bonds in the molecule. A covalent bond is formed by sharing of electrons between the atoms.
While a covalent compound contains covalent bonds, the ionic compound is made up of ionic bond which is formed by transference of electrons from one atom to the other.
Characteristics of covalent compounds:
(i) These compounds show low melting points and boiling points because the intermolecular forces are weak.
(ii) Such compounds are poor conductors of electricity.
(iii) Generally insoluble or less soluble in water but soluble in organic solvents.
Q30: Write chemical equation of the reaction of ethanoic acid with the following:
(а) Sodium; (b) Sodium hydroxide; (c) Ethanol.
Write the name of one main product of each reaction. (CBSE 2016)
 View Answer
View Answer 
Ans:
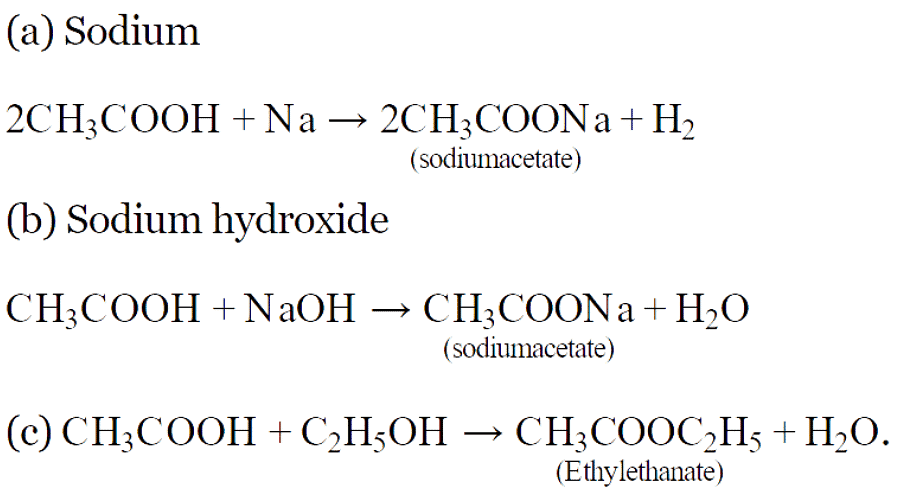
Q31: An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. Write their structures and name them. State the relationship between the two in the language of science. (CBSE 2016)
 View Answer
View Answer 
Ans: The structures of the aldehyde and ketone with the molecular formula C3H6O are
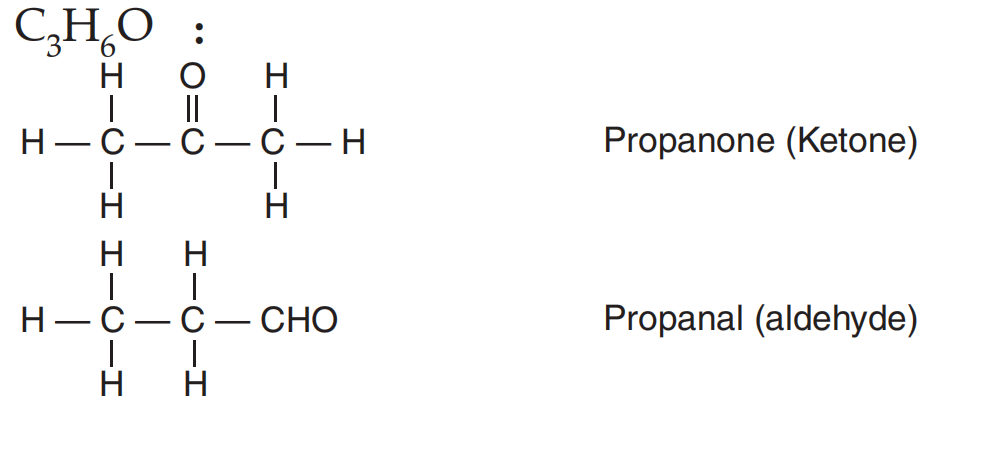
Both these compounds are isomers (because they have the same molecular formula but different structural formula).
Q32: (i) The chemical properties of ethanol are different from methyl ethanoate. Justify the statement with a proper reason.
(ii) Methyl ethanoate is used in making perfumes. Justify.
(iii) Ethanol is converted into ethene with excess of hot concentrated H2SO4. Justify with the help of chemical equation. (CBSE 2016)
 View Answer
View Answer 
Ans:
(i) Ethanol reacts with sodium metal whereas methyl ethanoate does not. They differ in functional group, and in chemical properties.
(ii) It is because it has pleasant fruity smell.
(iii) 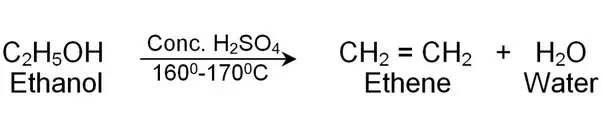
Ethanol gets dehydrated with conc. H2SO4 to form ethene and H2O.
Q33: (a) Define the term functional group. Identify the functional group present in
(i)
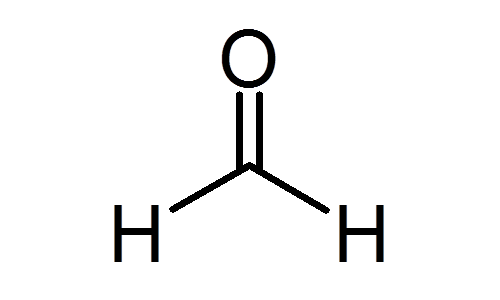
(ii)
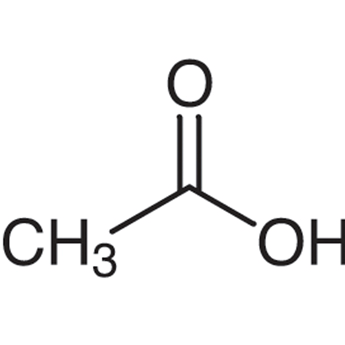
(b) What happens when 5% alkaline KMnO4 solution is added drop by drop to warm ethanol taken in a test tube? State the role of alkaline KMnO4 solution in this reaction. (Foreign 2016)
 View Answer
View Answer 
Ans:
(a) It is an atom or group of atoms or reactive part of compound which largely determine the chemical properties of compound (i) Methanol (ii) Ethanoic acid.
(b) 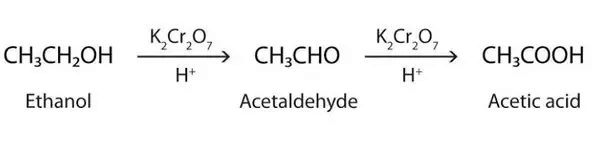
Alkaline KMnO4 acts as an oxidizing agent.
Q34: On dropping a small piece of sodium in a test tube containing carbon compound ‘X ’ With molecular formula C2H6O, a brisk effervescence is observed and a gas ‘Y' is produced, On bringing a burning splinter at the mouth of the test tube the gas evolved burns with a pop sound. Identify ‘X ’ and 'Y'. Also write the chemical equation for the reaction. Write the name and structure of the product formed, when you heat ‘X’ with excess cone, sulphuric acid. (AI 2016)
 View Answer
View Answer 
Ans:
‘X’ is C2H5OH
2C2H5OH + 2Na → C2H5Na + H2
‘Y’ is H2(g) which burns with ‘pop’ sound.
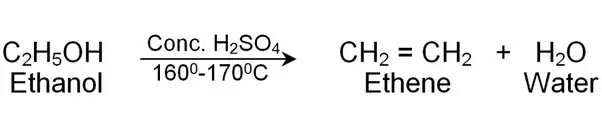
Ethene is the product.
Q35: An organic compound ‘P' is a constituent of wine. ‘P ’ on reacting with acidified K2Cr2O7 forms another compound ‘Q'. When a piece of sodium is added to ‘Q’ a gas ‘R’ evolves which burns with a pop sound. Identify P, Q and R and write the chemical equations of the reactions involved. (Foreign 2016)
 View Answer
View Answer 
Ans:
‘P ’ is C2H5OH, Ethanol
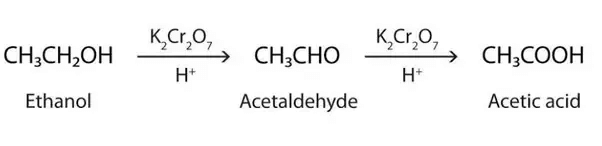
Q is Acetic Acid.
2CH3COOH + 2Na → 2CH3COONa + H2
‘R’ is hydrogen gas which burns with ‘pop’ sound.
Q36: (a) Give chemical tests to detect the presence of
(i) Ethanol
(ii) Ethanoic acid
(b) Why ethanoic acid is also called glacial acetic acid? (CBSE 2016)
 View Answer
View Answer 
Ans:
(a) Add sodium hydrogen carbonate. Ethanol will not react. Ethanoic acid will give brisk effervescence due to carbon dioxide.
(b) Pure ethanoic acid exists as solid like glaciers at 291 K, therefore, called glacial acetic acid.
Q37: Write a chemical equation of the action of ethanoic acid with the following: (a) Sodium; (b) Sodium hydroxide; (c) Ethanol.
Write the name of one main product of each reaction. (Al 2016)
 View Answer
View Answer 
Ans:
(a) 2CH3COOH + 2Na → 2CH3COONa + H2
Sodium ethoxide is the main product formed.
(b) CH3COOH + NaOH→ CH3COONa + H2O
Sodium ethoxide is the main product formed.
(c)
Ethyl ethanoate (ester) is the main product formed.
Q38: Under what conditions an oxidation reaction can be called a combustion reaction? Illustrate your answer with examples. (CBSE 2016)
 View Answer
View Answer 
Ans: When complete oxidation of fuel takes place with the release of high amount of heat and light, it is called combustion reaction. It is highly exothermic and generally accompanied by evolution of heat and light.
Examples - Burning of coal
C + O2 → CO2 + heat + light
Burning of the rocket fuel (liquid H2)
2H2O + O2 → 2H2O + heat + light
Burning of acetylene used in welding torches.
2C2H2 + 5O2 → 4CO2 + 2H2O + heat + light
Q39: C3H6, C4H8 and C5H10 belong to the same homologous series. (CBSE 2016)
(i) Define homologous series.
(ii) Why are the melting and boiling points of C5H10 is higher than C4H8?
(iii) Arrange these hydrocarbons in order of increasing boiling points.
 View Answer
View Answer 
Ans: (i) The series of organic compounds which have similar chemical properties, same functional group and same general formula is called homologous series.
(ii) It is because C5H10 has higher molecular weight, more Vander Wall’s force of attraction and higher boiling points and melting points.
(iii) C3H6 < C4H8 < C5H10 is increasing order of boiling point.
Q40: An aldehyde as well as ketone can be represented by the same molecular formula, say C3H6O. Write their structures and name them. State the relation between the two in the language of science. (Al 2016)
 View Answer
View Answer 
Ans:
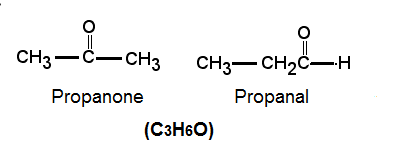
They are functional isomers.
Q41: Write the name and molecular formula of an organic compound having its name suffixed with ‘ol' and having two carbon atoms in its molecule. Write the balanced chemical equation to indicate what happens when this compound is heated with excess conc. H2SO4 and the name of main product formed. Also state the role of conc. H2SO4 in the reaction. (2016)
 View Answer
View Answer 
Ans:
Ethanol, C2H5OH
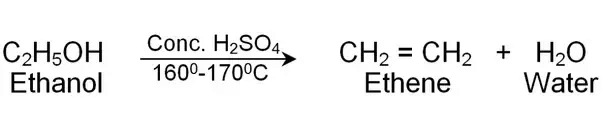
Conc. H2SO4 acts as dehydrating agent.
Q42: What is an oxidising agent? What happens when an oxidising agent is added to propanol? Explain with the help of a chemical equation. (Delhi 2016)
 View Answer
View Answer 
Ans:
Those substances which add oxygen are called oxidising agent and propanoic acid is formed.
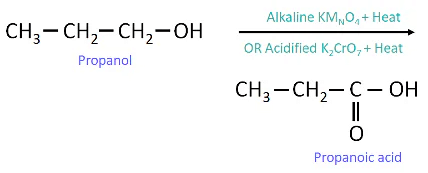
Reaction of Propanol with Oxidizing Agent
Propanol will get oxidised to propanoic acid by acidified KMnO4.
Q43: (a) You have three unlabelled test tubes containing ethanol, ethanoic acid and soap solution. Explain the method you would use to identify the compounds in different test tubes by chemical tests using litmus paper and sodium metal.
(b) Give the reason of formation of scum when soaps are used with hard water. (Foreign 2016)
 View Answer
View Answer 
(a) Ethanol will not be affected by blue litmus as well as red litmus paper. Ethanoic acid will turn blue litmus red whereas red litmus will remain as it is. Soap solution will turn red litmus blue but blue litmus will remain as it is. Sodium metal will liberate hydrogen gas with ethanol as well as ethanoic acid. Soap solution will not react with sodium metal.
(b) It is because soap will react with Ca2+ and Mg2+ ions present in hand water to form calcium salts of fatty acids which are insoluble and called scum.
Q44: (a) Give a chemical test to distinguish between saturated and unsaturated hydrocarbon.
(b) Name the products formed when ethane burns in air. Write the balanced chemical equation for the reaction showing the types of energies liberated.
(c) Why is reaction between methane and chlorine in the presence of sunlight considered a substitution reaction? (Delhi 2016)
 View Answer
View Answer 
Ans:
(a) Add bromine water. Unsaturated hydrocarbon will decolourise bromine water whereas saturated hydrocarbon will not.
(b) Carbon dioxide and Water are formed.
2C2H6 (g) + 7O2 (g) → 4CO2 (g) + 6H2O +Heat + Light
(c) It is because hydrogen atom of methane gets substituted by chlorine atom to form chloromethane, therefore, it is called a substitution reaction.
Q45: What are micelles? Why does it form when soap is added to water? Will a micelle be formed in other solvents such as ethanol also? State briefly how the formation of micelles help to clean the clothes having oily spots. (Foreign 2016)
 View Answer
View Answer 
Ans: Micelles: When molecular ions in soaps and detergents aggregate, they form micelles.
It is because a large number of molecular ions of soaps get aggregated and form colloidal solution. Soap has hydrophobic tail (hydrocarbon) which dissolves in hydrocarbon part and hydrophilic part dissolves in water. Ethanol is non-polar solvent therefore micelles are not formed because hydrocarbon part gets attracted towards ethanol and the ionic end will not dissolve in alcohol.
Q46: A carbon compound ‘P ’ on heating with excess cone. H2SO4 forms another carbon compound ‘Q’ which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound 'R'. One molecule of 'R’ on combustion forms two molecules of carbon dioxide and three molecules of water. Identify P, Q and R and write chemical equations for the reactions involved. (CBSE 2016)
 View Answer
View Answer 
Ans: The compounds are identified as under:
P = Ethanol (CH3CH2OH)
Q = Ethene (CH2 = CH2)
R = Ethane (CH3 – CH3)
Reactions are given as under:

Previous Year Questions 2015
Q47: Draw the electron-dot structure for ethyne. A mixture of ethyne and oxygen is burnt for welding. In your opinion, why cannot we use a mixture of ethyne and air for this purpose? (AI 2015)
 View Answer
View Answer 
Ans:

Electron-dot structure of Ethyne
Ethyne and air will not produce enough heat due to incomplete combustion needed for welding purpose. Ethyne and oxygen will produce a lot of heat due to complete combustion which can be used for welding purposes.
Q48: That is meant by isomers? Draw the structures of two isomers of butane, C4H10. Explain why we cannot have isomers of the first three members of alkane series. (DoE, Delhi 2015)
 View Answer
View Answer 
Ans: Isomers are those compounds which have the same molecular formula but different structural formula.
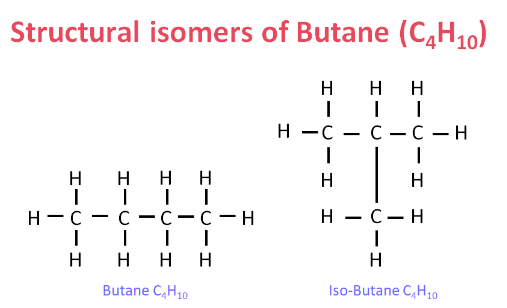
Butane & Iso-Butane are two isomers of C4H10. Isomers are not possible for the first three members because branching is not possible.
Q49: Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen is possible. State the essential conditions for an addition reaction. Stating this condition, write a chemical equation giving the name of the reactant and the product of the reaction. (AI 2015)
 View Answer
View Answer 
Ans: CnH2n and CnH2n- 2 are the general formula of alkene and alkynes in which addition reaction with hydrogen is possible. Hydrogen is added to unsaturated hydrocarbon (having double or triple bond) in presence of heated nickel as catalyst.
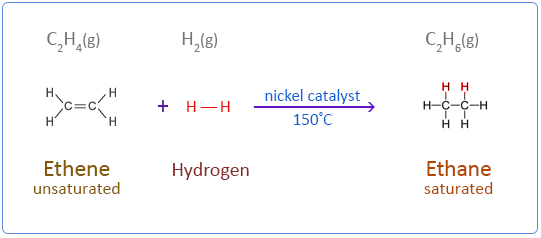
Q50: Write the molecular formula of the following compounds and draw their electron-dot structures: (Foreign 2015)
(i) Ethane
(ii) Ethene
(iii) Ethyne
 View Answer
View Answer 
Ans:
(i) Ethane (C2H6)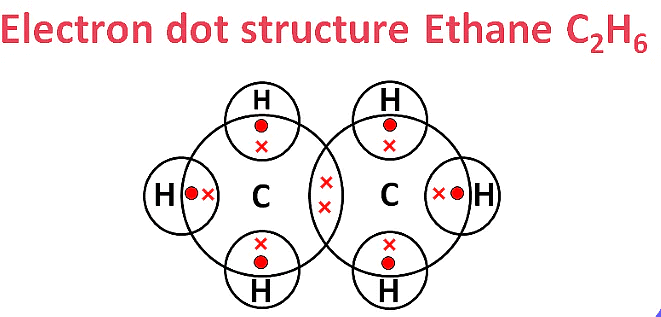
(ii) Ethene (C2H4)
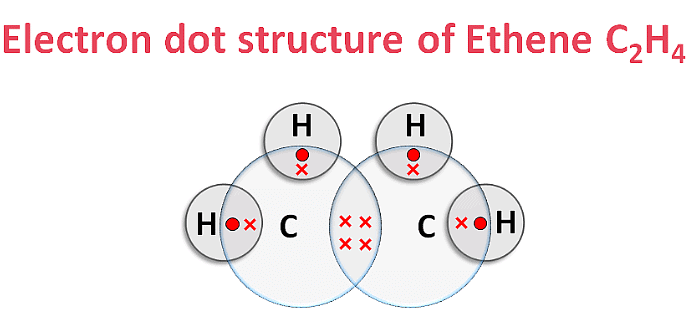
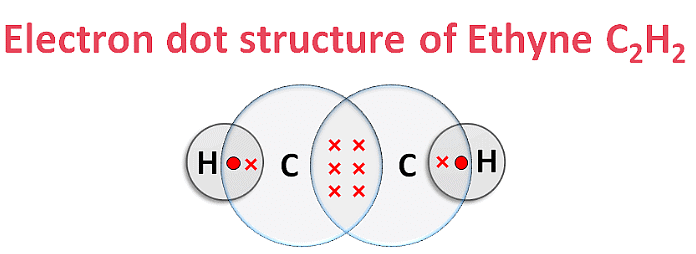
(iii) Ethyne (C2H2)
Q51: Write the respective chemical equations to show what happens when (Foreign 2015)
(i) Methane is burned in presence of oxygen?
(ii) Ethanol is heated with concentrated sulphuric acid at 443 K?
(iii) Ethanol reacts with ethanoic acid in the presence of an acid acting as a catalyst?
 View Answer
View Answer 
Ans:
(i) CH4 + 2O2 → 2H2O + CO2
Water and carbon dioxide formed.
(ii)
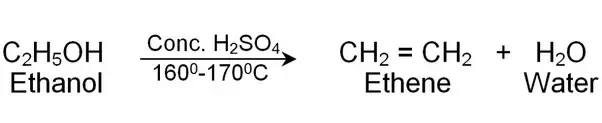
The concentrated sulphuric acid can be regarded as a dehydrating agent which removes water from ethanol.
(iii)
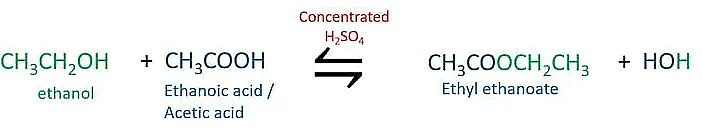
Esters are sweet smelling substances.
Q52: Write the chemical equation to explain what happens when ethanol is heated with alkaline solution of potassium permanganate. Mention two physical properties and two uses of ethanol. (Foreign 2015)
 View Answer
View Answer 
Ans:
Alkaline KMnO4 is dark pink in colour. So when it is added to ethanol and heated, the pink colour of the solution disappears. When excess of KMnO4 is added, the pink colour does not disappear, indicating that all the ethanol has been converted to ethanoic acid.
• Physical properties of ethanol:
(a) It is a colourless liquid with pleasant smell and burning taste.
(b) It is a volatile liquid with low boiling point.
• Uses of ethanol:
(a) It is used in the manufacture of medicines, varnished, paints, dyes, soap, etc.
(b) It is a good solvent. Many organic compounds which are insoluble in water are soluble in ethanol.
Q53: What is the difference between the molecules of soaps and detergents? Explain the cleansing action of soaps. (CBSE 2015)
 View Answer
View Answer 
Ans:
Soaps are sodium or potassium salts of long chain carboxylic acids.
Detergents are ammonium or sulphonate salts of long chain carboxylic acids.
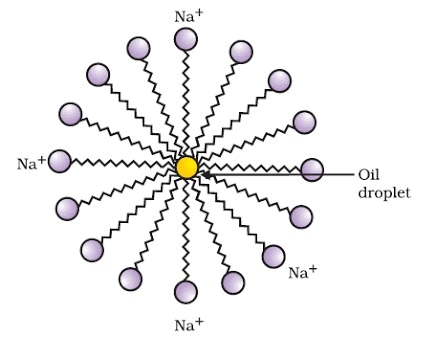
Cleansing action of soap: The ionic end of soap dissolves in water while the carbon chain dissolves in oil. Soap molecules form micelles where one end of the soap molecule is linked to oil droplet while the ionic part faces water. This forms an emulsion in water. On adding water, these micelles containing dirt are washed away and our clothes are washed clean. This is represented in the figure.
Q54: With the help of an example, explain the process of hydrogenation. Mention the essential conditions for the reaction and state the change in physical property with the formation of the product. (CBSE 2015)
 View Answer
View Answer 
Ans: Conversion of oil into fat by passing hydrogen gas in the presence of nickel as catalyst is called hydrogenation. This is how hydrogenation is carried out.
Conditions: The presence of a catalyst like nickel is essential.
Change in physical property: Oils are liquids while the fats obtained on hydrogenation are solids i.e., a change in the physical state takes place on hydrogenation.
Q55: Explain why carbon forms compounds mainly by covalent bond. Explain in brief two main reasons for carbon forming a large number of compounds. Why does carbon form strong bonds with most other elements? (CBSE 2015)
 View Answer
View Answer 
Ans: Carbon has no tendency to lose electrons easily. Similarly it has no tendency to gain electrons. It completes its octet by sharing the electrons with other atoms. Therefore, carbon forms compounds mainly by covalent bond.
Two main reasons for carbon forming a large number of compounds are given below:
Catenation: It is the property of carbon to link with other carbon atoms to form straight chain, branched chain or cyclic compounds. Thus, carbon forms a large number of compounds containing 2, 3, 4, 5,....etc., carbon atoms.
Tetravalency: Carbon has four electrons in the outermost shell. It shares these four electrons with four electrons from four other monovalent atoms. Carbon has the tendency to form bonds with hydrogen, oxygen, nitrogen and halogens. This increases the number of compounds of carbon.
Carbon forms strong bonds with most other elements: This is because carbon has a small atomic size. It can hold strongly the four pairs of electrons that it shares with other atoms. Therefore, it forms strong bonds with other elements.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science Chapter 4 Previous Year Questions - Carbon and its compounds
| 1. What are the different allotropes of carbon? |  |
| 2. How does carbon form covalent bonds in its compounds? |  |
| 3. What is the importance of carbon compounds in everyday life? |  |
| 4. How do carbon compounds exhibit isomerism? |  |
| 5. How do carbon compounds play a role in environmental issues like global warming? |  |





















