NEET Exam > NEET Notes > Chemistry Class 11 > Mind Map: Equilibrium
Mind Map: Equilibrium | Chemistry Class 11 - NEET PDF Download
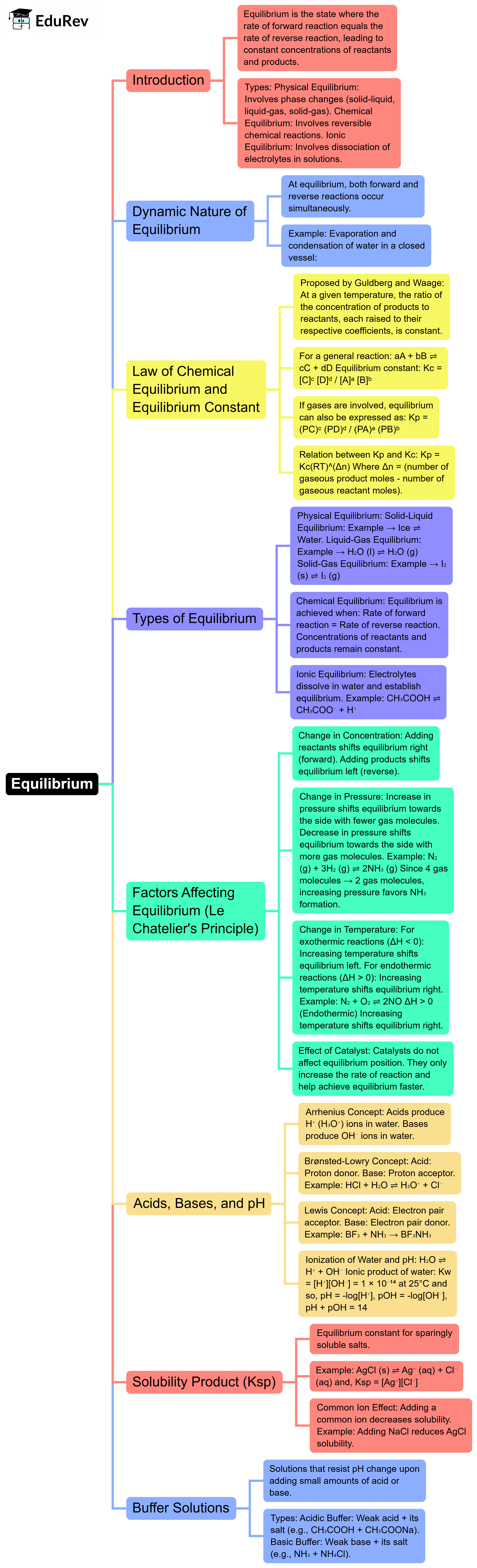
The document Mind Map: Equilibrium | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
114 videos|263 docs|74 tests
|
FAQs on Mind Map: Equilibrium - Chemistry Class 11 - NEET
| 1. What is equilibrium? |  |
Ans. Equilibrium refers to a state in which opposing forces or processes are balanced, resulting in a stable and unchanging condition. In the context of the article, equilibrium is likely referring to chemical equilibrium, where the rates of forward and reverse reactions are equal, resulting in a constant concentration of reactants and products.
| 2. How is equilibrium achieved in chemical reactions? |  |
Ans. Equilibrium in chemical reactions is achieved when the rates of the forward and reverse reactions become equal. This can occur through various factors such as adjusting the temperature, pressure, or concentration of reactants and products. When equilibrium is reached, the concentrations of reactants and products remain constant over time.
| 3. What are the factors that can shift the equilibrium position of a reaction? |  |
Ans. Several factors can shift the equilibrium position of a reaction, including changes in temperature, pressure, and concentration of reactants and products. Additionally, the presence of catalysts can also affect the equilibrium position. These factors can cause the reaction to shift either towards the products or the reactants, altering the equilibrium concentrations.
| 4. How does Le Chatelier's principle explain the behavior of equilibrium systems? |  |
Ans. Le Chatelier's principle states that when a system at equilibrium is subjected to a change, it will respond by shifting in a way that minimizes the effect of the change. For example, if the concentration of a reactant is increased, the equilibrium will shift towards the product side to counteract the increase. Similarly, if the temperature is increased, the equilibrium will shift in the direction that absorbs heat.
| 5. Can equilibrium be disturbed permanently? |  |
Ans. Equilibrium is a dynamic state, and it can be disturbed temporarily but not permanently. If a system at equilibrium is subjected to a change in conditions, it will shift to counteract the change and establish a new equilibrium position. However, once the new equilibrium is reached, the concentrations of reactants and products will remain constant unless further changes are introduced.
Related Searches






















