Class 10 Science Chapter 3 Question Answers - Metals and Non-metals
Q1: How is the method of extraction of metals high up in the reactivity series different from that for metal in the middle? Why the same process cannot be applied for them? Explain giving equations, the extraction of sodium.
Ans: The metals placed high up in the reactivity series (e.g., Na, K, Ca, Mg, Al) are highly reactive and strong reducing agents and are extracted by carrying out the electro-reduction of their molten salts. On the other hand, metals placed in the middle of the series (e.g., Fe, Zn, Cd, Co, Ni, etc.) are comparatively less reactive and are extracted by roasting or calcination.
Sodium is extracted by electro-reduction process.
NaCl ⇌ Na+ + Cl–
Reaction at the cathode: Na+ + e+ → Na+
Reaction at the anode: Cl– – e– → Cl
Cl + Cl → Cl2
Q2: A non-metal X exists in two different forms Y and Z. Y is the hardest natural substance, whereas Z is a good conductor of electricity. Identify X, Y and Z.
Ans: Non-metal ‘X’ must be carbon. It exist in two forms, diamond and graphite. Diamond is hardest natural substance. Hence, ‘Y’ is diamond. Graphite is a good conductor of electricity. Hence, ‘Z’ is graphite.
Q3: You are provided with three metals: Sodium, magnesium and copper. Using only water as the reactant, how will you identify them?
Ans:
- The metal which reacts violently with cold water and catches fire is sodium.
- The metal which evolves hydrogen gas upon heating with water is magnesium.
- The metal which does not react with water even on strong heating is copper.
Q4: When a metal X is treated with cold water, it gives a basic salt Y with molecular formula XOH (Molecular mass = 40) and liberates a gas Z which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Ans: The base with molecular mass 40 is NaOH. Hence, the metal X must be sodium (Na). It reacts with H2O to form the base NaOH and liberates H2 gas which easily catches fire.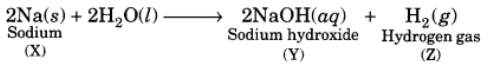
Q5: What is an alloy? How is it prepared? Name the alloy which is used for welding electrical wires together.
Ans: Alloy is a mixture of two or more metals or non-metals. Alloys are made by mixing the metals in their molten state. It is prepared by first melting the primary metal and then dissolving the other metal in definite proportion. It is then cooled to room temperature. Solder is used for welding electrical wires together.
Q6: What is an amalgam? Write the constituent metals of brass.
Ans: An amalgam is an alloy in which one of the metals is mercury. Brass is an alloy of copper and zinc (Cu and Zn). It contains 80% copper and 20% zinc.
Q7: Every ore is a mineral but not every mineral is an ore. Explain.
Ans: Every mineral is not suitable for the extraction of the metal. The mineral from which the metal is economically and conveniently extracted is called an ore.
Q8: Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Ans: Zinc and magnesium can displace hydrogen from dilute acids whereas copper and silver cannot. The metals lying above hydrogen in the reactivity series can displace hydrogen on reaction with dilute acids.
Q9: Name two metals which react violently with cold water. Write any three observations you would make when such a metal is dropped into water. How would you identify the gas evolved, if any, during the reaction?
Ans: Sodium, Potassium
When these metals are dropped in water, bubbles will be evolved due to evolution of hydrogen gas.
The gas will catch fire and the solution will be alkaline, i.e., it will turn red litmus blue.
Test: When a burning matchstick is brought near the gas, it burns explosively with a ‘pop’ sound and the splinter is extinguished.
Q10: Using the electronic configurations, explain how magnesium atom combines with oxygen atom to form magnesium oxide by transfer of electrons.
Ans: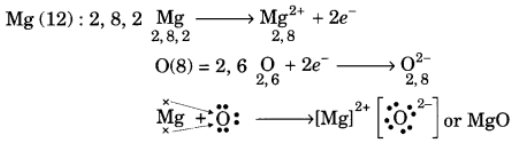
Q11: Write balanced chemical equation for the reactions taking place when
(i) zinc carbonate is calcinated.
(ii) zinc sulphide is roasted or heated in air.
(iii) zinc oxide is reduced to zinc.
Ans:
Q12: Explain how the following metals are obtained from their compounds by reduction process:
(i) Metal X which is low in reactivity series
(ii) Metal Y which is in the middle of the series
(iii) Metal Z which is high up in the reactivity series.
Give one example of each type.
Ans:
(i) Metals which are low in reactivity series can be obtained by heating their compounds. For example, mercury is obtained by heating its ore, cinnabar (HgS), in air.
HgS + O2 → Hg + SO2
(ii) Metals which are in the middle of the series are generally obtained by heating their compounds with some reducing agent such as carbon. For example, iron is obtained from haematite (Fe2O3) by reduction with carbon.
2Fe2O3 + 3C → 4Fe + 3CO2
(iii) Metals which are high up in the series are obtained by electrolytic reduction. For example, sodium is obtained by electrolysis of molten sodium chloride.
Q13: A metal E is stored under kerosene. When a small piece of it is left open in air, it catches fire. When the product formed is dissolved in water, it turns red litmus to blue.
(i) Name the metal E.
(ii) Write the chemical equation for the reaction when it is exposed to air and when the product is dissolved in water.
(iii) Explain the process by which the metal E is obtained from its molten chloride.
Ans:
(i) The available information suggests that the metal (E) is sodium (Na).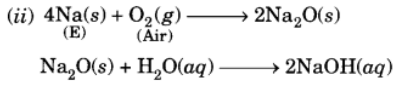
The solution is basic and it turns red litmus blue.
(iii) The metal is obtained by the process of electrolytic reduction.
Q14: A non-metal A is an important constituent of our food and forms two oxides B and C. Oxide B is toxic whereas C causes global warming.
(a) Identify A, B and C.
(b) To which Group of Periodic Table does A belong?
Ans: The non-metal A is carbon. It is an important constituent of our food in different forms. For example, glucose (C6H12O6) contains carbon. In fact, all food materials are organic compounds and these contain carbon as an essential constituent. The two oxides of carbon are, carbon monoxide (B) and carbon dioxide (C). Carbon dioxide causes global warming.
(a) A = Carbon (C); B = Carbon monoxide (CO); C = Carbon dioxide (CO2)
(b) Carbon is the first member of group 14 in the long form of periodic table.
Q15: Give reasons for the following observations:
(i) Ionic compounds in general have high melting and boiling points.
(ii) Highly reactive metals cannot be obtained from their oxides by heating them with carbon.
(iii) Copper vessels get a green coat when left exposed to air in the rainy season.
Ans: (i) Ionic compounds have high melting and boiling points due to strong force of attraction between oppositely charged ions.
(ii) It is because these metals, have great affinity towards oxygen and so cannot be reduced by carbon or carbon monoxide or hydrogen.
(iii) Copper vessels react with CO2, O2 and moisture to form green coloured basic copper carbonate [CuCO3. CU(OH)2] on their surface when exposed to moist air.
Q16: Write chemical equations for the reactions taking place when
(a) Magnesium ribbon is burnt in a jar containing oxygen
(b) Sodium metal falls into a sink containing water
Ans:
(a)
(b) 2Na + 2H2O → 2NaOH + H2
Q17: The following reaction takes place when aluminium powder is heated with MnO2
3MnO2(s) + 4Al(s) → 3Mn(l) + 2Al2O3(l) + Heat
(a) Is aluminium getting reduced?
(b) Is MnO2 getting oxidised?
Ans:
(a) Aluminium is getting oxidised to Al2O3.
(b) Manganese dioxide is getting reduced to Mn.
Q18: Give the reaction involved during extraction of zinc from its ore by
(a) roasting of zinc ore
(b) calcination of zinc ore
Ans:
(a) Roasting is done for the sulphide ore (zinc blende). (b) Calcination is done for the carbonate ore (calamine).
(b) Calcination is done for the carbonate ore (calamine).
Q19: Name two metals which can be used to reduce metal oxides to metal.
Ans: (i) Aluminium (Al) (ii) Magnesium (Mg)
(ii) Magnesium (Mg)
Q20: Give reason for the following:
(i) Iron grills are frequently painted.
(ii) Gold ornaments retain their lustre even after several years of use.
Ans:
(i) Iron metal easily gets rusted by air containing moisture and oxygen. Therefore, iron grills are frequently painted with rust proof paints.
(ii) Gold is a noble metal and is not affected by chemicals or by air. Therefore, gold ornaments retain their lustre even after several years.
|
666 docs
|
















