Class 8 Exam > Class 8 Notes > Science Class 8 > NCERT Summary: Chemical Effects of Electric Current
Chemical Effects of Electric Current Summary Class 8 NCERT Summary Chapter 11
Introduction
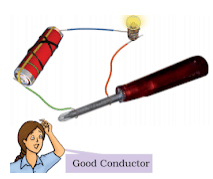
- Materials, which allow electric current to pass through them, are good conductors of electricity.
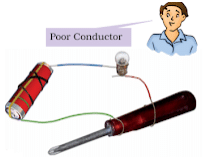
- Materials, which do not allow electric current to pass through them easily, are poor conductors of electricity.
- Metals such as copper and aluminium conduct electricity whereas materials such as rubber, plastic and wood do not conduct electricity.
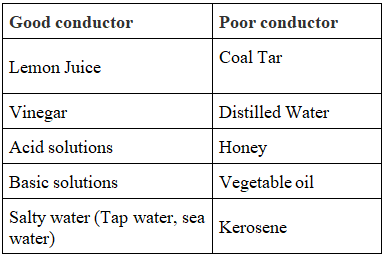
Electrolyte
- Conducting liquids are also called electrolytes.
- The electric current passing through a conducting liquid (electrolyte) causes chemical reactions (electrolysis).
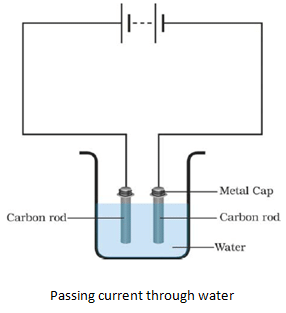
- A chemical reaction takes place when electric current passes through a conducting solution.
Magnetic Effect of current
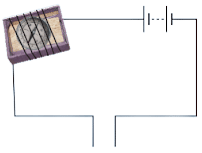
- The bulb will glow or the magnetic needle will show deflection if the liquid in the beaker is a good conductor of electricity.
- Greater the deflection of needle or brighter the light, better is the conductivity of the liquid.
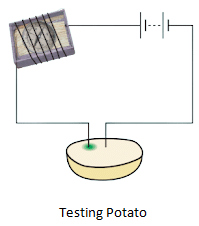
- A greenish-blue spot on the potato around the positive electrode is seen if current is passed through wire.
- The chemical effects of electric current are used in the working of electrochemical cells.
- Electrochemical cells which generate current are called voltaic cells or galvanic cells.
- Common batteries also consist of one or more such cells.
Electroplating
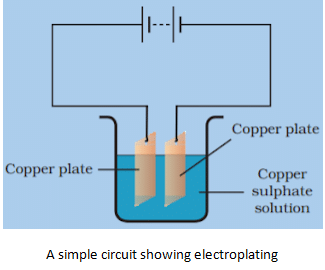
- Electroplating is the process of coating a metal using electric current and electrolyte.
- The above picture explains how copper can be deposited on a different metal using electroplating.
- Electroplating can be used to give shiny and scratch proof finish (properties of chromium) to objects made from cheaper metals.

- A coating of zinc is deposited on iron to protect it from formation of rust.
Environmental Concerns
In electroplating facilities, managing the disposal of used conductive solutions is critical due to its potential to pollute. There are strict guidelines in place to ensure the protection of the environment.
The document Chemical Effects of Electric Current Summary Class 8 NCERT Summary Chapter 11 is a part of the Class 8 Course Science Class 8.
All you need of Class 8 at this link: Class 8
|
92 videos|296 docs|44 tests
|
FAQs on Chemical Effects of Electric Current Summary Class 8 NCERT Summary Chapter 11
| 1. What are the chemical effects of electric current? |  |
Ans. The chemical effects of electric current refer to the changes that occur in substances when an electric current passes through them. This can lead to processes such as electrolysis, where chemical compounds are broken down into their constituent elements. For example, when an electric current is passed through water, it can separate into hydrogen and oxygen gases.
| 2. How does electrolysis work in the context of the chemical effects of electric current? |  |
Ans. Electrolysis is a process that uses electric current to drive a non-spontaneous chemical reaction. In this context, when an electric current is passed through an electrolyte solution, it causes the ions in the solution to migrate towards the electrodes. Positively charged ions move towards the cathode (negative electrode) and gain electrons, while negatively charged ions move towards the anode (positive electrode) and lose electrons, leading to the formation of new substances.
| 3. What are some common applications of the chemical effects of electric current? |  |
Ans. The chemical effects of electric current have several important applications, including electroplating, where a thin layer of metal is deposited onto a surface to enhance its appearance and resistance to corrosion. Other applications include the production of chemicals through electrolysis, such as chlorine gas and sodium hydroxide from brine, and the extraction of metals from their ores.
| 4. What safety precautions should be taken when studying the chemical effects of electric current? |  |
Ans. When studying the chemical effects of electric current, it is important to take safety precautions. These include wearing safety goggles and gloves, ensuring that all electrical equipment is properly insulated, working in a well-ventilated area, and being aware of the risks of electric shock. It is also crucial to handle chemicals with care and dispose of them according to safety guidelines.
| 5. How can the strength of an electric current affect its chemical effects? |  |
Ans. The strength of an electric current can significantly affect its chemical effects. A stronger current typically increases the rate of electrolysis, leading to more rapid production of gases or deposition of metals. However, if the current is too strong, it can cause overheating or unwanted side reactions. Therefore, it is important to use an appropriate current strength to achieve the desired chemical reaction safely and effectively.
Related Searches
















