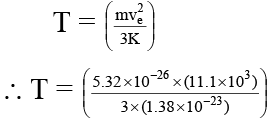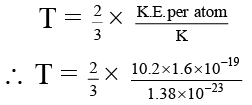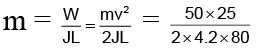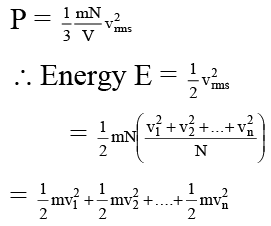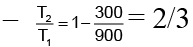JEE Advanced (Single Correct Type): Heat & Thermodynamics | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. In the following figure A certain mass of gas traces three paths 1, 2 and 3 from state A to state B. If the work done by the gas along these paths are W1, W2 and W3 respectively. Then-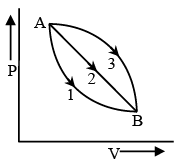 (a) W1< W2< W3
(a) W1< W2< W3
(b) W1> W2> W3
(c) W1 = W2 = W3
(d) W1< W2 but W3< W2
Correct Answer is option (a)
The work done by the gas system depends upon the area enclosed between P-V curve and volume axis. The area enclosed by curve 3 is maximum and that enclosed by curve 1 is minimum. Hence W3> W2> W1
Q.2. At what temperature will the root mean square velocity of oxygen molecules be sufficient so as the escape from the earth?
(a) 1.6 × 105 K
(b) 16 × 105K
(c) .16 × 105 K
(d) 160 × 105 K
Correct Answer is option (a)
∴ 3/2 KT = 1/2 mve2
Where ve = escape velocity of earth = 11.1 km/sec
m = mass of 1 molecule of oxygen = 5.34 × 10–26
⇒ T = 1.6 × 105 K
Q.3. In the following indicator diagram (Figure), the net amount of work done will be -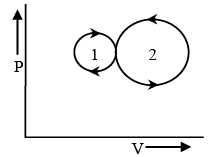 (a) Positive
(a) Positive
(b) negative
(c) Zero
(d) infinity
Correct Answer is option (b)
The cyclic process 1 is clockwise and the process 2 is anticlockwise. Therefore W, will be positive and W2 will be negative. Area 2 > area 1, Hence the network will be negative.
Q.3. In the following figure two indicator diagrams are shown. If the amounts of work done in them are W1 and W2 respectively, then –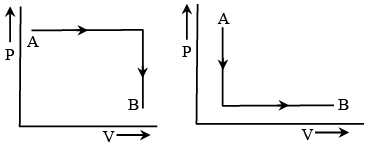 (a) W1> W2
(a) W1> W2
(b) W1< W2
(c) W1 = W2
(d) W1 = W2/4
Correct Answer is option (a)
The area enclosed by first P-V curve is greater than that of by the second curve, Therefore, W1> W2.
Q.4. The first excited state of hydrogen atom is 10.2 eV above its ground state. What temperature is needed to excite hydrogen atoms to first excited level –
(a) 7.88 × 104 K
(b) .788 × 104 K
(c) 78.8 × 104 K
(d) 788 × 104 K
Correct Answer is option (a)
K.E. Per atom = 3/2 KT
K.E. of the hydrogen atom = 10.2 eV
10.2 eV = 10.2 × (1.6 × 10–19) Joule
Where, k = 1.38 × 10–23 J/mole. ºK
⇒ T = 7.88 × 104 K
Q.6. The atomic weight of iodine is 127. A standing wave in a tube filled with iodine gas at 400 K has nodes that are 6.77cm apart when the frequency is 1000 vib/sec. iodine is
(a) Monoatomic
(b) Diatomic
(c) Triatomic
(d) None of these
Correct Answer is option (b)
∴ λ = 2 × 6.77 cm = 13. 54cm
v = nλ = 1000 × 13.54 = 1.354 × 104cm/sec.We know that
where molecular weight
M = Ax with x = 1 if iodine is monoatomic x = 2 it diatomic and A is atomic weight
∴ γ = Axv2/RT = 0.7
Where x = 2 as iodine is diatomic
∴ γ = 1.4 (right value of diatomic gas)
Q.7. A block of ice of mass 50 Kg. is pushed out on a horizontal plane with a velocity of 5 M/s. Due to friction it comes to rest after covering a distance of 25 meter. How much ice will melt?
(a) 0.86 gm
(b) 1.86 gm
(c) 100 gm
(d) 1000 gm
Correct Answer is option (b)
m = 1.86 gm.
Q.8. M kilograms of a material are to be kept in melted state at melting point and power required for this purpose is P. When the power source is disconnected then the sample completely solidifies in t second. The latent heat of fusion of the material will be -
(a) M/Pt
(b) Pt/M
(c) PtM
(d) Pt
Correct Answer is option (b)
Heat released in t second Q = ML ...(A)
Loss of heat per second P = Q/t ...(B)From equation (A) and (B) P = ML/t
∴ L = Pt/M
Q.9. Isothermal curves are obtained by drawing –
(a) P against V
(b) P against T
(c) PV against R
(d) PV against V
Correct Answer is option (b)
In an isothermal process, temperature remains constant and process equation is, PV = constant So a graph is drawn between P and V.
Q.10. dU = – dW is true for –
(a) Isothermal process
(b) Adiabatic process
(c) Isobaric process
(d) Isochoric process
Correct Answer is option (b)
In an adiabatic process, heat cannot be exchanged between the system and surrounding so,
dQ = 0
By Ist law of thermodynamics
dQ = dU + dW
dU= –dW
Q.11. In a certain process the pressure of one mole ideal gas varies with volume according to the relation P = 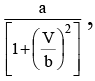 where a, b are constants, when the volume of gas V = b, then temperature of the gas will be -
where a, b are constants, when the volume of gas V = b, then temperature of the gas will be -
(a) ab
(b) ab/R
(c) ab
(d) zero
Correct Answer is option (b)
∴ T = PV/RAt V = b, P = a/(1 + 1) = a/2
∴ ab/ 2R
Q.12. The internal energy of monatomic and diatomic gases are respectively due to
(a) Linear motion and rolling motion
(b) Rolling motion and linear motion
(c) Linear motion and rotatory motion
(d) Rotatory motion and linear motion
Correct Answer is option (a)
The internal energy of a monatomic gas is due to linear motion only and that of the diatomic gas is due to rolling (linear rotatory) motion.
Q.13. In the figure (A) indicator diagram, the net amount of work done will be :
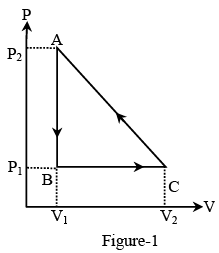 (a) Positive
(a) Positive
(b) Negative
(c) Zero
(d) Infinity
Correct Answer is option (b)
The cyclic process 1 is clockwise and the process 2 is anticlockwise. Therefore w1 will be positive and w2 will be negative area2> area1. Hence, the network will be negative.
Q.14. The pressure of an ideal gas is written as P = 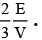 Here E refers to –
Here E refers to –
(a) Translational kinetic energy
(b) Rotational kinetic energy
(c) Vibrational kinetic energy
(d) Total kinetic energy
Correct Answer is option (a)
Pressure of the gas
So energy is basically the sum of energies of all molecules which represents translational kinetic energy.
Q.15. Which of the following parameters is the same for molecules of all gases at a given temperature?
(a) Mass
(b) Speed
(c) Momentum
(d) Kinetic energy
Correct Answer is option (a)
Kinetic energy according to kinetic theory of gases is given by,
E = 3/2 RT
R is a universal constant and temperature remains same for all gases. So kinetic energy is same for molecules of all gases at a given temperature.
Q.16. In the above question find out heat given to the system or taken out from the system in the process C → A and network done in complete cycle.
(a) – 200J, 50J
(b) – 200J, 60J
(c) 60J, – 200J
(d) +200J , – 69J
Correct Answer is option (a)
For the process C → A, ΔU = UA - UC = 0 - 80
⇒ ΔU = - 80Jand W = area ACED = area ACB + area ABED
∴ W = ( 1/2 × AB × BC) + (DE × DA)∴ W = (1/2 × 2 × 60) + ( 2 × 30) = 120 J
Since, in this process the volume decreases, the work will be negative (w = 120Joule). That is, the work will be done on the system. Now, by the first law of thermodynamics, will have
Q = ΔU + W = - 80 - 120 = - 200J
Since it is negative, this heat is given out by the system and work done in the whole cycle = area ABC = 1/2 × 2 × 60 = 60J
Since, the cyclic process is traced anticlowise, the net work will be done on the system.
Q.17. A Carnot engine takes 103 kilocalories of heat from a reservoir at 627ºC and exhausts it to a sink at 27ºC. The efficiency of the engine will be
(a) 22.2%
(b) 33.3%
(c) 44.4%
(d) 66.6%
Correct Answer is option (d)
Efficiency of Carnot engine
η = 1∴ η = 66.6 %
Q.18. From the relation Cp-Cv = R/J it is inferred that
(a) the gas is monatomic
(b) gas is diatomic
(c) gas obeys ideal gas equation irrespective of whether it is mono or diatomic
(d) gas is monatomic and it can be ideal or real
Correct Answer is option (c)
Gas obeys ideal gas equation irrespective of whether it is mono or diatomic.
Q.19. The ratio of number of collisions per second at the walls of containers by H2 and Ne gas molecules kept at same volume and temperature is –
(a) 10 : 1
(b) 1 : 10
(c) 1 : √10
(d) √10 : 1
Correct Answer is option (c)
Let's consider the wall perpendiculars to x-axis number of collisions per second are given by vx/2L Now, vx = vrms/√3
Q.20. In a given process on an ideal gas dW = 0 and dQ < 0, then for the gas
(a) the temperature will decreases.
(b) the volume will increases.
(c) the pressure will remain constant.
(d) the temperature will increase
Correct Answer is option (a)
dQ < 0
dU < 0 (or dW = 0)
T2 < T1
Hence, (a) is correct.
|
446 docs|930 tests
|