JEE Exam > JEE Notes > Physics for JEE Main & Advanced > Revision Notes: Thermodynamics
Revision Notes: Thermodynamics | Physics for JEE Main & Advanced PDF Download
 Introduction to Thermodynamics
Introduction to Thermodynamics
- Thermodynamics is the branch of physics focused on the transformation of heat into other forms of energy and vice versa.
- It is a macroscopic science, dealing with bulk systems and not delving into the molecular constitution of matter.
- A thermodynamic system is defined as a collection of an extremely large number of atoms or molecules confined within certain boundaries.
- The system has specific values for pressure (P), volume (V), and temperature (T).
Thermal Equilibrium and Zeroth Law
Thermal Equilibrium
- A thermodynamic system is in an equilibrium state when macroscopic variables like pressure, volume, temperature, and mass composition remain constant over time.
- In thermal equilibrium, the temperatures of two systems are equal.
Zeroth Law of Thermodynamics
- This law establishes thermal equilibrium and introduces temperature as a key parameter for identifying equilibrium.
- The law states, "If two systems are in thermal equilibrium with a third system, then those two systems are in equilibrium with each other."
Heat, Work, and Internal Energy
Heat Transfer
- Energy transferred between a system and surroundings due to temperature difference.
- Occurs when the system and surroundings have different temperatures.
Work Done
- Work occurs when a system moves through a distance in the direction of applied force.
- Expressed as dW = P dV, where P is the gas pressure in the cylinder.
Internal Energy
- For a bulk system with numerous molecules, internal energy (U) is the sum of kinetic (Ek) and potential (Ep) energies.
- Internal energy (U=Ek+Ep) is due to molecular motion and configuration.
- Macroscopic variable dependent only on the system's state.
- Value depends solely on the system's given state, independent of the path taken to reach that state.
Question for Revision Notes: ThermodynamicsTry yourself: Which of the following statements best describes internal energy in a thermodynamic system?View Solution
First Law of Thermodynamics
- Essentially the conservation of energy applied to any system.
- States that the total heat energy change in a system is the sum of internal energy change and work done.
- Mathematically expressed as dQ=dU+dW.
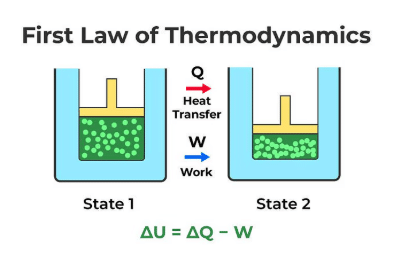
Heat Transfer in Systems
- When heat dQ is applied to a system, part is used to increase internal energy (dU) and part is used for external work (dW).
Specific Heat Capacities for Gases
- Specific heat capacity for gases varies based on the process or conditions of heat transfer.
- Two principal specific heat capacities for a gas
- Specific heat capacity at constant volume (Cυ).
- Specific heat capacity at constant pressure (Cp).
Relation between Specific Heats
- First Law establishes a relationship between the two principal specific heats of an ideal gas.
- Relation:Cp−Cυ=R, where Cp and Cυ are molar specific heats under constant pressure and constant volume conditions, respectively.
Specific Heat Capacities of Gases
- Cp>Cυ for a gas.
- Reason:At constant volume, all supplied heat raises the temperature, while at constant pressure, heat is used for temperature increase and work against external pressure.
Heat Transfer at Constant Volume
- When heat is supplied at constant volume (Cυ)
- No work is done by the gas against external pressure.
- All energy is used to raise gas temperature.
Heat Transfer at Constant Pressure
- When heat is supplied at constant pressure (Cp):
- Volume increases.
- Heat energy used for temperature increase and work against external pressure.
Difference in Specific Heats
- Difference (Cp−Cυ) is the thermal equivalent of work done during gas expansion against external pressure.
Expression for Relation between Cp and Cυ
- Let P, V, and T be initial pressure, volume, and absolute temperature of one mole of ideal gas.
Case (i): Heat Transfer at Constant Volume
- Heat (dQ) supplied, temperature increases to T+dT.
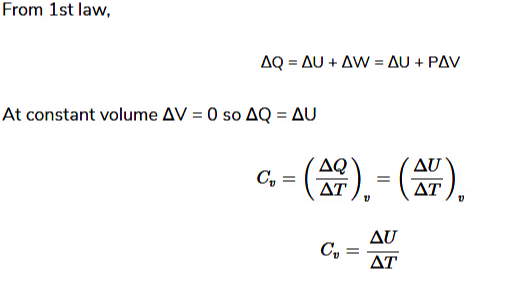
On the other hand, at constant pressure,
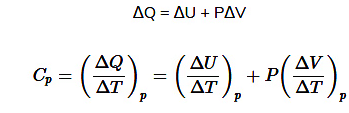
Now, for a mole of an ideal gas
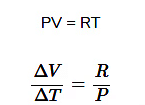
Thermodynamic State Variables
- Parameters describing equilibrium states of a system.
- Examples:pressure, volume, temperature, mass, and composition for a gas.
Equation of State
- Represents the connection between a system's state variables.
- Example:Ideal gas equation - PV=μRT, where P is pressure, V is volume, μ is the number of moles, R is the gas constant, and T is temperature.
Extensive Variables
- Indicate the size of the system.
- Examples: Volume, mass, internal energy.
Intensive Variables
- Do not indicate the size of the system.
- Examples: Pressure, temperature, density.
Question for Revision Notes: ThermodynamicsTry yourself:Which of the following equations represents the relationship between pressure, volume, temperature, and the number of moles for an ideal gas?
View Solution
Thermodynamic Processes
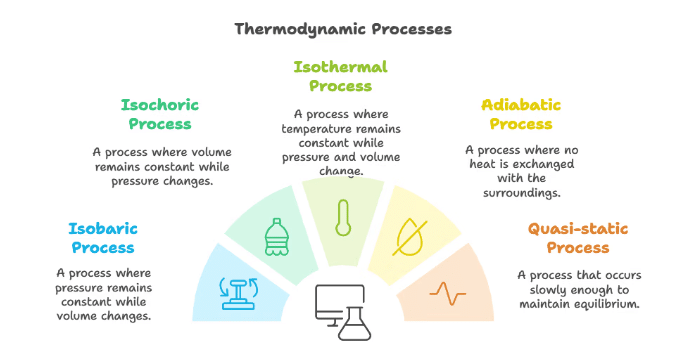
- Any change in the thermodynamic variables of a system.
Quasi-Static Processes
- Processes sufficiently slow, avoiding accelerated motion of piston and large temperature gradients.
- Small changes in pressure, volume, or temperature.
Isothermal Process
- Change in pressure and volume without altering the temperature.
- Involves free exchange of heat between the gas and surroundings.
Adiabatic Process
- No heat exchange between the gas and surroundings.
Work Done in Isothermal Change
- The work done dW under isothermal change is involved.
- For adiabatic process, Q = 0
From first law of Thermodynamics,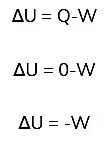
P-V Diagram and Reversible Processes
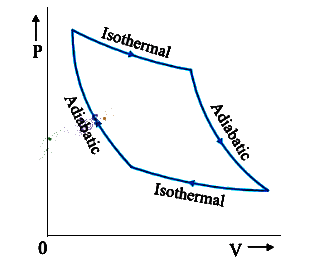 P-V Diagram
P-V Diagram
- Graph representing pressure variation with volume.
- Work done by the system equals the area under the P-V diagram.
Reversible Process
- Process can retrace, passing through the same states.
- If not reversible, it's irreversible.
Causes of Irreversibility
- Many processes lead to non-equilibrium states (e.g., free expansion, explosive reactions).
- Irreversibility often results from friction, viscosity, and dissipative effects.
Second Law of Thermodynamics
- Governs phenomena not allowed by the First Law.
Kelvin-Planck Statement
- No engine can operate in a cycle, extracting heat from a hot body, converting it entirely into work without any residual change.
- 100% conversion of heat into work is impossible.
Clausius Statement
- A self-acting machine in a cycle, without external energy, cannot transfer heat from a cold body to a hot body.
- Heat cannot spontaneously flow from a colder body to a hotter body.
Question for Revision Notes: ThermodynamicsTry yourself: Which thermodynamic process involves no heat exchange between the gas and surroundings?View Solution
Heat Engines and Carnot's Engine
Heat Engine
- Device facilitating a cyclic process converting heat to work.
- Components
- Heat source
- Heat sink
- Working substance
Carnot’s Engine
- Hypothetical engine using a cyclic/reversible process between two temperatures.
- Efficiency formula: η = 1 - T2/T1 (where T1 is source temperature, T2 is sink temperature).
Carnot’s Theorem
- No engine working between T1 and T2 can surpass Carnot engine's efficiency.
- Carnot engine's efficiency is independent of the working substance.
Refrigerators and Coefficient of Performance
Refrigeration Process
- Removal of heat from bodies colder than their surroundings.
- Device for this process is called a refrigerator.
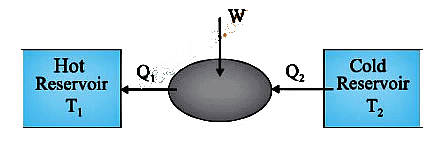
Refrigerator Functionality
- Absorbs heat at low temperature.
- Rejects heat at higher temperature.
- External mechanical work is involved.
- Functions as a heat engine in reverse, termed as a heat pump.
Reverse Carnot Process
- Refrigerator operates in the reverse process of a Carnot engine.
- System extracts heat from a low-temperature sink (T2) and transfers it to a high-temperature source (T1).
Coefficient of Performance
- Represents the efficiency of the refrigerator.
- Involves the work done on the system.
- Functionality based on the reverse Carnot process
Important Table
Here is the summarised table:
The document Revision Notes: Thermodynamics | Physics for JEE Main & Advanced is a part of the JEE Course Physics for JEE Main & Advanced.
All you need of JEE at this link: JEE
|
297 videos|948 docs|172 tests
|
FAQs on Revision Notes: Thermodynamics - Physics for JEE Main & Advanced
| 1. What is thermal equilibrium and how does it relate to the Zeroth Law of Thermodynamics? |  |
Ans.Thermal equilibrium is a condition where two or more systems in thermal contact no longer exchange heat, meaning they are at the same temperature. The Zeroth Law of Thermodynamics states that if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This law allows us to define temperature as a measurable property of matter.
| 2. What is the First Law of Thermodynamics and how is it applied in real-world scenarios? |  |
Ans.The First Law of Thermodynamics, also known as the law of energy conservation, states that the change in internal energy of a closed system is equal to the heat added to the system minus the work done by the system. In real-world scenarios, this can be applied in heat engines, refrigerators, and other energy conversion systems to analyze how energy is conserved and transformed between heat and work.
| 3. How do specific heat capacities differ for gases and why is this important? |  |
Ans.Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. For gases, specific heat capacities can vary based on whether the process occurs at constant volume (Cv) or constant pressure (Cp). This distinction is important for understanding thermodynamic processes and for calculating heat transfer in systems involving gases.
| 4. What are the differences between reversible and irreversible processes in thermodynamics? |  |
Ans.Reversible processes are idealized processes that occur infinitely slowly, allowing the system to remain in equilibrium at all times, and can be reversed without leaving any change in the system or surroundings. Irreversible processes, on the other hand, occur spontaneously and cannot be reversed without external intervention. The distinction is crucial for understanding efficiency in real-world thermodynamic cycles.
| 5. How does the Second Law of Thermodynamics relate to the efficiency of heat engines? |  |
Ans.The Second Law of Thermodynamics states that the total entropy of an isolated system can never decrease over time. This law implies that no heat engine can be 100% efficient because some energy is always converted to a less useful form, usually heat, which increases the entropy of the surroundings. The efficiency of a heat engine is determined by the ratio of work output to heat input, and the Carnot efficiency sets an upper limit based on the temperatures of the heat reservoirs involved.
Related Searches





















