All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of January Week 1 for NEET Exam
Value of gas constant, R for one mole of a gas is independent of the- a)Atomicity of the gas
- b)Mass of gas
- c)Distance between two molecules of gas at 273 K
- d)Volume of gas
Correct answer is option 'A'. Can you explain this answer?
Value of gas constant, R for one mole of a gas is independent of the
a)
Atomicity of the gas
b)
Mass of gas
c)
Distance between two molecules of gas at 273 K
d)
Volume of gas

|
Trisha Vashisht answered |
We know that PV=nRT also PM=dRT
So in the equation The value of R depends on P , V , n , T , d , M
except atomicity
so the ans is A
So in the equation The value of R depends on P , V , n , T , d , M
except atomicity
so the ans is A
In which part of nephron, reabsorption of glucose is maximum from filtrate?- a)Henle’s loop
- b)Distal convoluted tubule
- c)seminiferous tubules
- d)Proximal convoluted tubule
Correct answer is option 'D'. Can you explain this answer?
In which part of nephron, reabsorption of glucose is maximum from filtrate?
a)
Henle’s loop
b)
Distal convoluted tubule
c)
seminiferous tubules
d)
Proximal convoluted tubule

|
Shyamal Modak. answered |
The ans is D).PCT.
Acc. to NCERT nearly all the essential nutrients and 70-80% of the electrolytes and water are reabsorbed by this segment.i.e. PCT
Acc. to NCERT nearly all the essential nutrients and 70-80% of the electrolytes and water are reabsorbed by this segment.i.e. PCT
The observed order of the stability of the cabocation is:- a)(CH3)2CH+ < (CH3)3C+ 3+ < CH3CH2+
- b)CH3+ < CH3CH2+ <(CH3)2CH+ < (CH3)3C+
- c)CH3CH2+ <(CH3)2CH+ < (CH3)3C+ < CH3+
- d)CH3+ < CH3CH2+ < (CH3)3C+ <(CH3)2CH+
Correct answer is option 'B'. Can you explain this answer?
The observed order of the stability of the cabocation is:
a)
(CH3)2CH+ < (CH3)3C+ 3+ < CH3CH2+
b)
CH3+ < CH3CH2+ <(CH3)2CH+ < (CH3)3C+
c)
CH3CH2+ <(CH3)2CH+ < (CH3)3C+ < CH3+
d)
CH3+ < CH3CH2+ < (CH3)3C+ <(CH3)2CH+
|
|
Rajesh Gupta answered |
Alkyl groups directly attached to the +vely charged carbon stabilize the carbocations due to inductive and hyperconjugation effects.
Inductive effect:
→ Stability of carbocation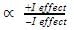
→ More number of +I group more stable carbocation.
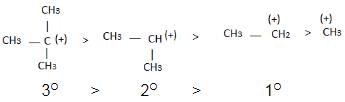
Hyperconjugation:
Inductive effect:
→ Stability of carbocation
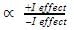
→ More number of +I group more stable carbocation.
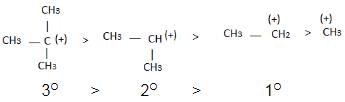
Hyperconjugation:
Stability
∝Number of canonical structures
∝Number of H (alpha hydrogen)
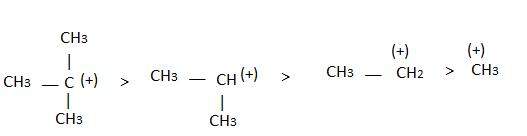
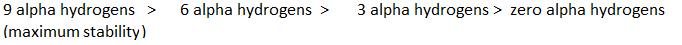
∝Number of canonical structures
∝Number of H (alpha hydrogen)
The kinetic energy of one mole of an ideal gas is E=3/2 RT. Then Cρ will be- a)0.5 R
- b)1.5 R
- c)2.5 R
- d)2.2 R
Correct answer is option 'C'. Can you explain this answer?
The kinetic energy of one mole of an ideal gas is E=3/2 RT. Then Cρ will be
a)
0.5 R
b)
1.5 R
c)
2.5 R
d)
2.2 R
|
|
Naina Sharma answered |
We know that,
Cp=cv+R
E=3Rt/2
also,
E=Cv
cp=R+3RT/2
=5/2 RT
=2.5R
Cp=cv+R
E=3Rt/2
also,
E=Cv
cp=R+3RT/2
=5/2 RT
=2.5R
Which blood vessel takes blood away from the kidney? - a)Renal portal vein
- b)Renal vein
- c)Afferent arteriole
- d)Efferent arteriole
Correct answer is option 'B'. Can you explain this answer?
Which blood vessel takes blood away from the kidney?
a)
Renal portal vein
b)
Renal vein
c)
Afferent arteriole
d)
Efferent arteriole

|
Ramesh Chand answered |
The renal vein takes blood away from the kidney. The process starts with renal artery which enters the kidney as afferent arteriole. It carries the urea loaded blood into the glomerulus of the kidney. The blood is filtered by the glomerulus into the Bowman's capsule and runs parallel to the loop of Henle. The urea is absorbed into the nephric filtrate by the process of tubular secretion in the loop of Henle, distal convulated tubule and collecting duct. The process of tubular secretion helps to secrete the urea from the blood to the collecting duct which is finally excreted in form of urine. The purified blood comes from the kidney through the renal vein and drained into vena cava outside kidney.
So, the correct answer is 'Renal vein'.
So, the correct answer is 'Renal vein'.
Heterolytic cleavage is a way to cleave the:- a)Non-ionic bonds
- b)Ionic bonds
- c)Covalent bonds
- d)Polar bonds
Correct answer is option 'C'. Can you explain this answer?
Heterolytic cleavage is a way to cleave the:
a)
Non-ionic bonds
b)
Ionic bonds
c)
Covalent bonds
d)
Polar bonds
|
|
Rahul Bansal answered |
In heterolytic cleavage, a covalent bond breaks in such a way that one fragment gets both of the shared electrons. In homolytic cleavage, a covalent bond breaks in such a way that each fragment gets one of the shared electrons. The word heterolytic comes from the Greek heteros, "different", and lysis, "loosening".
The hormone with enzymatic action which catalyses conversion of angiotensinogen into angiotensin is :- a)ANF
- b)Renin
- c)JFA
- d)Aldosterone
Correct answer is option 'B'. Can you explain this answer?
The hormone with enzymatic action which catalyses conversion of angiotensinogen into angiotensin is :
a)
ANF
b)
Renin
c)
JFA
d)
Aldosterone
|
|
Sujata Chandel answered |
Renin also known as an angiotensinogenase is an aspartic protease protein and enzyme secreted by the kidneys in to the circulation in the response to the renal hypotension and hypernatremia sympathetic nerve activation
The organic reaction which proceeds through heterolytic bond cleavage are known as:- a)Covalent reactions
- b)Ionic reactions
- c)Free radical reaction
- d)Polar reactions
Correct answer is option 'B'. Can you explain this answer?
The organic reaction which proceeds through heterolytic bond cleavage are known as:
a)
Covalent reactions
b)
Ionic reactions
c)
Free radical reaction
d)
Polar reactions
|
|
Hansa Sharma answered |
In heterolytic bond cleavage the bond breaks unevenly and the shared pair of electrons is accommodated by one of the products, which produces one or more ions.As heterolytic bond cleavage gives ions. So the reaction which proceeds through heterolytic bond cleavage is an ionic reaction.
Hence B is the correct answer.
A species having a carbon atom possessing a sextet of electrons and a positive charge is called as:- a)Chirality
- b)Metamerism
- c)Carbocation
- d)Carbaanion
Correct answer is option 'C'. Can you explain this answer?
A species having a carbon atom possessing a sextet of electrons and a positive charge is called as:
a)
Chirality
b)
Metamerism
c)
Carbocation
d)
Carbaanion

|
Rounak Desai answered |
Carbocation:
A carbocation is a species that contains a positively charged carbon atom. It is formed when a carbon atom loses a pair of electrons, leaving it with only six electrons in its valence shell. This electron deficiency makes the carbon atom highly reactive and prone to attracting electrons from nearby atoms or molecules.
Structure and Bonding:
In a carbocation, the carbon atom is attached to three other atoms or groups. It forms three sigma bonds by overlapping its atomic orbitals with the orbitals of the other atoms or groups. These sigma bonds provide stability to the positively charged carbon atom.
The carbon atom in a carbocation possesses only six electrons in its valence shell, which results in an incomplete octet. It is called a sextet because it has six valence electrons instead of the usual eight. The positive charge on the carbon atom indicates that it has lost an electron, resulting in a deficiency of negative charge.
Reactivity:
Carbocations are highly reactive species due to their electron deficiency. They are electron acceptors and can react with nucleophiles, which are electron-rich species. The nucleophile donates a pair of electrons to the positively charged carbon atom, neutralizing the charge and forming a new bond.
The reactivity of a carbocation depends on its stability. Carbocations can be classified into three types based on the number of alkyl groups attached to the positively charged carbon atom:
1. Primary carbocation: It has one alkyl group attached to the carbon atom.
2. Secondary carbocation: It has two alkyl groups attached to the carbon atom.
3. Tertiary carbocation: It has three alkyl groups attached to the carbon atom.
Tertiary carbocations are more stable than secondary carbocations, and secondary carbocations are more stable than primary carbocations. This stability is due to the electron-donating nature of the alkyl groups, which helps to disperse the positive charge on the carbon atom.
Applications:
Carbocations are intermediates in many organic reactions, including electrophilic additions, rearrangements, and substitution reactions. Understanding their reactivity and stability is crucial in organic chemistry for predicting reaction mechanisms and designing synthetic routes.
A carbocation is a species that contains a positively charged carbon atom. It is formed when a carbon atom loses a pair of electrons, leaving it with only six electrons in its valence shell. This electron deficiency makes the carbon atom highly reactive and prone to attracting electrons from nearby atoms or molecules.
Structure and Bonding:
In a carbocation, the carbon atom is attached to three other atoms or groups. It forms three sigma bonds by overlapping its atomic orbitals with the orbitals of the other atoms or groups. These sigma bonds provide stability to the positively charged carbon atom.
The carbon atom in a carbocation possesses only six electrons in its valence shell, which results in an incomplete octet. It is called a sextet because it has six valence electrons instead of the usual eight. The positive charge on the carbon atom indicates that it has lost an electron, resulting in a deficiency of negative charge.
Reactivity:
Carbocations are highly reactive species due to their electron deficiency. They are electron acceptors and can react with nucleophiles, which are electron-rich species. The nucleophile donates a pair of electrons to the positively charged carbon atom, neutralizing the charge and forming a new bond.
The reactivity of a carbocation depends on its stability. Carbocations can be classified into three types based on the number of alkyl groups attached to the positively charged carbon atom:
1. Primary carbocation: It has one alkyl group attached to the carbon atom.
2. Secondary carbocation: It has two alkyl groups attached to the carbon atom.
3. Tertiary carbocation: It has three alkyl groups attached to the carbon atom.
Tertiary carbocations are more stable than secondary carbocations, and secondary carbocations are more stable than primary carbocations. This stability is due to the electron-donating nature of the alkyl groups, which helps to disperse the positive charge on the carbon atom.
Applications:
Carbocations are intermediates in many organic reactions, including electrophilic additions, rearrangements, and substitution reactions. Understanding their reactivity and stability is crucial in organic chemistry for predicting reaction mechanisms and designing synthetic routes.
A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as:- a)Reaction mechanism
- b)Reaction kinetics
- c)Thermodynamics
- d)Equation
Correct answer is option 'A'. Can you explain this answer?
A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as:
a)
Reaction mechanism
b)
Reaction kinetics
c)
Thermodynamics
d)
Equation

|
Rounak Desai answered |
Reaction Mechanism
The correct answer is option 'A', reaction mechanism. A reaction mechanism provides a detailed account of each step involved in a chemical reaction, including the movement of electrons, the energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products. It explains how reactant molecules rearrange and bond with each other to form products.
Electron Movement
In a chemical reaction, electrons play a crucial role in the formation and breaking of chemical bonds. The movement of electrons determines the reactivity and stability of molecules. The reaction mechanism describes how electrons are transferred or shared between atoms during a reaction. It explains the movement of electrons from high energy orbitals to low energy orbitals, leading to the formation of new bonds and the breaking of existing bonds.
Energetics during Bond Cleavage and Bond Formation
During a chemical reaction, bonds between atoms are broken and new bonds are formed. The reaction mechanism explains the energy changes associated with these processes. Bond cleavage requires an input of energy, known as bond dissociation energy, as bonds are broken. This energy is absorbed from the surroundings. On the other hand, bond formation releases energy, known as bond formation energy, as new bonds are created. The reaction mechanism describes the energy changes during these processes and how they influence the overall energetics of the reaction.
Rates of Transformation (Kinetics)
The reaction mechanism also provides information about the rates of transformation of reactants into products. It describes the individual steps involved in the reaction and the order in which they occur. Each step in the mechanism has a specific rate constant associated with it, which determines how fast that step proceeds. The overall rate of the reaction is determined by the slowest step, known as the rate-determining step. The reaction mechanism helps to identify this step and understand the factors that control the reaction rate.
Conclusion
In summary, a reaction mechanism is a detailed account of the steps involved in a chemical reaction. It describes the movement of electrons, the energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products. Understanding the reaction mechanism is crucial for predicting and controlling chemical reactions.
The correct answer is option 'A', reaction mechanism. A reaction mechanism provides a detailed account of each step involved in a chemical reaction, including the movement of electrons, the energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products. It explains how reactant molecules rearrange and bond with each other to form products.
Electron Movement
In a chemical reaction, electrons play a crucial role in the formation and breaking of chemical bonds. The movement of electrons determines the reactivity and stability of molecules. The reaction mechanism describes how electrons are transferred or shared between atoms during a reaction. It explains the movement of electrons from high energy orbitals to low energy orbitals, leading to the formation of new bonds and the breaking of existing bonds.
Energetics during Bond Cleavage and Bond Formation
During a chemical reaction, bonds between atoms are broken and new bonds are formed. The reaction mechanism explains the energy changes associated with these processes. Bond cleavage requires an input of energy, known as bond dissociation energy, as bonds are broken. This energy is absorbed from the surroundings. On the other hand, bond formation releases energy, known as bond formation energy, as new bonds are created. The reaction mechanism describes the energy changes during these processes and how they influence the overall energetics of the reaction.
Rates of Transformation (Kinetics)
The reaction mechanism also provides information about the rates of transformation of reactants into products. It describes the individual steps involved in the reaction and the order in which they occur. Each step in the mechanism has a specific rate constant associated with it, which determines how fast that step proceeds. The overall rate of the reaction is determined by the slowest step, known as the rate-determining step. The reaction mechanism helps to identify this step and understand the factors that control the reaction rate.
Conclusion
In summary, a reaction mechanism is a detailed account of the steps involved in a chemical reaction. It describes the movement of electrons, the energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products. Understanding the reaction mechanism is crucial for predicting and controlling chemical reactions.
According to kinetic theory of gases, 0K is that temperature at which for an ideal gas- a)pressure is not zero
- b)internal energy is zero
- c)nearly all molecular motion becomes very rapid
- d)volume is not zero
Correct answer is option 'B'. Can you explain this answer?
According to kinetic theory of gases, 0K is that temperature at which for an ideal gas
a)
pressure is not zero
b)
internal energy is zero
c)
nearly all molecular motion becomes very rapid
d)
volume is not zero

|
Sara Saldi answered |
Because at temperature 0K nearly all molecular motion happen to stop. insisting the internal energy to be simple.
I hope it helped
I hope it helped
Which among the following is a very unstable and reactive species:- a)Carbaanion
- b)Polar ions
- c)Carbocation
- d)Free radical
Correct answer is option 'C'. Can you explain this answer?
Which among the following is a very unstable and reactive species:
a)
Carbaanion
b)
Polar ions
c)
Carbocation
d)
Free radical

|
Geethika Reddy answered |
Actually carbanions are filled with octet, so they are stable and less reactive, in case of polar ions already they are stable, and free radicle is heptet i.e, near to octet whereas carbocation is sextet in nature, so in order to gain to octet it is more reactive and less stable
At absolute zero temperature may be defined as that temperature at which- a)Volume is maximum
- b)Root mean square velocity of the gas molecule reduces to zero
- c)Temperature is 273 K
- d)Mass of molecules of gas is zero
Correct answer is option 'B'. Can you explain this answer?
At absolute zero temperature may be defined as that temperature at which
a)
Volume is maximum
b)
Root mean square velocity of the gas molecule reduces to zero
c)
Temperature is 273 K
d)
Mass of molecules of gas is zero
|
|
Gaurav Kumar answered |
At 0K temperature we know that there is no molecular motion, that is the KE of the particles gets 0. Thus we can say the combined KE of a gaseous system is zero, but as there combined mass cant be zero thus the combined of the square of velocities of the particles is zero, which means that the root mean square velocity of the gas is zero.
Towards the centre of the inner concave surface of the kidney is a notchcalled:- a)Medulla
- b)Hilum
- c)Cortex
- d)Calyces
Correct answer is option 'B'. Can you explain this answer?
Towards the centre of the inner concave surface of the kidney is a notchcalled:
a)
Medulla
b)
Hilum
c)
Cortex
d)
Calyces
|
|
Vivek Patel answered |
Towards the centre of the inner concave surface of the kidney is a notch called hilum through which ureter, blood vessels and nerves enter.
In a free radical reaction, free radicals are formed at- a)initiation step
- b)propagation step
- c)termination step
- d)both A and B
Correct answer is option 'D'. Can you explain this answer?
In a free radical reaction, free radicals are formed at
a)
initiation step
b)
propagation step
c)
termination step
d)
both A and B

|
Nitin Nair answered |
Once a reactive free radical is generated, it can react with stable molecules to form new free radicals. These new free radicals go on to generate yet more free radicals, and so on. Propagation steps often involve hydrogen abstraction or addition of the radical to double bonds.
In human excretory system :
i. kidneys and ureters are paired structures but urinary bladder is single.
ii. Kidneys are situated between the levels of last thoracic and third lumbar vertebra.- a)both are wrong
- b)Statement ii) is wrong and i) is correct.
- c)both are correct
- d)Statement i) is correct and ii) is wrong.
Correct answer is option 'C'. Can you explain this answer?
In human excretory system :
i. kidneys and ureters are paired structures but urinary bladder is single.
ii. Kidneys are situated between the levels of last thoracic and third lumbar vertebra.
i. kidneys and ureters are paired structures but urinary bladder is single.
ii. Kidneys are situated between the levels of last thoracic and third lumbar vertebra.
a)
both are wrong
b)
Statement ii) is wrong and i) is correct.
c)
both are correct
d)
Statement i) is correct and ii) is wrong.
|
|
Shreya Das answered |
Both the statements are correct
kidney and ureter are paired structure whereas urinary bladder is a single structure
and kidneys typically extend from T12 to L3, although the right kidney is often situated slightly lower due to the presence of the liver. Each kidney is approximately three vertebrae in length.
kidney and ureter are paired structure whereas urinary bladder is a single structure
and kidneys typically extend from T12 to L3, although the right kidney is often situated slightly lower due to the presence of the liver. Each kidney is approximately three vertebrae in length.
People with chronic kidney disease are usually affected by anemia. It happens because damaged kidney doesn’t secrete sufficient :- a)Erythropoietin
- b)Angiotensin I
- c)Atrial natriuretic factor
- d)Angiotensin II
Correct answer is option 'A'. Can you explain this answer?
People with chronic kidney disease are usually affected by anemia. It happens because damaged kidney doesn’t secrete sufficient :
a)
Erythropoietin
b)
Angiotensin I
c)
Atrial natriuretic factor
d)
Angiotensin II
|
|
Bhaskar Yadav answered |
The person suffering from chronic kidney disease are usually affected by anemia. The damage kidney is not able to secrete sufficient erythropoietin in blood that help in absorption of iron.
A carbon species carrying a negative charge on carbon atom is known as:- a)Polar ions
- b)Carbanion
- c)Free radical
- d)Carbocation
Correct answer is option 'B'. Can you explain this answer?
A carbon species carrying a negative charge on carbon atom is known as:
a)
Polar ions
b)
Carbanion
c)
Free radical
d)
Carbocation

|
Nitin Nair answered |
A carbanion has a negatively charged, trivalent carbon atom that has eight electrons in its valence shell. Thus, a carbanion is not electron deficient. As a result, carbanions have pyramidal geometry. Carbocations, radicals, and carbanions can be stabilized by resonance.
The average kinetic energy of a molecule in an ideal gas is- a)proportional to the pressure
- b)depends on the nature of the ideal gas
- c)proportional to the absolute temperature of the gas
- d)proportional to the volume
Correct answer is option 'C'. Can you explain this answer?
The average kinetic energy of a molecule in an ideal gas is
a)
proportional to the pressure
b)
depends on the nature of the ideal gas
c)
proportional to the absolute temperature of the gas
d)
proportional to the volume
|
|
Priya Patel answered |
SHOW THAT THE AVERAGE TRANSLATIONAL KINETIC ENERGY OF THE MOLECULES OF A GAS IS DIRECTLY PROPORTIONAL TO ABSOLUTE TEMPERATURE. kinetic energy of the molecules of a gas is directly proportional to absolute temperature.
The part of a nephron which adds HCO-3 to the filtrate is :- a)Bowman’s capsule
- b)Loop of Henle
- c)Collecting Duct
- d)Distal convoluted tubule
Correct answer is option 'D'. Can you explain this answer?
The part of a nephron which adds HCO-3 to the filtrate is :
a)
Bowman’s capsule
b)
Loop of Henle
c)
Collecting Duct
d)
Distal convoluted tubule
|
|
Ritika Nair answered |
Understanding the Nephron's Function
The nephron is the functional unit of the kidney, responsible for filtering blood and producing urine. Each nephron has several segments, each with specific roles in maintaining the body’s electrolyte balance and acid-base homeostasis.
Role of the Distal Convoluted Tubule
The distal convoluted tubule (DCT) plays a crucial role in the reabsorption of bicarbonate (HCO3-), which is essential for maintaining the body's pH balance. Here’s how it works:
- Bicarbonate Reabsorption: The DCT is responsible for the reabsorption of bicarbonate ions from the filtrate back into the blood. This process helps to buffer the acid-base balance in the body.
- Acid-Base Regulation: By adding HCO3- to the filtrate, the DCT helps neutralize excess hydrogen ions (H+), thereby preventing acidosis.
- Hormonal Influence: The reabsorption of bicarbonate in the DCT is regulated by hormones such as aldosterone, which increases sodium reabsorption and indirectly influences bicarbonate levels.
Other Nephron Components
- Bowman's Capsule: This structure is primarily involved in the initial filtration of blood but does not add bicarbonate to the filtrate.
- Loop of Henle: Its main functions include concentrating urine and reabsorbing water and salts, but it does not directly manage bicarbonate levels.
- Collecting Duct: Primarily involved in the final adjustments of water and electrolyte balance but not in the direct addition of bicarbonate.
Conclusion
The key function of adding bicarbonate to the filtrate occurs in the distal convoluted tubule, making option 'D' the correct answer. Understanding this function is vital for comprehending kidney physiology and its role in maintaining acid-base balance in the body.
The nephron is the functional unit of the kidney, responsible for filtering blood and producing urine. Each nephron has several segments, each with specific roles in maintaining the body’s electrolyte balance and acid-base homeostasis.
Role of the Distal Convoluted Tubule
The distal convoluted tubule (DCT) plays a crucial role in the reabsorption of bicarbonate (HCO3-), which is essential for maintaining the body's pH balance. Here’s how it works:
- Bicarbonate Reabsorption: The DCT is responsible for the reabsorption of bicarbonate ions from the filtrate back into the blood. This process helps to buffer the acid-base balance in the body.
- Acid-Base Regulation: By adding HCO3- to the filtrate, the DCT helps neutralize excess hydrogen ions (H+), thereby preventing acidosis.
- Hormonal Influence: The reabsorption of bicarbonate in the DCT is regulated by hormones such as aldosterone, which increases sodium reabsorption and indirectly influences bicarbonate levels.
Other Nephron Components
- Bowman's Capsule: This structure is primarily involved in the initial filtration of blood but does not add bicarbonate to the filtrate.
- Loop of Henle: Its main functions include concentrating urine and reabsorbing water and salts, but it does not directly manage bicarbonate levels.
- Collecting Duct: Primarily involved in the final adjustments of water and electrolyte balance but not in the direct addition of bicarbonate.
Conclusion
The key function of adding bicarbonate to the filtrate occurs in the distal convoluted tubule, making option 'D' the correct answer. Understanding this function is vital for comprehending kidney physiology and its role in maintaining acid-base balance in the body.
How does the counter current mechanism contribute to the concentration of urine in mammals?- a) By promoting the flow of filtrate in the same direction in the Henle's loop and vasa recta
- b) By maintaining a decreasing osmolarity towards the inner medullary interstitium
- c) By facilitating the exchange of NaCl and urea between the ascending limb of Henle's loop and the descending limb of vasa recta
- d) By preventing the reabsorption of essential substances in the nephron
Correct answer is option 'C'. Can you explain this answer?
How does the counter current mechanism contribute to the concentration of urine in mammals?
a)
By promoting the flow of filtrate in the same direction in the Henle's loop and vasa recta
b)
By maintaining a decreasing osmolarity towards the inner medullary interstitium
c)
By facilitating the exchange of NaCl and urea between the ascending limb of Henle's loop and the descending limb of vasa recta
d)
By preventing the reabsorption of essential substances in the nephron

|
Top Rankers answered |
The counter current mechanism, established by the flow of filtrate in opposite directions in the Henle's loop and the vasa recta, plays a crucial role in the concentration of urine in mammals. This mechanism allows for the exchange of NaCl and urea between the ascending limb of Henle's loop and the descending limb of vasa recta, contributing to the increasing osmolarity towards the inner medullary interstitium. This process aids in the maintenance of a concentration gradient necessary for the reabsorption of water and essential substances in the nephron, thereby enabling the production of concentrated urine.
Topic in NCERT: Counter current mechanism
Line in NCERT: "The flow of filtrate in the two limbs of Henle's loop is in opposite directions and thus forms a counter current."
In a mixture of ideal gases at a fixed temperature the heavier molecule has the lower average speed. This is easiest to conclude from the equation- a)
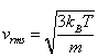
- b)
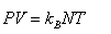
- c)
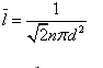
- d)
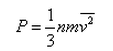
Correct answer is option 'A'. Can you explain this answer?
In a mixture of ideal gases at a fixed temperature the heavier molecule has the lower average speed. This is easiest to conclude from the equation
a)
b)
c)
d)
|
|
Lavanya Menon answered |
In option A, v is inversely proportional to mass. Hence, the condition of the question is seen accurately in that option.
Vasa recta is absent or reduced in:- a)PCT
- b)Juxtamedullary nephrons
- c)Cortical nephrons
- d)Bowman’s capsule
Correct answer is option 'C'. Can you explain this answer?
Vasa recta is absent or reduced in:
a)
PCT
b)
Juxtamedullary nephrons
c)
Cortical nephrons
d)
Bowman’s capsule
|
|
Rahul Kumar answered |
Cortical nephrons are the nephron which lies on the cortex region or most of the parts lies in the cortical region. Therefore, in cortical nephrons, vasa recta is absent or reduced in medulla part.
Which of the following statements is/are false?a. The collecting duct extends from the cortex of the kidney to the inner parts of the medulla.
b. Each human kidney has nearly one million complex tubular structures called Columns of Bertini.
c. The collecting duct can reabsorb large amounts of water to produce a concentrated urine.- a)Only a
- b)Only b
- c)Only a and b
- d)Only b and c
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements is/are false?
a. The collecting duct extends from the cortex of the kidney to the inner parts of the medulla.
b. Each human kidney has nearly one million complex tubular structures called Columns of Bertini.
c. The collecting duct can reabsorb large amounts of water to produce a concentrated urine.
b. Each human kidney has nearly one million complex tubular structures called Columns of Bertini.
c. The collecting duct can reabsorb large amounts of water to produce a concentrated urine.
a)
Only a
b)
Only b
c)
Only a and b
d)
Only b and c

|
Mohit Rajpoot answered |
- Nephrons are the functional unit of the kidney.
- These are the structures which actually produce urine in the process of removing waste and excess substances from the blood.
- There are about 10,00,000 nephrons in each human kidney.
Topic in NCERT: Collecting duct
Line in NCERT: "Collecting Duct: This long duct extends from the cortex of the kidney to the inner parts of the medulla. Large amounts of water could be reabsorbed from this region to produce a concentrated urine."
Which of the following substances is/are secreted by the active transport into the filtrate in the distal convoluted tubule?a. K+ ions
b. Creatinine
c. H+
d. HCO3- ions
e. Hippuric acid- a)Only b and e
- b)Only a and c
- c)Only c
- d)Only b and c
Correct answer is option 'B'. Can you explain this answer?
Which of the following substances is/are secreted by the active transport into the filtrate in the distal convoluted tubule?
a. K+ ions
b. Creatinine
c. H+
d. HCO3- ions
e. Hippuric acid
b. Creatinine
c. H+
d. HCO3- ions
e. Hippuric acid
a)
Only b and e
b)
Only a and c
c)
Only c
d)
Only b and c

|
Stepway Academy answered |
K+ and HCO3- ions are secreted by active transport into the filtrate in the distal convoluted tubule.
Topic in NCERT: Distal convoluted tubule
Line in NCERT: "the distal convoluted tubule (DCT) is also capable of reabsorption of HCO3 and selective secretion of hydrogen and potassium ions"
Reema is on dialysis and she is unable to conceive due to high levels of waste products in her body fluids. Her doctor can suggest her- a)Laser therapy
- b)Operation of damaged kidney
- c)more frequent hemodialysis
- d)Kidney transplant
Correct answer is option 'D'. Can you explain this answer?
Reema is on dialysis and she is unable to conceive due to high levels of waste products in her body fluids. Her doctor can suggest her
a)
Laser therapy
b)
Operation of damaged kidney
c)
more frequent hemodialysis
d)
Kidney transplant

|
Shreya Saini answered |
I think kidney transplant... because increase in body wastes could be due to malfunctioning of kidney.
The perfect gas equation is PV = nRT where n is the- a)mass of molecules
- b)number of moles
- c)number of molecules
- d)number of atoms
Correct answer is option 'B'. Can you explain this answer?
The perfect gas equation is PV = nRT where n is the
a)
mass of molecules
b)
number of moles
c)
number of molecules
d)
number of atoms
|
|
Rajat Patel answered |
In SI units, P is measured in pascals, V is measured in cubic metres, n is measured in moles, and T in kelvins (the Kelvin scale is a shifted Celsius scale, where 0.00 K = −273.15 degreeC, the lowest possible temperature). R has the value 8.314 J/(K. mol) ≈ 2 cal/(K. mol), or 0.08206 L.
Chapter doubts & questions for January Week 1 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of January Week 1 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup