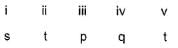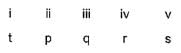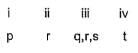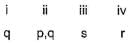All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of August Week 1 for NEET Exam
An electron with a speed of 1.8 x 106 m/s is moving in a circular orbit in a uniform magnetic field of 10-4 Wb/m², the radius of the circular path of the electron is
- a)10.63 m
- b)1.063 m
- c)106.3 m
- d)0.1063 m
Correct answer is option 'D'. Can you explain this answer?
An electron with a speed of 1.8 x 106 m/s is moving in a circular orbit in a uniform magnetic field of 10-4 Wb/m², the radius of the circular path of the electron is
a)
10.63 m
b)
1.063 m
c)
106.3 m
d)
0.1063 m

|
Learners Habitat answered |


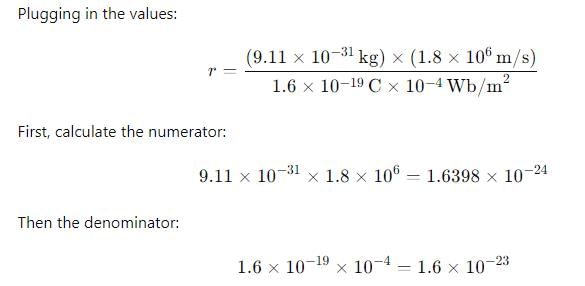
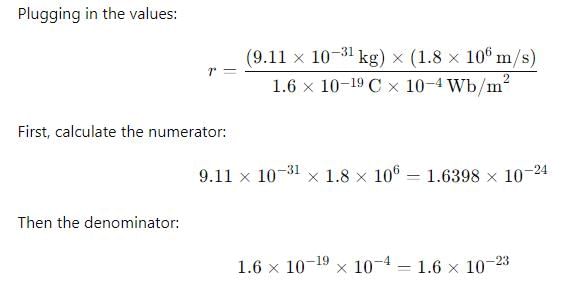


4. 0.1063 m
A proton with 1MeV kinetic energy is moving in a circular path of radius R in a uniform magnetic field. What should be the energy of an α – particle to describe a circle of same radius in the same magnetic field?- a)2 MeV
- b)0.5 MeV
- c)1 MeV
- d)4 MeV
Correct answer is option 'C'. Can you explain this answer?
A proton with 1MeV kinetic energy is moving in a circular path of radius R in a uniform magnetic field. What should be the energy of an α – particle to describe a circle of same radius in the same magnetic field?
a)
2 MeV
b)
0.5 MeV
c)
1 MeV
d)
4 MeV

|
Shraddha Singh answered |
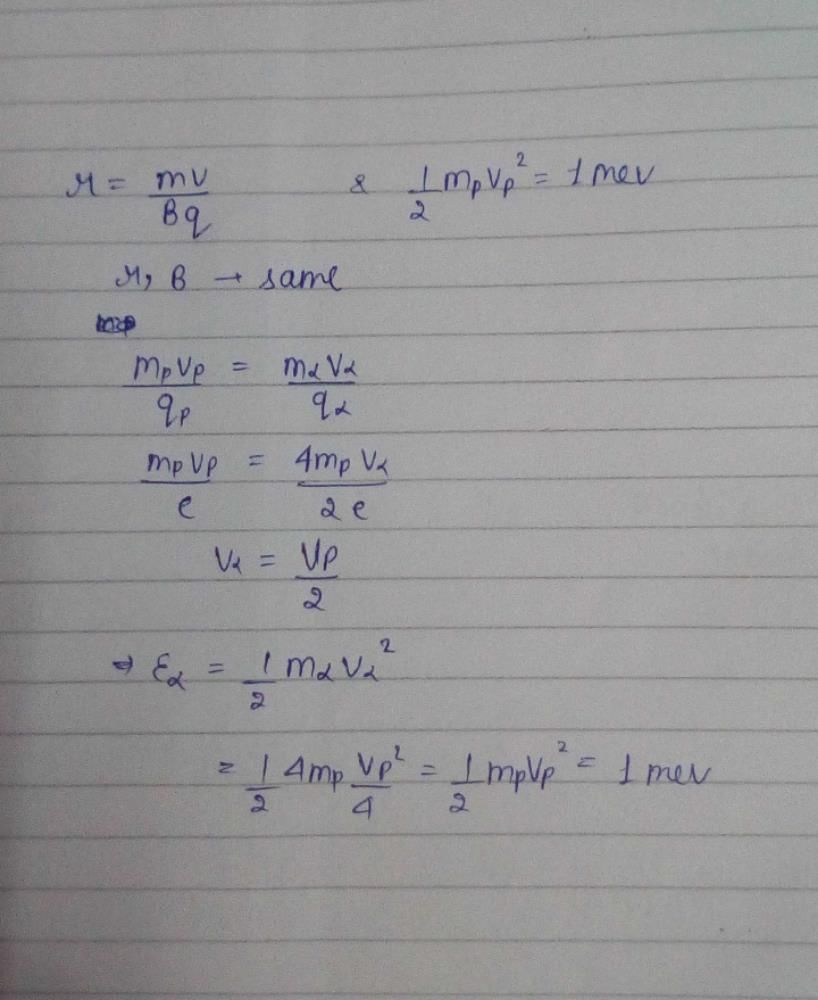
The frequency (v) of charged particle, moving at right angles to the magnetic field is independent of- a)radius of circular trajectory (r)
- b)magnetic induction (B)
- c)speed of the particle (v)
- d)both speed of the particle (v) and radius of trajectory (r).
Correct answer is option 'D'. Can you explain this answer?
The frequency (v) of charged particle, moving at right angles to the magnetic field is independent of
a)
radius of circular trajectory (r)
b)
magnetic induction (B)
c)
speed of the particle (v)
d)
both speed of the particle (v) and radius of trajectory (r).

|
Infinity Academy answered |
Frequency (v) = qB/2πm.
Frequency is independent of radius of trajectory of particle and speed of particle.
Frequency is independent of radius of trajectory of particle and speed of particle.
A particle of charge 1.6 x 10-19 C and mass 1.8 x 10-27 kg is moving around the path of radius 2 x 104 m with velocity 2.4 x 106 m/s. The magnetic field necessary is (in Wb/m²)- a)13.5 x 10-6
- b)135 x 10-6
- c)0.135 x 10
- d)1.35 x 10-6
Correct answer is option 'D'. Can you explain this answer?
A particle of charge 1.6 x 10-19 C and mass 1.8 x 10-27 kg is moving around the path of radius 2 x 104 m with velocity 2.4 x 106 m/s. The magnetic field necessary is (in Wb/m²)
a)
13.5 x 10-6
b)
135 x 10-6
c)
0.135 x 10
d)
1.35 x 10-6
|
|
Sreemoyee Choudhury answered |
Explanation:
When an electron is projected in a uniform electric field and a uniform magnetic field, both pointing in the same direction as the electron's velocity, the following happens:
1. Electric field:
The electric field exerts a force on the electron in the direction of the field. Since the electron is negatively charged, it experiences a force opposite to the direction of the electric field. Therefore, the electric field does not affect the direction of the electron's motion.
2. Magnetic field:
The magnetic field exerts a force on the electron perpendicular to both the field direction and the electron's velocity. The force is given by the Lorentz force equation:
F = q(v x B)
where F is the force, q is the charge of the electron, v is its velocity, and B is the magnetic field.
In this case, the force is directed inward, towards the center of the circular path. The magnitude of the force is given by:
|F| = qvB
where |F| is the magnitude of the force.
Since the force is perpendicular to the velocity, it causes the electron to move in a circular path around the magnetic field lines. The radius of the path is given by:
r = mv/qB
where r is the radius of the path, m is the mass of the electron, and v is its velocity.
3. Combined effect:
Since the electric field does not affect the direction of the electron's motion, the only effect is due to the magnetic field. As the electron moves in a circular path, it loses kinetic energy due to the work done by the magnetic force. Therefore, its velocity decreases in magnitude.
Hence, the correct option is D- The electron velocity will decrease in magnitude.
When an electron is projected in a uniform electric field and a uniform magnetic field, both pointing in the same direction as the electron's velocity, the following happens:
1. Electric field:
The electric field exerts a force on the electron in the direction of the field. Since the electron is negatively charged, it experiences a force opposite to the direction of the electric field. Therefore, the electric field does not affect the direction of the electron's motion.
2. Magnetic field:
The magnetic field exerts a force on the electron perpendicular to both the field direction and the electron's velocity. The force is given by the Lorentz force equation:
F = q(v x B)
where F is the force, q is the charge of the electron, v is its velocity, and B is the magnetic field.
In this case, the force is directed inward, towards the center of the circular path. The magnitude of the force is given by:
|F| = qvB
where |F| is the magnitude of the force.
Since the force is perpendicular to the velocity, it causes the electron to move in a circular path around the magnetic field lines. The radius of the path is given by:
r = mv/qB
where r is the radius of the path, m is the mass of the electron, and v is its velocity.
3. Combined effect:
Since the electric field does not affect the direction of the electron's motion, the only effect is due to the magnetic field. As the electron moves in a circular path, it loses kinetic energy due to the work done by the magnetic force. Therefore, its velocity decreases in magnitude.
Hence, the correct option is D- The electron velocity will decrease in magnitude.
The force acting on a charge q moving with velocity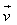 in a magnetic field
in a magnetic field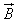 is given by
is given by- a)
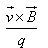
- b)
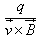
- c)
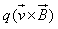
- d)
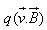
Correct answer is option 'C'. Can you explain this answer?
The force acting on a charge q moving with velocity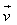 in a magnetic field
in a magnetic field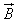 is given by
is given by
a)
b)
c)
d)
|
|
Neha Sharma answered |
The magnetic force on a free moving charge is perpendicular to both the velocity of the charge and the magnetic field with direction given by the right hand rule . The force is given by the charge times the vector product of velocity and magnetic field.
Among the following compounds, the number of compounds which have oxidation states of S is +4 ?PbS, SO2, SF6, Na2S2O3, H2SO3
Correct answer is '2'. Can you explain this answer?
Among the following compounds, the number of compounds which have oxidation states of S is +4 ?
PbS, SO2, SF6, Na2S2O3, H2SO3
|
|
Shreya Singh answered |
SO2...x+2(-2)=0.x=4..H2SO3.2(1)+x+3(-2)=0.2+x-6=0.-4+x=0.x=4...
A Charge is fired through a magnetic field. The magnetic force acting on it is maximum when the angle between the direction of motion and magnetic field is- a)π
- b)zero
- c)π/2
- d)π/4
Correct answer is option 'C'. Can you explain this answer?
A Charge is fired through a magnetic field. The magnetic force acting on it is maximum when the angle between the direction of motion and magnetic field is
a)
π
b)
zero
c)
π/2
d)
π/4
|
|
Krishna Iyer answered |
The force will have a magnitude F=qvB sin q, thus it will be maximum if sin q is maximum. Thus, angle between velocity and magnetic field should be 90o or the charge particle moves perpendicular to the velocity vector.
When a charged particle moves in a magnetic field, its kinetic energy always- a)remain constant
- b)first increases then decreases.
- c)decreases
- d)increases
Correct answer is option 'A'. Can you explain this answer?
When a charged particle moves in a magnetic field, its kinetic energy always
a)
remain constant
b)
first increases then decreases.
c)
decreases
d)
increases
|
|
Rajeev Saxena answered |
The magnetic field does no work, so the kinetic energy and speed of a charged particle in a magnetic field remain constant. The magnetic force, acting perpendicular to the velocity of the particle, will cause circular motion.
The correct statements among the following are
- a)Bond lengths in O2 ,
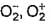 are 121 , 134, 149 pm
are 121 , 134, 149 pm
- b)Ozone is stronger oxidising agent than dioxygen
- c)O2 acts as reducing agent when it reacts with powerful oxidising agents like PtF6
- d)Ozone is much more stable than oxygen
Correct answer is option 'A,B,C'. Can you explain this answer?
The correct statements among the following are
a)
Bond lengths in O2 , 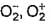 are 121 , 134, 149 pm
are 121 , 134, 149 pm
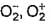 are 121 , 134, 149 pm
are 121 , 134, 149 pmb)
Ozone is stronger oxidising agent than dioxygen
c)
O2 acts as reducing agent when it reacts with powerful oxidising agents like PtF6
d)
Ozone is much more stable than oxygen

|
Srishti Kaur answered |
The correct option is Option A, B and C.
Bond length is inversely proportional to bond order. O2+ has the highest bond order among these three, so it should have the shortest bond length.
Ozone is a powerful oxidizing agent as compared to oxygen. This is due to the unstable nature of ozone and the nascent oxygen that is released during the reaction.
O2 when gas makes others like H2 gas to lose electrons, therefore, O2 gas is an oxidizing agent and H2 when gas loses electrons in redox reaction, therefore H2 gas is a reducing agent.
Oxygen is more stable than ozone. On heating, ozone readily dissociates and forms oxygen and free radicals of oxygen known as nascent oxygen which take part in reaction, thus ozone is more reactive than oxygen
Two straight horizontal parallel wires are carrying the same current in same direction, d is the distnace between the wires. You are povided with a small freely suspended magnetic needle. At which of the following positions will the orientation of the needle be independent of the magnitude of current in the wires?- a)At a distance d/2 from any of the wires.
- b)At a distance d/2 from any of the wires in the horizontal plane.
- c)Anywhere on the circumference of a vertical circle of radius d ans centre half way between the wires.
- d)At points half way between the wires in the horizontal plane.
Correct answer is option 'A'. Can you explain this answer?
Two straight horizontal parallel wires are carrying the same current in same direction, d is the distnace between the wires. You are povided with a small freely suspended magnetic needle. At which of the following positions will the orientation of the needle be independent of the magnitude of current in the wires?
a)
At a distance d/2 from any of the wires.
b)
At a distance d/2 from any of the wires in the horizontal plane.
c)
Anywhere on the circumference of a vertical circle of radius d ans centre half way between the wires.
d)
At points half way between the wires in the horizontal plane.
|
|
Nandini Patel answered |
The answer is d.
At these points, the resultant field =0
At these points, the resultant field =0
Direction (Q. Nos. 16 and 17) This section contains a paragraph, each describing theory, experiments, data, etc. Two questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageSulphur and the rest of the elements of 16th group are less electronegative than oxygen. They can acquire ns2np6 by sharing two electrons with the atoms of other elements and thus, exhibit +2 oxidation state in their compounds, in addition to this, atoms have vacant d-orbitals in their valence shell due to which electrons I can be promoted from the s- and p-orbitals of the same shell. As a result, they can show +4 and +6 oxidation states. The oxidation states of elements never exceed +6.Q.Oxidation states of sulphur in H2S2O7 and H2S2O8 respectively are- a)+ 6 and + 7
- b)+ 6 and + 6
- c)+ 5 and + 6
- d)+ 5 and + 7
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 16 and 17) This section contains a paragraph, each describing theory, experiments, data, etc. Two questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Sulphur and the rest of the elements of 16th group are less electronegative than oxygen. They can acquire ns2np6 by sharing two electrons with the atoms of other elements and thus, exhibit +2 oxidation state in their compounds, in addition to this, atoms have vacant d-orbitals in their valence shell due to which electrons I can be promoted from the s- and p-orbitals of the same shell. As a result, they can show +4 and +6 oxidation states. The oxidation states of elements never exceed +6.
Q.
Oxidation states of sulphur in H2S2O7 and H2S2O8 respectively are
a)
+ 6 and + 7
b)
+ 6 and + 6
c)
+ 5 and + 6
d)
+ 5 and + 7
|
|
Om Desai answered |
H2S2O7 has no peroxy bond while H2S2O8 has one peroxy bond.
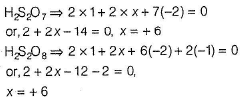
Which has lowest bond energy (single bond)?- a)O—H
- b)O—O
- c)S—H
- d)S—S
Correct answer is option 'B'. Can you explain this answer?
Which has lowest bond energy (single bond)?
a)
O—H
b)
O—O
c)
S—H
d)
S—S
|
|
Rahul Bansal answered |
Sulfur atoms are larger than oxygen atoms.
Pi bonds are formed by overlapping of two parallel p orbitals. The further the distance between atoms, the lesser the overlapping and weaker the bond.
But sigma bonds in case of Oxygen and Nitrogen are not strong enough because you are bringing two very small atoms (with large no. of electrons in the outer shell) too close which makes the sigma bond comparatively unstable than that of S-S bond where sigma bond is more stable due to lesser electro static repulsion of non-bonding electrons.
Harmful UV radiations emitted from the sun are prevented from reaching the earth by the presence of ozone in the- a)mesosphere
- b)thermosphere
- c)stratosphere
- d)troposphere
Correct answer is option 'C'. Can you explain this answer?
Harmful UV radiations emitted from the sun are prevented from reaching the earth by the presence of ozone in the
a)
mesosphere
b)
thermosphere
c)
stratosphere
d)
troposphere

|
Pooja Pillai answered |
Stratosphere layer strongly absorb harmful UV-radiations (λ. 255 nm).
The correct order of thermal stability of the hydrides of group 16 elements is- a)H2Po> H2Fe> H2Se> H2S > H2O
- b)H2O<H2S>H2Se>H2Te>H2Po
- c)H2O=H2S=H2Se-H2Te=H2Po
- d)H2O>H2S>H2SE>H2Te>H2Po
Correct answer is option 'D'. Can you explain this answer?
The correct order of thermal stability of the hydrides of group 16 elements is
a)
H2Po> H2Fe> H2Se> H2S > H2O
b)
H2O<H2S>H2Se>H2Te>H2Po
c)
H2O=H2S=H2Se-H2Te=H2Po
d)
H2O>H2S>H2SE>H2Te>H2Po

|
Gauri Khanna answered |
Thermal stability ∝ bond dissociation energy
(where, E = group 16 elements)
On moving down the group, bond dissociation energy decreases due to increase in bond length. Thus, the order of bond dissociation energy or thermal stability is
H2O > H2S > H2Se > H2Te > H2PO
Select the incorrect statement regarding DNA replication.- a)Leading strand is formed in 5'→3' direction
- b)Okazaki fragments are formed in 5'→3' direction
- c)DNA polymerase catalyses polymerisation in 5'→3' direction
- d)DNA polymerase catalyses polymerisation in 3'→5' direction
Correct answer is option 'D'. Can you explain this answer?
Select the incorrect statement regarding DNA replication.
a)
Leading strand is formed in 5'→3' direction
b)
Okazaki fragments are formed in 5'→3' direction
c)
DNA polymerase catalyses polymerisation in 5'→3' direction
d)
DNA polymerase catalyses polymerisation in 3'→5' direction
|
|
Priya Menon answered |
Synthesis of DNA by DNA polymerases occurs only in 5'→3' direction. One strand called leading strand , is copied in the same direction as the unwinding helix. The other strand is known as lagging strand. Replication of lagging strand is in a dicontinuous way , and in the direction of growth of lagging stand is 3'→5' though in short segments of DNA which are always in the 5'→3' direction. These short segments are called Okazaki fragments joined together by the action od DNA ligase.
Which of the following do not stick to the poles of a magnet?- a)Liquid oxygen
- b)Liquid nitrogen
- c)Liquid SO2
- d)Liquid ozone
Correct answer is option 'B,C,D'. Can you explain this answer?
Which of the following do not stick to the poles of a magnet?
a)
Liquid oxygen
b)
Liquid nitrogen
c)
Liquid SO2
d)
Liquid ozone

|
Juhi Deshpande answered |
Liquid oxygen is attracted to and actually sticks to the poles of the magnet. It is due to electron distribution as unpaired electrons are present while other do not stick to the poles of magnet.
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. Which is incorrect statement?- a)Element with atomic number 52 belong to 5th period and 16th group
- b)Number of electrons in the n and (n - 1) shells of selenium are 6 and 18
- c)Among chalcogens oxygen and sulphur exist in free as well as in combined state
- d)The differentiating electron in tellurium enters into 6p subshell
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which is incorrect statement?
a)
Element with atomic number 52 belong to 5th period and 16th group
b)
Number of electrons in the n and (n - 1) shells of selenium are 6 and 18
c)
Among chalcogens oxygen and sulphur exist in free as well as in combined state
d)
The differentiating electron in tellurium enters into 6p subshell
|
|
Anand Saha answered |
In tellurium, differentiating electron enters in to 5p subshell. Its valence shell configuration is 5s25p4.
In earth’s crust, sulphur exists as- a)elemental sulphur
- b)sulphide ore
- c)sulphate ores
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
In earth’s crust, sulphur exists as
a)
elemental sulphur
b)
sulphide ore
c)
sulphate ores
d)
All of these

|
Ishani Yadav answered |
Sulphur exists in elemental form which is extracted by Frasch process.
Sulphide ores are zinc blende (ZnS), galena (PbS), copper pyrites (CuFeS2), cinnabar (HgS),
Sulphate ores are gypsum (CaSO4. 2H2O), epsom (MgSO4 .7 H2O) .
Sulphide ores are zinc blende (ZnS), galena (PbS), copper pyrites (CuFeS2), cinnabar (HgS),
Sulphate ores are gypsum (CaSO4. 2H2O), epsom (MgSO4 .7 H2O) .
Direction (Q. Nos. 21-24) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Q. Number of chemicals that produce dioxygen on heatingKCIO3, (NH4)2 Cr2O7, NH4NO3, NaNO3, KMnO4, K2Cr2O7, H2O2, Pb3O4, NH4NO2
Correct answer is '6'. Can you explain this answer?
Direction (Q. Nos. 21-24) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
Number of chemicals that produce dioxygen on heating
KCIO3, (NH4)2 Cr2O7, NH4NO3, NaNO3, KMnO4, K2Cr2O7, H2O2, Pb3O4, NH4NO2
|
|
Ashwini Shah answered |
Question Analysis:
The question asks us to determine the number of chemicals that produce dioxygen on heating. Dioxygen, commonly known as oxygen, is a gas that is essential for various biological processes. We are given a list of chemicals and we need to identify the ones that produce oxygen when heated.
Solution:
To solve this question, we need to identify the chemicals that undergo a decomposition reaction when heated, resulting in the release of oxygen gas.
List of Chemicals:
1. KCIO3
2. (NH4)2 Cr2O7
3. NH4NO3
4. NaNO3
5. KMnO4
6. K2Cr2O7
7. H2O2
8. Pb3O4
9. NH4NO2
Chemicals That Produce Dioxygen on Heating:
1. KCIO3: Potassium chlorate (KCIO3) undergoes decomposition when heated, producing oxygen gas (O2).
2. (NH4)2 Cr2O7: Ammonium dichromate ((NH4)2 Cr2O7) is a highly unstable compound that decomposes when heated, giving off nitrogen gas (N2), water vapor (H2O), and oxygen gas (O2).
3. NH4NO3: Ammonium nitrate (NH4NO3) is a compound commonly used as a fertilizer and an oxidizing agent. It decomposes explosively when heated, producing nitrogen gas (N2) and oxygen gas (O2).
4. NaNO3: Sodium nitrate (NaNO3) is a stable compound that does not produce oxygen gas when heated. Therefore, it does not fulfill the requirement.
5. KMnO4: Potassium permanganate (KMnO4) is a strong oxidizing agent that does not produce oxygen gas when heated. Therefore, it does not fulfill the requirement.
6. K2Cr2O7: Potassium dichromate (K2Cr2O7) is a compound commonly used as an oxidizing agent. It does not produce oxygen gas when heated. Therefore, it does not fulfill the requirement.
7. H2O2: Hydrogen peroxide (H2O2) is a compound that readily decomposes when heated, releasing oxygen gas (O2).
8. Pb3O4: Lead(II,IV) oxide (Pb3O4) is a compound that does not produce oxygen gas when heated. Therefore, it does not fulfill the requirement.
9. NH4NO2: Ammonium nitrite (NH4NO2) is a compound that decomposes when heated, releasing nitrogen gas (N2) and oxygen gas (O2).
Conclusion:
Out of the given chemicals, the following 6 chemicals produce dioxygen on heating:
1. KCIO3
2. (NH4)2 Cr2O7
3. NH4NO3
4. H2O2
5. NH4NO2
The question asks us to determine the number of chemicals that produce dioxygen on heating. Dioxygen, commonly known as oxygen, is a gas that is essential for various biological processes. We are given a list of chemicals and we need to identify the ones that produce oxygen when heated.
Solution:
To solve this question, we need to identify the chemicals that undergo a decomposition reaction when heated, resulting in the release of oxygen gas.
List of Chemicals:
1. KCIO3
2. (NH4)2 Cr2O7
3. NH4NO3
4. NaNO3
5. KMnO4
6. K2Cr2O7
7. H2O2
8. Pb3O4
9. NH4NO2
Chemicals That Produce Dioxygen on Heating:
1. KCIO3: Potassium chlorate (KCIO3) undergoes decomposition when heated, producing oxygen gas (O2).
2. (NH4)2 Cr2O7: Ammonium dichromate ((NH4)2 Cr2O7) is a highly unstable compound that decomposes when heated, giving off nitrogen gas (N2), water vapor (H2O), and oxygen gas (O2).
3. NH4NO3: Ammonium nitrate (NH4NO3) is a compound commonly used as a fertilizer and an oxidizing agent. It decomposes explosively when heated, producing nitrogen gas (N2) and oxygen gas (O2).
4. NaNO3: Sodium nitrate (NaNO3) is a stable compound that does not produce oxygen gas when heated. Therefore, it does not fulfill the requirement.
5. KMnO4: Potassium permanganate (KMnO4) is a strong oxidizing agent that does not produce oxygen gas when heated. Therefore, it does not fulfill the requirement.
6. K2Cr2O7: Potassium dichromate (K2Cr2O7) is a compound commonly used as an oxidizing agent. It does not produce oxygen gas when heated. Therefore, it does not fulfill the requirement.
7. H2O2: Hydrogen peroxide (H2O2) is a compound that readily decomposes when heated, releasing oxygen gas (O2).
8. Pb3O4: Lead(II,IV) oxide (Pb3O4) is a compound that does not produce oxygen gas when heated. Therefore, it does not fulfill the requirement.
9. NH4NO2: Ammonium nitrite (NH4NO2) is a compound that decomposes when heated, releasing nitrogen gas (N2) and oxygen gas (O2).
Conclusion:
Out of the given chemicals, the following 6 chemicals produce dioxygen on heating:
1. KCIO3
2. (NH4)2 Cr2O7
3. NH4NO3
4. H2O2
5. NH4NO2
Direction (Q. No. 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.Q. Statement I : Oxygen is more electronegative than sulphur, yet H2S is acidic, while H2O is neutral.Statement II : H— S bond is weaker than O—H bond.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. No. 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Oxygen is more electronegative than sulphur, yet H2S is acidic, while H2O is neutral.
Statement II : H— S bond is weaker than O—H bond.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Sinjini Bajaj answered |
H—S bond is weaker than H—O bond hence, H2S is more acidic than H2O.
Other than DNA polymerase, which of the following enzymes involved in DNA synthesis?- a)Topoisomerase
- b)Helicase
- c)RNA primase
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
Other than DNA polymerase, which of the following enzymes involved in DNA synthesis?
a)
Topoisomerase
b)
Helicase
c)
RNA primase
d)
All of these
|
|
Vivek Patel answered |
Process of DNA synthesis whereby a parent DNA molecule is faithfully copied , giving rise to two identical daughter molecules id called DNA replication. In DNA synthesis, DNA polymerase plays an important role having the capability to elongate an existing DNA strand but cannot initiate the synthesis. So, the synthesis is initiated with the help of RNA primer formed by RNA primase. RNA primase synthesises the short RNA primer of about 10 nucleotides that is elongated by DNA polymerase to form an Okazaki fragment of DNA during DNA replication. Helicase unzips the two strands of DNA and toposiomerase reduces the coiling tension developed due to the unwinding of the two strands of DNA and topoisomerase reduces the coiling tension developed due to the unwinding of the two strands.
The number of bond pairs and lone pairs in rhombic sulphur molecule are- a)6 and 6
- b)8 and 8
- c)8 and 16
- d)16 and 8
Correct answer is option 'C'. Can you explain this answer?
The number of bond pairs and lone pairs in rhombic sulphur molecule are
a)
6 and 6
b)
8 and 8
c)
8 and 16
d)
16 and 8
|
|
Shail Chawla answered |
Bond pairs and Lone pairs in Rhombic Sulphur molecule
Point out the incorrect statement among the following.- a)In OF2, oxidation state of oxygen is + 2
- b)Sulphur shows strong tendency to catenation while oxygen shows this tendency to a limited extent
- c)The transition temperature of rhombic and monoclinic sulphur is 369°C
- d)Oxygen is most abundant element in the earth's crust followed by sulphur in 16th group
Correct answer is option 'C'. Can you explain this answer?
Point out the incorrect statement among the following.
a)
In OF2, oxidation state of oxygen is + 2
b)
Sulphur shows strong tendency to catenation while oxygen shows this tendency to a limited extent
c)
The transition temperature of rhombic and monoclinic sulphur is 369°C
d)
Oxygen is most abundant element in the earth's crust followed by sulphur in 16th group

|
Ishani Yadav answered |
It is 369 K or 96°C. The stable form at room temperature is rhombic sulphur which transforms to monoclinic sulphur when heated about 369 K.
The element used in the production of photocells and solar cells and also used in zerox machines is- a)sulphur
- b)selenium
- c)tellurium
- d)polonium
Correct answer is option 'B'. Can you explain this answer?
The element used in the production of photocells and solar cells and also used in zerox machines is
a)
sulphur
b)
selenium
c)
tellurium
d)
polonium

|
Srishti Kaur answered |
Se is non-conductor in dark but acts as conductor when exposed to light.
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Which of the following property do not increase down the group from oxygen to polonium?- a)Atomic size
- b)Density
- c)Electron affinity
- d)Ionisation energy
Correct answer is option 'C,D'. Can you explain this answer?
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Which of the following property do not increase down the group from oxygen to polonium?
a)
Atomic size
b)
Density
c)
Electron affinity
d)
Ionisation energy

|
Pooja Pillai answered |
Electron affinity and ionisation energy decreases down the group due to increase in size.
Which of the following statements regarding ozone are correct?- a)Conversion of ozone to oxygen is endothermic
- b)O—O bonds in ozone have considerable double bond character
- c)Pure ozone is pale blue gas and dark blue liquid
- d)Nitric oxide and freons are responsible for the depletion of ozone layer
Correct answer is option 'B,C,D'. Can you explain this answer?
Which of the following statements regarding ozone are correct?
a)
Conversion of ozone to oxygen is endothermic
b)
O—O bonds in ozone have considerable double bond character
c)
Pure ozone is pale blue gas and dark blue liquid
d)
Nitric oxide and freons are responsible for the depletion of ozone layer

|
Preethi Kaur answered |
Bonds in Ozone
- Ozone (O3) is a molecule composed of three oxygen atoms.
- Each oxygen atom is connected to the adjacent atoms by a double bond.
- The bonds in ozone have a considerable double bond character (option B is correct).
- This means that the oxygen atoms are not fully bonded to each other like in a single bond, but instead, they share electrons more like a double bond.
- The double bond character in ozone gives it unique properties and reactivity.
Physical Properties of Ozone
- Ozone is a pale blue gas (option C is correct) with a pungent odor.
- It is heavier than air and can be condensed into a dark blue liquid.
- At very low temperatures, ozone can solidify into a violet-black crystalline solid.
- Ozone is highly reactive and can decompose into oxygen molecules and atomic oxygen.
Ozone Conversion and Endothermicity
- Ozone can be converted back to oxygen through a process called ozone decomposition or Ozone-Oxygen Conversion.
- This process can occur spontaneously or can be induced by various factors such as heat, UV radiation, and certain catalysts.
- Conversion of ozone to oxygen is an exothermic reaction, meaning it releases heat.
- Conversely, the conversion of oxygen to ozone is an endothermic reaction, meaning it absorbs heat (option A is correct).
- The endothermic nature of ozone formation is due to the need to break the double bonds in oxygen molecules and create new bonds in ozone.
Depletion of Ozone Layer
- Nitric oxide (NO) and chlorofluorocarbons (CFCs) are responsible for the depletion of the ozone layer (option D is correct).
- Nitric oxide is primarily released into the atmosphere through human activities, such as combustion processes and industrial emissions.
- CFCs, commonly used as refrigerants and aerosol propellants, were found to be major contributors to ozone depletion.
- When released into the atmosphere, CFCs can reach the stratosphere, where they are broken down by UV radiation, releasing chlorine atoms.
- These chlorine atoms can then catalytically destroy ozone molecules, leading to the thinning of the ozone layer.
In summary, the correct statements regarding ozone are:
- The bonds in ozone have considerable double bond character (option B is correct).
- Ozone is a pale blue gas and dark blue liquid (option C is correct).
- Nitric oxide and freons (CFCs) are responsible for the depletion of the ozone layer (option D is correct).
- Ozone (O3) is a molecule composed of three oxygen atoms.
- Each oxygen atom is connected to the adjacent atoms by a double bond.
- The bonds in ozone have a considerable double bond character (option B is correct).
- This means that the oxygen atoms are not fully bonded to each other like in a single bond, but instead, they share electrons more like a double bond.
- The double bond character in ozone gives it unique properties and reactivity.
Physical Properties of Ozone
- Ozone is a pale blue gas (option C is correct) with a pungent odor.
- It is heavier than air and can be condensed into a dark blue liquid.
- At very low temperatures, ozone can solidify into a violet-black crystalline solid.
- Ozone is highly reactive and can decompose into oxygen molecules and atomic oxygen.
Ozone Conversion and Endothermicity
- Ozone can be converted back to oxygen through a process called ozone decomposition or Ozone-Oxygen Conversion.
- This process can occur spontaneously or can be induced by various factors such as heat, UV radiation, and certain catalysts.
- Conversion of ozone to oxygen is an exothermic reaction, meaning it releases heat.
- Conversely, the conversion of oxygen to ozone is an endothermic reaction, meaning it absorbs heat (option A is correct).
- The endothermic nature of ozone formation is due to the need to break the double bonds in oxygen molecules and create new bonds in ozone.
Depletion of Ozone Layer
- Nitric oxide (NO) and chlorofluorocarbons (CFCs) are responsible for the depletion of the ozone layer (option D is correct).
- Nitric oxide is primarily released into the atmosphere through human activities, such as combustion processes and industrial emissions.
- CFCs, commonly used as refrigerants and aerosol propellants, were found to be major contributors to ozone depletion.
- When released into the atmosphere, CFCs can reach the stratosphere, where they are broken down by UV radiation, releasing chlorine atoms.
- These chlorine atoms can then catalytically destroy ozone molecules, leading to the thinning of the ozone layer.
In summary, the correct statements regarding ozone are:
- The bonds in ozone have considerable double bond character (option B is correct).
- Ozone is a pale blue gas and dark blue liquid (option C is correct).
- Nitric oxide and freons (CFCs) are responsible for the depletion of the ozone layer (option D is correct).
Direction (Q. Nos. 18-20) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.Q. Match the Column I with Column II and mark the correct option from the codes given below.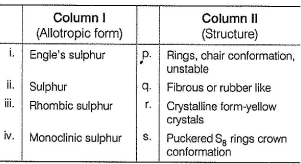

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 18-20) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
a)
a
b)
b
c)
c
d)
d

|
Maulik Mehra answered |
PassageSulphur and the rest of the elements of 16th group are less electronegative than oxygen. They can acquire ns2np6 by sharing two electrons with the atoms of other elements and thus, exhibit +2 oxidation state in their compounds, in addition to this, atoms have vacant d-orbitals in their valence shell due to which electrons I can be promoted from the s- and p-orbitals of the same shell. As a result, they can show +4 and +6 oxidation states. The oxidation states of elements never exceed +6.Q. Like sulphur, oxygen does not show +4 and +6 oxidation states .The reason is- a)that oxygen is a gas while sulphur is solid
- b)that oxygen has high ionisation energy in comparison to sulphur
- c)that sulphur has high electron affinity in comparison to oxygen
- d)that oxygen has no d-orbitals in its valence shell like sulphur
Correct answer is option 'D'. Can you explain this answer?
Passage
Sulphur and the rest of the elements of 16th group are less electronegative than oxygen. They can acquire ns2np6 by sharing two electrons with the atoms of other elements and thus, exhibit +2 oxidation state in their compounds, in addition to this, atoms have vacant d-orbitals in their valence shell due to which electrons I can be promoted from the s- and p-orbitals of the same shell. As a result, they can show +4 and +6 oxidation states. The oxidation states of elements never exceed +6.
Q.
Like sulphur, oxygen does not show +4 and +6 oxidation states .The reason is
a)
that oxygen is a gas while sulphur is solid
b)
that oxygen has high ionisation energy in comparison to sulphur
c)
that sulphur has high electron affinity in comparison to oxygen
d)
that oxygen has no d-orbitals in its valence shell like sulphur

|
Janhavi Kaur answered |
Oxygen does not show +4 and +6 oxidation states because oxygen has no cf-orbitals in its valence shell.
DNA replication takes place at ________ phase of the cell cycle- a)G1
- b)S
- c)G2
- d)M
Correct answer is option 'B'. Can you explain this answer?
DNA replication takes place at ________ phase of the cell cycle
a)
G1
b)
S
c)
G2
d)
M
|
|
Meera Singh answered |
In eukaryotes, the replication of DNA takes place at S-phase of the cell cycle. The replication of DNA and cell division cycle should be coordinated. A failure in cell division after DNA replication results into chromosomal anomaly.
Number of chemical species having bent shape in
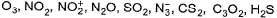
Correct answer is '4'. Can you explain this answer?
Number of chemical species having bent shape in
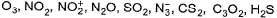

|
Kavya Das answered |
O3, NO2, SO2 and H2S show bent shape.
First experimental proof for semi-conservative DNA replication was shown in- a)Streptococcus pneumoniae
- b)Escherichia coli
- c)Neurospora crassa
- d)Rattus rattus
Correct answer is option 'B'. Can you explain this answer?
First experimental proof for semi-conservative DNA replication was shown in
a)
Streptococcus pneumoniae
b)
Escherichia coli
c)
Neurospora crassa
d)
Rattus rattus
|
|
Suresh Iyer answered |
Mathew Meselson and Franklin Stahl (1958) conducted various experiments using isotopically labelled DNA of Escherichia oli to provide evidence in favour of semije mode of DNA replication. various experiments Escherichia coli to on conservative mode notes the renlisation of DNA replication.
Chapter doubts & questions for August Week 1 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of August Week 1 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup