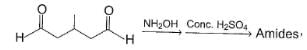
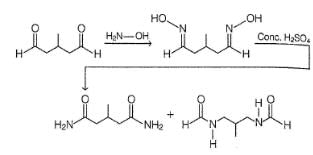
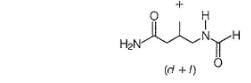
All Exams >
JEE >
6 Months Preparation for JEE >
All Questions
All questions of Aldehydes, Ketones and Carboxylic Acids for JEE Exam
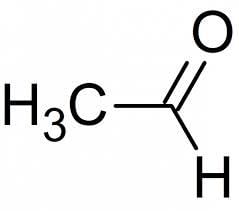
The IUPAC name of CH3CHO is:- a)Acetaldehyde
- b)Ethanal
- c)Formaldehyde
- d)Methanal
Correct answer is option 'A'. Can you explain this answer?
The IUPAC name of CH3CHO is:
a)
Acetaldehyde
b)
Ethanal
c)
Formaldehyde
d)
Methanal

|
Ambition Institute answered |
- The functional group is an aldehyde; −CHO and the given compound has two carbon atoms.
- Thus, the IUPAC name of the compound is ethanal.
Write the IUPAC name of (CH3)2CHCHO?- a)2,2-Dimethylpropanal
- b)3-Hydroxypropanal
- c)But-3-en-2-one
- d)None of these
Correct answer is option 'D'. Can you explain this answer?
Write the IUPAC name of (CH3)2CHCHO?
a)
2,2-Dimethylpropanal
b)
3-Hydroxypropanal
c)
But-3-en-2-one
d)
None of these
|
|
Neha Sharma answered |
The IUPAC name of (CH3)2CHCHO is 2-methylpropanal and its chemical name is Isobutyraldehyde.
Which of the following statements are correct in case of the carbonyl bond between carbon and oxygen?- a)Carbon is the nucleophilic centre and Oxygen is the electrophilic centre.
- b)Oxygen is the nucleophilic centre and Carbon is the electrophilic centre.
- c)Carbon and Oxygen double bond is polarised.
- d)Both ‘b’ and ‘c’ are correct
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements are correct in case of the carbonyl bond between carbon and oxygen?
a)
Carbon is the nucleophilic centre and Oxygen is the electrophilic centre.
b)
Oxygen is the nucleophilic centre and Carbon is the electrophilic centre.
c)
Carbon and Oxygen double bond is polarised.
d)
Both ‘b’ and ‘c’ are correct
|
|
Nandini Patel answered |
The double bonds in alkenes and double bonds in carbonyl groups are VERY different in terms of reactivity. The C=C is less reactive due to C=O electronegativity attributed to the oxygen and its two lone pairs of electrons. One pair of the oxygen lone pairs are located in 2s while the other pair are in 2p orbital where its axis is directed perpendicular to the direction of the pi orbitals. The Carbonyl groups properties are directly tied to its electronic structure as well as geometric positioning. For example, the electronegativity of oxygen also polarizes the pi bond allowing the single bonded substituent connected to become electron withdrawing.
Which of the following statement about C=O and C=C is correct?- a)Both consist of a sigma and pi bond
- b)C=O is polar but C=C is non-polar
- c)Both a and b are correct
- d)Both C=O and C=C undergo nucleophilic addition reactions
Correct answer is option 'C'. Can you explain this answer?
Which of the following statement about C=O and C=C is correct?
a)
Both consist of a sigma and pi bond
b)
C=O is polar but C=C is non-polar
c)
Both a and b are correct
d)
Both C=O and C=C undergo nucleophilic addition reactions

|
Knowledge Hub answered |
- The first bond formed is a sigma bind and the second one is a pi bond.
- O has a higher electronegativity than C and hence the electron cloud will be shifted towards the O atom, making the compound polar.
- This is not possible in C=C.
Among the following functional groups, which of these is not a carbonyl compound?- a)alcohols
- b)aldehydes
- c)Carboxylic acid
- d)ketones
Correct answer is option 'A'. Can you explain this answer?
Among the following functional groups, which of these is not a carbonyl compound?
a)
alcohols
b)
aldehydes
c)
Carboxylic acid
d)
ketones
|
|
Pari answered |
In carbonyl compound C=O is present bt in alchol OH is present. So alchol is not a carbonyl compound
Acetone is isomeric to:- a)n-propyl alcohol
- b)propanal
- c)ethyl methyl ether
- d)isopropyl alcohol
Correct answer is option 'B'. Can you explain this answer?
Acetone is isomeric to:
a)
n-propyl alcohol
b)
propanal
c)
ethyl methyl ether
d)
isopropyl alcohol
|
|
Lavanya Menon answered |
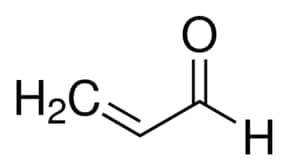
Acetone (CH3COCH3) and Propanal (CH3CH2CHO) are functional isomers.
- Acetone:
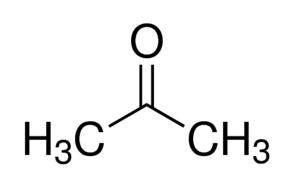
- Propanal:

What is the IUPAC name of Acrolein?- a)pent-2-enal
- b)but-1-enal
- c)prop-2-enal
- d)but-2-enal
Correct answer is option 'C'. Can you explain this answer?
What is the IUPAC name of Acrolein?
a)
pent-2-enal
b)
but-1-enal
c)
prop-2-enal
d)
but-2-enal
|
|
Nandini Iyer answered |
IUPAC name of acrolein is prop-2-enal, a name derived from that of acrylic acid, the parent carboxylic acid.

Propanone and prop-2-en-1-ol are examples of which type of isomerism?
- a)Functional isomers
- b)Chain isomers
- c)Tautomers
- d)Position isomers
Correct answer is option 'A'. Can you explain this answer?
Propanone and prop-2-en-1-ol are examples of which type of isomerism?
a)
Functional isomers
b)
Chain isomers
c)
Tautomers
d)
Position isomers
|
|
Gayatri Banerjee answered |
Functional isomers
Explanation: Propanone (CH3COCH3) and prop-2-en-1-ol (CH2=CHCH2OH) are examples of functional isomers because they have the same molecular formula (C3H6O) but different functional groups. Propanone has a carbonyl group (C=O) while prop-2-en-1-ol has an alcohol group (OH) and a carbon-carbon double bond (C=C).
Explanation: Propanone (CH3COCH3) and prop-2-en-1-ol (CH2=CHCH2OH) are examples of functional isomers because they have the same molecular formula (C3H6O) but different functional groups. Propanone has a carbonyl group (C=O) while prop-2-en-1-ol has an alcohol group (OH) and a carbon-carbon double bond (C=C).
How many structural isomers can compound with molecular formula ‘C3H6O’ have?- a)6
- b)4
- c)5
- d)11
Correct answer is option 'D'. Can you explain this answer?
How many structural isomers can compound with molecular formula ‘C3H6O’ have?
a)
6
b)
4
c)
5
d)
11
|
|
Ritu Singh answered |
The Isomers of C3H6O include:
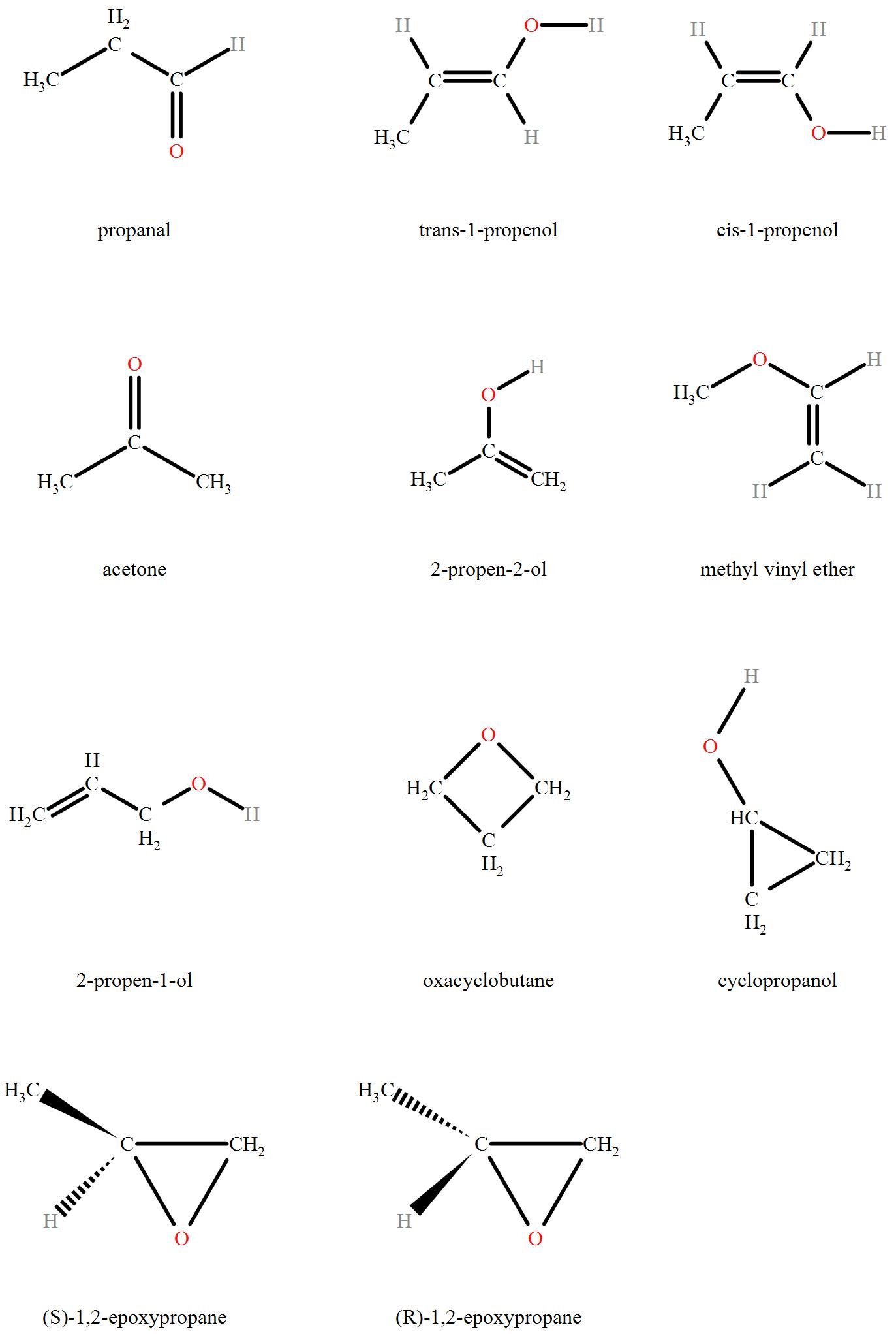
Hence we can see that a total of 11 isomers of C3H6O are possible.
What is the common name of 2-methyl-propanal?- a)formaldehyde
- b)Isobutyraldehyde
- c)carbaldehyde
- d)acetaldehyde
Correct answer is 'B'. Can you explain this answer?
What is the common name of 2-methyl-propanal?
a)
formaldehyde
b)
Isobutyraldehyde
c)
carbaldehyde
d)
acetaldehyde
|
|
Nikita Singh answered |
- Isobutyraldehyde is the chemical compound with the formula (CH₃)₂CHCHO.

- It is an aldehyde, isomeric with n-butyraldehyde.
- Isobutyraldehyde is manufactured, often as a side-product, by the hydroformylation of propene. Its odour is described as that of wet cereal or straw.
Only One Option Correct TypeDirection (Q, Nos. 1-9) This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. Which of the following will give a racemic mixture on reduction with NaBH4 followed by acid work-up?- a)
- b)
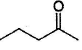
- c)
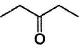
- d)
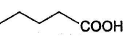
Correct answer is option 'B'. Can you explain this answer?
Only One Option Correct Type
Direction (Q, Nos. 1-9) This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which of the following will give a racemic mixture on reduction with NaBH4 followed by acid work-up?
a)
b)
c)
d)

|
Amisha answered |
The ans is B . coz after the action of NaBH4 ,chiral carbon is present only in the product of B.And hence it can give both R and S configuration.
Which of the following could result as a product in the aldol condensation reaction?- a)4-methyl-3-penten-2-on
- b)4-methyl-4-penten-2-one
- c)4-methyl-5-hexen-2-one
- d)3-methyl-4-hexen-2-one
Correct answer is option 'A'. Can you explain this answer?
Which of the following could result as a product in the aldol condensation reaction?
a)
4-methyl-3-penten-2-on
b)
4-methyl-4-penten-2-one
c)
4-methyl-5-hexen-2-one
d)
3-methyl-4-hexen-2-one

|
Maulik Verma answered |
It is an α, β-unsaturated ketone which can be formed in an aldol condensation followed by dehydration.
Which is the most suitable reagent for the following transformation?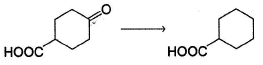
- a)Zn(Hg)-HCI
- b)N2H4/NaOH/Heat
- c)LiAIH4
- d)NaBH4
Correct answer is option 'A'. Can you explain this answer?
Which is the most suitable reagent for the following transformation?
a)
Zn(Hg)-HCI
b)
N2H4/NaOH/Heat
c)
LiAIH4
d)
NaBH4
|
|
Geetika Shah answered |
Clemmensen reduction is suitable for reductio n of carbonyls containing additional acidic functional group.
An organic compound A (C7H16O) shows both enantiomerism and diastereomerism. Treatment of a pure enantiomer of A with Na2CrO4 /Dil. H2SO4 gives B (C7H14O) - Also A on dehydration with concentrated H2SO4 gives a single alkene C (C7H14). Ozonolysis of C followed by work-up with Zn-H2O gives D (C5H10O) as one of the product which gives racemic mixture on reduction with NaBH4.Q. If B is reduced with LiAIH4 followed by acid hydrolysis will give- a)a pure enantiomer
- b)a racemic mixture
- c)a pair of diastereomers
- d)an achiral alcohol
Correct answer is option 'C'. Can you explain this answer?
An organic compound A (C7H16O) shows both enantiomerism and diastereomerism. Treatment of a pure enantiomer of A with Na2CrO4 /Dil. H2SO4 gives B (C7H14O) - Also A on dehydration with concentrated H2SO4 gives a single alkene C (C7H14). Ozonolysis of C followed by work-up with Zn-H2O gives D (C5H10O) as one of the product which gives racemic mixture on reduction with NaBH4.
Q.
If B is reduced with LiAIH4 followed by acid hydrolysis will give
a)
a pure enantiomer
b)
a racemic mixture
c)
a pair of diastereomers
d)
an achiral alcohol

|
Preethi Kaur answered |
Explanation:
Enantiomerism:
Enantiomerism refers to the phenomenon where two molecules are mirror images of each other and are non-superimposable. In this case, compound A shows enantiomerism, which means it exists in two different mirror-image forms.
Diastereomerism:
Diastereomerism refers to the phenomenon where two molecules have the same connectivity but are not mirror images of each other and are non-superimposable. In this case, compound A shows diastereomerism, which means it exists in multiple forms that are not mirror images of each other.
Reaction 1: Treatment of A with Na2CrO4/Dil. H2SO4:
When compound A is treated with Na2CrO4 and dilute H2SO4, it undergoes oxidation reaction and gives compound B. The reaction is as follows:
C7H16O (A) + Na2CrO4 / Dil. H2SO4 → C7H14O (B)
Reaction 2: Dehydration of A with concentrated H2SO4:
When compound A is dehydrated with concentrated H2SO4, it undergoes elimination reaction and gives a single alkene C. The reaction is as follows:
C7H16O (A) + H2SO4 (conc.) → C7H14 (C)
Reaction 3: Ozonolysis of C followed by work-up with Zn-H2O:
When alkene C is subjected to ozonolysis followed by treatment with Zn-H2O, it undergoes oxidative cleavage and gives compound D as one of the products. The reaction is as follows:
C7H14 (C) + O3 → C5H10O (D)
Reaction 4: Reduction of D with NaBH4:
When compound D is reduced with NaBH4, it undergoes reduction reaction and gives a racemic mixture. This means that both enantiomers of D are formed in equal amounts. The reaction is as follows:
C5H10O (D) + NaBH4 → Racemic mixture of D
Reaction 5: Reduction of B with LiAlH4 followed by acid hydrolysis:
When compound B is reduced with LiAlH4 followed by acid hydrolysis, it undergoes reduction reaction and gives a pair of diastereomers. This means that two different diastereomers of B are formed. The reaction is as follows:
C7H14O (B) + LiAlH4 → Diastereomers of B
Therefore, the correct answer is option c) a pair of diastereomers.
Enantiomerism:
Enantiomerism refers to the phenomenon where two molecules are mirror images of each other and are non-superimposable. In this case, compound A shows enantiomerism, which means it exists in two different mirror-image forms.
Diastereomerism:
Diastereomerism refers to the phenomenon where two molecules have the same connectivity but are not mirror images of each other and are non-superimposable. In this case, compound A shows diastereomerism, which means it exists in multiple forms that are not mirror images of each other.
Reaction 1: Treatment of A with Na2CrO4/Dil. H2SO4:
When compound A is treated with Na2CrO4 and dilute H2SO4, it undergoes oxidation reaction and gives compound B. The reaction is as follows:
C7H16O (A) + Na2CrO4 / Dil. H2SO4 → C7H14O (B)
Reaction 2: Dehydration of A with concentrated H2SO4:
When compound A is dehydrated with concentrated H2SO4, it undergoes elimination reaction and gives a single alkene C. The reaction is as follows:
C7H16O (A) + H2SO4 (conc.) → C7H14 (C)
Reaction 3: Ozonolysis of C followed by work-up with Zn-H2O:
When alkene C is subjected to ozonolysis followed by treatment with Zn-H2O, it undergoes oxidative cleavage and gives compound D as one of the products. The reaction is as follows:
C7H14 (C) + O3 → C5H10O (D)
Reaction 4: Reduction of D with NaBH4:
When compound D is reduced with NaBH4, it undergoes reduction reaction and gives a racemic mixture. This means that both enantiomers of D are formed in equal amounts. The reaction is as follows:
C5H10O (D) + NaBH4 → Racemic mixture of D
Reaction 5: Reduction of B with LiAlH4 followed by acid hydrolysis:
When compound B is reduced with LiAlH4 followed by acid hydrolysis, it undergoes reduction reaction and gives a pair of diastereomers. This means that two different diastereomers of B are formed. The reaction is as follows:
C7H14O (B) + LiAlH4 → Diastereomers of B
Therefore, the correct answer is option c) a pair of diastereomers.
Only One Option Correct TypeDirection (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. Arrange the following in the increasing order of reactivity with NH3.I. CH2O
II. CH3CHO
III. CH3—CO—CH3
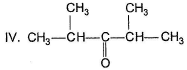
- a)I < II < III < IV
- b)IV < III < II < I
- c)III < IV < I < II
- d)II < I < IV < III
Correct answer is option 'B'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Arrange the following in the increasing order of reactivity with NH3.
I. CH2O
II. CH3CHO
III. CH3—CO—CH3

II. CH3CHO
III. CH3—CO—CH3
a)
I < II < III < IV
b)
IV < III < II < I
c)
III < IV < I < II
d)
II < I < IV < III

|
Harsharma answered |
I think this is because of steric hindrance.... because NH3 attack carbonyl carbon as nucleophile (Sn2)...and then steric hindrance is main factor
If glyoxal glycol is treated with a mixture of CH3MgBr and C2H5MgBr in diethyl ether followed by acid hydrolysis, how many different diols would be formed, which are simultaneously optically active?
Correct answer is '8'. Can you explain this answer?
If glyoxal glycol is treated with a mixture of CH3MgBr and C2H5MgBr in diethyl ether followed by acid hydrolysis, how many different diols would be formed, which are simultaneously optically active?

|
Ishani Yadav answered |
One pair of enantiomers for each (I) and (II) while two pairs of enantiomers for (III).
 A is optically active and C is one of the several aldol possible in the above reaction.Q. The product B is stereomeric. If a mixture containing all stereoisomers of B is treated with excess of LiAIH4 followed by the acidification will give how many different isomeric diols ?
A is optically active and C is one of the several aldol possible in the above reaction.Q. The product B is stereomeric. If a mixture containing all stereoisomers of B is treated with excess of LiAIH4 followed by the acidification will give how many different isomeric diols ?- a)2
- b)4
- c)6
- d)8
Correct answer is option 'D'. Can you explain this answer?

A is optically active and C is one of the several aldol possible in the above reaction.
Q.
The product B is stereomeric. If a mixture containing all stereoisomers of B is treated with excess of LiAIH4 followed by the acidification will give how many different isomeric diols ?
a)
2
b)
4
c)
6
d)
8

|
Niti Saha answered |
In which of the following compounds, enol form exist? - a)C6H5COCH3
- b)C6H5CHO
- c)

- d)Both (a) and (c)
Correct answer is option 'D'. Can you explain this answer?
In which of the following compounds, enol form exist?
a)
C6H5COCH3
b)
C6H5CHO
c)
d)
Both (a) and (c)
|
|
Preeti Khanna answered |
Both option (a) and option (c) forms enol but option (b) does not form enol.
Comprehension Type
Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Aldol condensation is an important reaction in organic chemistry, particularly for the formation of carbon-carbon bonds. This reaction typically involves the enolate ion derived from an aldehyde or ketone reacting with another carbonyl compound to form a β-hydroxy aldehyde or β-hydroxy ketone. This product can undergo dehydration to form an α,β-unsaturated carbonyl compound. The reaction is usually catalyzed by a base, although acid-catalyzed versions are also known. The presence of at least one α-hydrogen in the reactant is crucial for the aldol condensation to occur. Acetaldehyde, for example, can undergo aldol condensation to yield 3-hydroxybutanal, which can then dehydrate to form crotonaldehyde.
Ques: In the aldol condensation reaction of acetaldehyde (CH₃CHO), the initial product formed before dehydration is:
- a)2-Hydroxybutanal
- b)Crotonaldehyde
- c)3-Hydroxybutanal
- d)Acetone
Correct answer is option 'C'. Can you explain this answer?
Comprehension Type
Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Aldol condensation is an important reaction in organic chemistry, particularly for the formation of carbon-carbon bonds. This reaction typically involves the enolate ion derived from an aldehyde or ketone reacting with another carbonyl compound to form a β-hydroxy aldehyde or β-hydroxy ketone. This product can undergo dehydration to form an α,β-unsaturated carbonyl compound. The reaction is usually catalyzed by a base, although acid-catalyzed versions are also known. The presence of at least one α-hydrogen in the reactant is crucial for the aldol condensation to occur. Acetaldehyde, for example, can undergo aldol condensation to yield 3-hydroxybutanal, which can then dehydrate to form crotonaldehyde.
Ques: In the aldol condensation reaction of acetaldehyde (CH₃CHO), the initial product formed before dehydration is:
a)
2-Hydroxybutanal
b)
Crotonaldehyde
c)
3-Hydroxybutanal
d)
Acetone

|
Om Kumar answered |
The initial product formed in the aldol condensation reaction of acetaldehyde (CH₃CHO) is 3-hydroxybutanal. Here’s how:
- Acetaldehyde (CH₃CHO) has an α-hydrogen, which is necessary for forming the enolate ion.
- The enolate ion then reacts with another acetaldehyde molecule.
- This reaction forms 3-hydroxybutanal as the initial product
- Crotonaldehyde is formed after the dehydration of 3-hydroxybutanal.
Correct Answer: c
Consider the isomeric aldehydes with molar mass 100, if all the isomers (only structural) are treated independently with NH2OH, how many of them would give more than two stereomeric oximes?
Correct answer is '3'. Can you explain this answer?
Consider the isomeric aldehydes with molar mass 100, if all the isomers (only structural) are treated independently with NH2OH, how many of them would give more than two stereomeric oximes?

|
Janhavi Kaur answered |
MW = 100 indicates that molecular formula of aldehyde is C6H12O . For obtain ing more than two oximes, aldehyde must exist stereomeric.
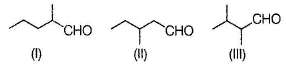
All the above has one chiral carbon each. Hence, when reacted with H2NOH, forms more than two oximes.
All the above has one chiral carbon each. Hence, when reacted with H2NOH, forms more than two oximes.
Which can be deduced correctly regarding keto-enol tautomerism in general?- a)Increasing temperature increases the enol content at equilibrium
- b)Mono-enols are usually more stable than dienols
- c)Enols of ketones are generally more stable than enols of aliphatic aldehydes
- d)Keto-enol taytomerism is catalysed by both acidic and basic catalys
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Which can be deduced correctly regarding keto-enol tautomerism in general?
a)
Increasing temperature increases the enol content at equilibrium
b)
Mono-enols are usually more stable than dienols
c)
Enols of ketones are generally more stable than enols of aliphatic aldehydes
d)
Keto-enol taytomerism is catalysed by both acidic and basic catalys

|
Ishita Deshpande answered |
Increasing temperature increases equilibrium content of less stable enol tautomers.
Enolisation decreases stability, hence introducing two or more enol groups are further more difficult.
Enols of ketones are more substituted at double bond, hence more stable.
Both acid and base catalyses keto-enol tautomerism.
Enolisation decreases stability, hence introducing two or more enol groups are further more difficult.
Enols of ketones are more substituted at double bond, hence more stable.
Both acid and base catalyses keto-enol tautomerism.
One integer Value Correct Type Direction (Q. Nos. 17-22) This section contains 6 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Q. If all the aldehyde isomers of C5H10O is independently treated with HCN/NaCN solution, how many of them will give racemic mixture of cyanohydrin?
Correct answer is '3'. Can you explain this answer?
One integer Value Correct Type
Direction (Q. Nos. 17-22) This section contains 6 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
If all the aldehyde isomers of C5H10O is independently treated with HCN/NaCN solution, how many of them will give racemic mixture of cyanohydrin?

|
Ishani Yadav answered |
IV is enantiomeric. Its pure enantiomer, with HCN/NaCN, would produce pair of diastereomers.
The compound(s) below that gives yellow precipitate with KOH/I2 is/are- a)CH3CH2OH
- b)

- c)

- d)Cl—CH2—CHO
Correct answer is option 'A,C'. Can you explain this answer?
The compound(s) below that gives yellow precipitate with KOH/I2 is/are
a)
CH3CH2OH
b)
c)
d)
Cl—CH2—CHO

|
Shraddha Gupta answered |
Aldehydes and ketones with  groups form iodoform with NaOH/I2. Besides, aldehydes and ketones, alcohols with
groups form iodoform with NaOH/I2. Besides, aldehydes and ketones, alcohols with  group also form iodofom in the same reaction.
group also form iodofom in the same reaction.
What is the correct order of equilibrium enol content of the following compounds?I. CH3COCH3
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH- a)I > II > III > IV
- b)I > IV > III > II
- c)IV > II > III > I
- d)III > IV > II > I
Correct answer is option 'D'. Can you explain this answer?
What is the correct order of equilibrium enol content of the following compounds?
I. CH3COCH3
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH
a)
I > II > III > IV
b)
I > IV > III > II
c)
IV > II > III > I
d)
III > IV > II > I

|
Asha Nair answered |
A 1,3-diketo compound forms more stable enol than a monocarbonyls. Also ester group forms less stable enol than carbonyls. Hence, III, a 1 , 3-diketo ne form s highest enol content while I (monocarbonyl) forms least enol content at equilibrium.
An organic compound A (C7H16O) shows both enantiomerism and diastereomerism. Treatment of a pure enantiomer of A with Na2CrO4 /Dil. H2SO4 gives B (C7H14O) - Also A on dehydration with concentrated H2SO4 gives a single alkene C (C7H14). Ozonolysis of C followed by work-up with Zn-H2O gives D (C5H10O) as one of the product which gives racemic mixture on reduction with NaBH4.Q. The correct statement regarding the compound D is- a)With CH3MgBr followed by acid hydrolysis gives racemic mixture
- b)With CH3CH2CH2MgBr followed by acid hydrolysis gives racemic mixture
- c)With CH3CH2MgBr followed by acid hydrolysis gives racemic mixture
- d)Both (b) and (c) are correct
Correct answer is option 'C'. Can you explain this answer?
An organic compound A (C7H16O) shows both enantiomerism and diastereomerism. Treatment of a pure enantiomer of A with Na2CrO4 /Dil. H2SO4 gives B (C7H14O) - Also A on dehydration with concentrated H2SO4 gives a single alkene C (C7H14). Ozonolysis of C followed by work-up with Zn-H2O gives D (C5H10O) as one of the product which gives racemic mixture on reduction with NaBH4.
Q.
The correct statement regarding the compound D is
a)
With CH3MgBr followed by acid hydrolysis gives racemic mixture
b)
With CH3CH2CH2MgBr followed by acid hydrolysis gives racemic mixture
c)
With CH3CH2MgBr followed by acid hydrolysis gives racemic mixture
d)
Both (b) and (c) are correct

|
Pooja Pillai answered |
Since, A is completely saturated, it must contain more than one chiral carbon atoms in order to show both enantiomerism and diastereomerism. Also, C on ozonolysis gives D (C5H10O) as one product, other product must be CH3SHO. Hence, C must be 
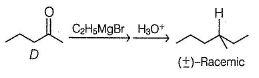
Statement I : When a mixture of ethanal and propanal is treated with aqueous Na2CO3, four aldol (excluding stereoisomers) are formed.
Statement II : In mixed aldol condensation, two self and two cross condensation products are always formed.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'C'. Can you explain this answer?
Statement I : When a mixture of ethanal and propanal is treated with aqueous Na2CO3, four aldol (excluding stereoisomers) are formed.
Statement II : In mixed aldol condensation, two self and two cross condensation products are always formed.
Statement II : In mixed aldol condensation, two self and two cross condensation products are always formed.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Rishika Chauhan answered |
It would be true only if both carbonyls are capable of forming enolates, i.e. possesses α-H
Only One Option Correct TypeDirection (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. In hexane-2,4-dione, how many different mono-enols are possible?- a)2
- b)3
- c)4
- d)7
Correct answer is option 'D'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
In hexane-2,4-dione, how many different mono-enols are possible?
a)
2
b)
3
c)
4
d)
7
|
|
Devanshi Mehta answered |
Possible Mono-enols in Hexane-2,4-dione
Hexane-2,4-dione, also known as Acetylacetone, has the following structure:
CH3COCH2COCH3
Mono-enol is the product obtained when one enolizable hydrogen atom of a compound is replaced by a hydroxyl group (-OH).
To determine the number of different mono-enols possible in hexane-2,4-dione, we need to identify the enolizable hydrogen atoms. These are the hydrogen atoms attached to the carbon atoms that are adjacent to the carbonyl groups (-CO-).
In hexane-2,4-dione, there are two such hydrogen atoms, one on each side of the molecule. Therefore, there are two possible enols that can be formed:
- The first enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ketone (-CO-) group on the left side of the molecule. This enol is called the alpha-enol or 1-enol.
CH3COCH=C(OH)CH3
- The second enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ester (-COO-) group on the right side of the molecule. This enol is called the beta-enol or 3-enol.
CH3C(OH)=CHCOCH3
However, each of these enols can exist in two different tautomeric forms: keto and enol. Tautomerism is the phenomenon where a compound exists in two or more isomeric forms that differ only in the position of a hydrogen atom and a double bond.
- The keto form is the one in which the compound has a carbonyl group (-CO-) and no hydroxyl (-OH) group.
- The enol form is the one in which the compound has a double bond (-C=C-) and a hydroxyl (-OH) group.
Therefore, there are four possible mono-enols in hexane-2,4-dione:
- The alpha-keto-enol form, also known as 1,3-diketone form.
- The alpha-hydroxy-ketone form or 1-enol form.
- The beta-keto-enol form, also known as the 3,5-diketone form.
- The beta-hydroxy-ketone form or 3-enol form.
However, each of these forms can also exist as a mixture of both tautomeric forms, keto and enol. Therefore, a total of seven different mono-enols are possible in hexane-2,4-dione:
- Alpha-keto-enol form (1,3-diketone)
- Alpha-enol-keto form (1-enol)
- Alpha-enol-enol form (1-enol)
- Beta-keto-enol form (3,5-diketone)
- Beta-enol-keto form (3-enol)
- Beta-enol-enol form (3-enol)
- Beta-enol-enol-keto form (3-enol)
Therefore, the correct answer is option D, seven.
Hexane-2,4-dione, also known as Acetylacetone, has the following structure:
CH3COCH2COCH3
Mono-enol is the product obtained when one enolizable hydrogen atom of a compound is replaced by a hydroxyl group (-OH).
To determine the number of different mono-enols possible in hexane-2,4-dione, we need to identify the enolizable hydrogen atoms. These are the hydrogen atoms attached to the carbon atoms that are adjacent to the carbonyl groups (-CO-).
In hexane-2,4-dione, there are two such hydrogen atoms, one on each side of the molecule. Therefore, there are two possible enols that can be formed:
- The first enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ketone (-CO-) group on the left side of the molecule. This enol is called the alpha-enol or 1-enol.
CH3COCH=C(OH)CH3
- The second enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ester (-COO-) group on the right side of the molecule. This enol is called the beta-enol or 3-enol.
CH3C(OH)=CHCOCH3
However, each of these enols can exist in two different tautomeric forms: keto and enol. Tautomerism is the phenomenon where a compound exists in two or more isomeric forms that differ only in the position of a hydrogen atom and a double bond.
- The keto form is the one in which the compound has a carbonyl group (-CO-) and no hydroxyl (-OH) group.
- The enol form is the one in which the compound has a double bond (-C=C-) and a hydroxyl (-OH) group.
Therefore, there are four possible mono-enols in hexane-2,4-dione:
- The alpha-keto-enol form, also known as 1,3-diketone form.
- The alpha-hydroxy-ketone form or 1-enol form.
- The beta-keto-enol form, also known as the 3,5-diketone form.
- The beta-hydroxy-ketone form or 3-enol form.
However, each of these forms can also exist as a mixture of both tautomeric forms, keto and enol. Therefore, a total of seven different mono-enols are possible in hexane-2,4-dione:
- Alpha-keto-enol form (1,3-diketone)
- Alpha-enol-keto form (1-enol)
- Alpha-enol-enol form (1-enol)
- Beta-keto-enol form (3,5-diketone)
- Beta-enol-keto form (3-enol)
- Beta-enol-enol form (3-enol)
- Beta-enol-enol-keto form (3-enol)
Therefore, the correct answer is option D, seven.
One Integer Value Correct TypeDirection (Q. Nos. 23-27) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Consider the following aldol condensation reaction,Butanone + NaOH (Dil.) → AldolsQ. How many different isomers (including stereoisomers) are formed above?
Correct answer is '6'. Can you explain this answer?
One Integer Value Correct Type
Direction (Q. Nos. 23-27) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Consider the following aldol condensation reaction,
Butanone + NaOH (Dil.) → Aldols
Q.
How many different isomers (including stereoisomers) are formed above?

|
Tejas Desai answered |
If butanone is treated with D2O18/DCI, isotopic exchange occur. What maximum gain in molecular mass is possible in the present case?
Correct answer is '7'. Can you explain this answer?
If butanone is treated with D2O18/DCI, isotopic exchange occur. What maximum gain in molecular mass is possible in the present case?

|
Asha Nair answered |
Gain of 7 units in molar mass is observed, five units due to 'D' and tw o units due to `O18'.
One or More than One Options Correct Type
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Successful mixed aldol condensation will be favoured
- a)treatment of electrophilic carbonyl component with stoichiometric strong base prior to the addition of the pre-nucleophilic carbonyl component
- b)treatment of mixture of electrophilic carbonyl component and pre-nucleophilic component with stoichiometric strong base
- c)continuous removal of aldol product from reaction mixture
- d)treatment of pre-nucleophilic carbonyl component with stoichiometric strong base prior to the addition of electrophilic carbonyl component.
Correct answer is option 'C,D'. Can you explain this answer?
One or More than One Options Correct Type
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Successful mixed aldol condensation will be favoured
a)
treatment of electrophilic carbonyl component with stoichiometric strong base prior to the addition of the pre-nucleophilic carbonyl component
b)
treatment of mixture of electrophilic carbonyl component and pre-nucleophilic component with stoichiometric strong base
c)
continuous removal of aldol product from reaction mixture
d)
treatment of pre-nucleophilic carbonyl component with stoichiometric strong base prior to the addition of electrophilic carbonyl component.

|
Rishika Chauhan answered |
For successful mixed aldol condensation to be favored, the correct conditions should ensure that one carbonyl component acts as a nucleophile while the other acts as an electrophile. Here's the analysis of each option:
(a) Treatment of electrophilic carbonyl component with a stoichiometric strong base before the addition of the pre-nucleophilic carbonyl component
- This would likely deprotonate the electrophilic carbonyl component, making it a poor electrophile and favoring side reactions. This is not favorable for a successful mixed aldol reaction.
(Incorrect)
(b) Treatment of mixture of electrophilic carbonyl component and pre-nucleophilic component with stoichiometric strong base
- This could result in deprotonation on both components, leading to a mixture of products and possibly side reactions.
(Incorrect)
(c) Continuous removal of aldol product from the reaction mixture
- Removing the aldol product shifts the equilibrium towards product formation, which is a beneficial condition for successful mixed aldol condensation.
(Correct)
(d) Treatment of pre-nucleophilic carbonyl component with stoichiometric strong base prior to the addition of electrophilic carbonyl component
- This ensures that the pre-nucleophilic component forms an enolate, which can then attack the electrophilic carbonyl component without side reactions, favoring a successful aldol condensation.
(Correct)
Final Answer:
(c) and (d) are correct choices.
Statement TypeDirection (Q. Nos. 16-19) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.Q. Statement I : In aldol condensation, enoiates are nucleophilic and reversibly attack the carbonyl carbon of aldehyde or ketone in the aldoi condensation.Statement II : An aldoi on refluxing with dilute base gives back the carbonyl compound.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Statement Type
Direction (Q. Nos. 16-19) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : In aldol condensation, enoiates are nucleophilic and reversibly attack the carbonyl carbon of aldehyde or ketone in the aldoi condensation.
Statement II : An aldoi on refluxing with dilute base gives back the carbonyl compound.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Tarun Chakraborty answered |
Reversibility of aldol into carbonyls establishes that carbanion nucleophile is formed in first fast reversible step.
The correct statement(s) regarding hydrates of aldehyde and ketone is/are- a)Usually hydrates have lower thermodynamic stability than anhydrous form
- b)Hydrate content of acetone is greater in water than in hexane
- c)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18
- d)C6H5CHO has greater hydrate content than p-nitrobenzaldehyd
Correct answer is option 'A,B,C'. Can you explain this answer?
The correct statement(s) regarding hydrates of aldehyde and ketone is/are
a)
Usually hydrates have lower thermodynamic stability than anhydrous form
b)
Hydrate content of acetone is greater in water than in hexane
c)
CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18
d)
C6H5CHO has greater hydrate content than p-nitrobenzaldehyd
|
|
Nisha Kulkarni answered |
Hydrates of aldehydes and ketones are less stable than anhydrous form (gem diols are unstable).
Hydrates form H-bonds with water, hence hydrate content is more in water than in hexane.
This exchange occur via hydrate.
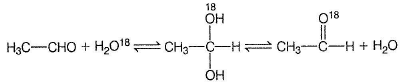
Hydrates form H-bonds with water, hence hydrate content is more in water than in hexane.
This exchange occur via hydrate.
Which compound (s) below can react via an intramolecular aldol condensation to give a six membered ring?- a)

- b)

- c)
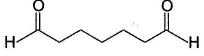
- d)

Correct answer is option 'A,C,D'. Can you explain this answer?
Which compound (s) below can react via an intramolecular aldol condensation to give a six membered ring?
a)
b)
c)
d)

|
Sinjini Tiwari answered |
All of these react in aldol reaction giving six-membered ring via intramolecular reaction.
Option (b) gives five and seven membered ring intramolecular aldol condensation.
Option (b) gives five and seven membered ring intramolecular aldol condensation.
The smallest acyclic ketone that gives pair of diastereomers with CH3NH2 in slightly acidic medium has how many carbon atoms?
Correct answer is '6'. Can you explain this answer?
The smallest acyclic ketone that gives pair of diastereomers with CH3NH2 in slightly acidic medium has how many carbon atoms?

|
Bhavana Banerjee answered |
The ketone must contain a chiral carbon.

The incorrect statement regarding aldol condensation is- a)It accomplishes the formation of a new C—C bond
- b)The enolate is favoured at equilibrium
- c)The key step in the mechanism of base catalysed reaction is attack of α-carbon of an enolate ion on the carbonyl carbo
- d)Dehydration of aldol gives α, β-unsaturated carbonyl compound
Correct answer is option 'B'. Can you explain this answer?
The incorrect statement regarding aldol condensation is
a)
It accomplishes the formation of a new C—C bond
b)
The enolate is favoured at equilibrium
c)
The key step in the mechanism of base catalysed reaction is attack of α-carbon of an enolate ion on the carbonyl carbo
d)
Dehydration of aldol gives α, β-unsaturated carbonyl compound

|
Niti Saha answered |
Enolate is formed in first fast step which undergo nucleophilic addition in second slow step.
A colourless liquid, at room temperature, reacts with soda-lime to form sodium salt of a carboxylic acid and ammonia gas. The liquid is- a)propanoic acid
- b)formamide
- c)propanamide
- d)methyl enthanoate
Correct answer is option 'B'. Can you explain this answer?
a)
propanoic acid
b)
formamide
c)
propanamide
d)
methyl enthanoate

|
Manish Aggarwal answered |
Only amides (but not acids and esters) undergo hydrolysis in presence of soda-lime to form sodium salt of a carboxylic acid and ammonia gas. Further, since the given compound is a liquid, it must be formamide, because propanamide is a solid.
Which of the following is/are true regarding aldol condensation ?- a)Both acid and base can act as catalyst
- b)A new carbon-carbon bond is always formed
- c)CH3CHO and D3C—CHO react at same rate if all other conditions are similar
- d)Prqpanal and propanone react at same rate if all other conditions are similar
Correct answer is option 'A,B,C'. Can you explain this answer?
Which of the following is/are true regarding aldol condensation ?
a)
Both acid and base can act as catalyst
b)
A new carbon-carbon bond is always formed
c)
CH3CHO and D3C—CHO react at same rate if all other conditions are similar
d)
Prqpanal and propanone react at same rate if all other conditions are similar

|
Aravind Mehra answered |
Both acid and base catalyses aldol reaction. In aldol reaction , a new α - β C—C bond is always formed . α - H is lost in first fast step, hence both CH3CHO and CD3CHO react at the same rate.
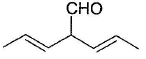
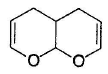
What is the major product in the following reaction?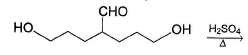
- a)
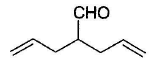
- b)
- c)
- d)
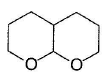
Correct answer is option 'D'. Can you explain this answer?
What is the major product in the following reaction?
a)
b)
c)
d)

|
Pragati Choudhury answered |
Acetal is form ed by cyclisation
Chapter doubts & questions for Aldehydes, Ketones and Carboxylic Acids - 6 Months Preparation for JEE 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Aldehydes, Ketones and Carboxylic Acids - 6 Months Preparation for JEE in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup