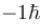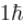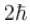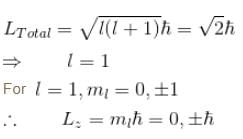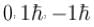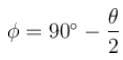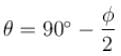All questions of The Quantum Model of the Atom for Grade 9 Exam
Linear momenta of a proton and an electron are equal. Relative to an electron- a)de-Broglie wavelength of proton is more
- b)Kinetic energy of proton is more
- c)de-Broglie wavelength of proton and electron are equal
- d)de-Broglie wavelength of proton is less
Correct answer is option 'C'. Can you explain this answer?
Linear momenta of a proton and an electron are equal. Relative to an electron
a)
de-Broglie wavelength of proton is more
b)
Kinetic energy of proton is more
c)
de-Broglie wavelength of proton and electron are equal
d)
de-Broglie wavelength of proton is less

|
Athul Menon answered |
If pe = pp,
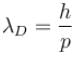
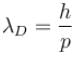
therefore, de-Broglie wavelength of proton and electron are equal.
The correct answer is: de-Broglie wavelength of proton and electron are equal
The correct answer is: de-Broglie wavelength of proton and electron are equal
Yellow light of 557 nm wavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (in nm) for photoelectric effect from cesium is.
Correct answer is '625'. Can you explain this answer?
Yellow light of 557 nm wavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (in nm) for photoelectric effect from cesium is.
|
|
Jayant Mishra answered |
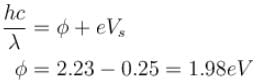
⇒
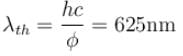
The correct answer is: 625
A particle is in an infinite square well potential with walls at x = 0 and x = L. If the particle is in the state 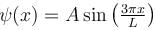 where A is a constant, what is the probability that the particle is between x = L/3 and x = 2L/3 upto 2 decimal places.
where A is a constant, what is the probability that the particle is between x = L/3 and x = 2L/3 upto 2 decimal places.
Correct answer is '0.333'. Can you explain this answer?
A particle is in an infinite square well potential with walls at x = 0 and x = L. If the particle is in the state 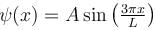 where A is a constant, what is the probability that the particle is between x = L/3 and x = 2L/3 upto 2 decimal places.
where A is a constant, what is the probability that the particle is between x = L/3 and x = 2L/3 upto 2 decimal places.
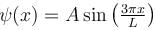 where A is a constant, what is the probability that the particle is between x = L/3 and x = 2L/3 upto 2 decimal places.
where A is a constant, what is the probability that the particle is between x = L/3 and x = 2L/3 upto 2 decimal places.|
|
Jayant Mishra answered |
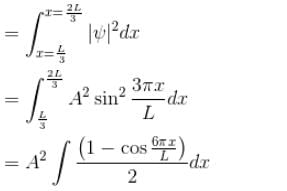
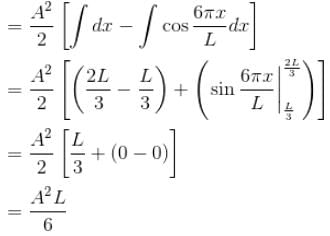
Now A2 = 2/L = from normalization of ψ
∴ Probability = 1/3 = 0.333
The correct answer is: 0.333
If the short wavelength limit of the continuous spectrum coming out of a coolidge tube is 10Å, then the de-Broglie wavelength (in Å)of the electrons reaching the largest metal in the coolidge tube is approximately.
Correct answer is '0.3'. Can you explain this answer?
If the short wavelength limit of the continuous spectrum coming out of a coolidge tube is 10Å, then the de-Broglie wavelength (in Å)of the electrons reaching the largest metal in the coolidge tube is approximately.
|
|
Jayant Mishra answered |
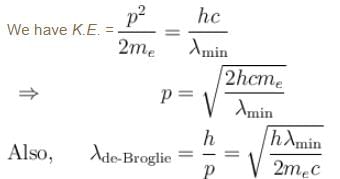

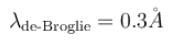
The correct answer is: 0.3
The kinetic energy of electrons ejected from the surface of a metal ranges from :- a)0 to hv - φ
- b) 0 to φ
- c)0 to eVs
- d)0 to φ/e
Correct answer is option 'A'. Can you explain this answer?
The kinetic energy of electrons ejected from the surface of a metal ranges from :
a)
0 to hv - φ
b)
0 to φ
c)
0 to eVs
d)
0 to φ/e

|
Pie Academy answered |
Correct Answer :- a
Explanation : In the photoelectric effect, each photon donates all of its energy hf to an electron in the metal. If this process occurs at the metal surface, the electron is released into the vacuum with a kinetic energy given by:
Kmax = hf - phi
The life time of a nucleus in excited state is 10–12s. The uncertainty in the energy and frequency of γ-ray photon emitted by it. (h = 6.63 × 10–34 Js) (in units of 1011 Hz) is a × 1011Hz. Find a.
Correct answer is '1.59'. Can you explain this answer?
The life time of a nucleus in excited state is 10–12s. The uncertainty in the energy and frequency of γ-ray photon emitted by it. (h = 6.63 × 10–34 Js) (in units of 1011 Hz) is a × 1011Hz. Find a.
|
|
Jayant Mishra answered |
From the energy time uncertainty
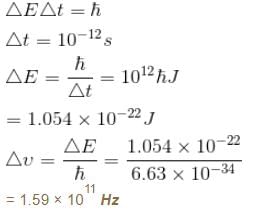
The correct answer is: 1.59 x 10^11 Hz
An atom has n = 1 and n = 2 levels filled. How many electrons does the atom have?- a)2
- b)10
- c)8
- d)6
Correct answer is option 'B'. Can you explain this answer?
An atom has n = 1 and n = 2 levels filled. How many electrons does the atom have?
a)
2
b)
10
c)
8
d)
6
|
|
Jayant Mishra answered |
Number of electrons in nth orbit is given by 2n2
∴ total electrons is = 2(1)2 + 2(22)
= 2 + 8 = 10
The correct answer is: 10
∴ total electrons is = 2(1)2 + 2(22)
= 2 + 8 = 10
The correct answer is: 10
A certain excited state of hydrogen atom is known to have a life of 2.5 × 10–4 s. The minimum error, with which the energy of the excited state can be measured is.- a)0.045 eV
- b)0.056 eV
- c)0.038 eV
- d)0.026 eV
Correct answer is option 'D'. Can you explain this answer?
A certain excited state of hydrogen atom is known to have a life of 2.5 × 10–4 s. The minimum error, with which the energy of the excited state can be measured is.
a)
0.045 eV
b)
0.056 eV
c)
0.038 eV
d)
0.026 eV
|
|
Vedika Singh answered |
The energy time uncertainty relation is
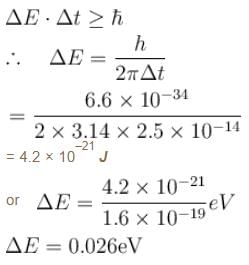
The correct answer is: 0.026 eV
de-Broglie hypothesized that the momentum and wavelength of a free massive particle are related by- a)Speed of light
- b)Boltzmann constant
- c)Rydberg’s constant
- d)Planck’s constant
Correct answer is option 'D'. Can you explain this answer?
de-Broglie hypothesized that the momentum and wavelength of a free massive particle are related by
a)
Speed of light
b)
Boltzmann constant
c)
Rydberg’s constant
d)
Planck’s constant
|
|
Vedika Singh answered |
de-Broglie wave length, λ = h/p, where h is the Planck's constant.
The correct answer is: Planck’s constant
Which of the following is true? When metallic surface is irradiated with white light- a)No photo-current observed
- b)Photo-current increases with time
- c)(K.E.)max of electrons increases with increase in time
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Which of the following is true? When metallic surface is irradiated with white light
a)
No photo-current observed
b)
Photo-current increases with time
c)
(K.E.)max of electrons increases with increase in time
d)
None of the above
|
|
Jayant Mishra answered |
White light does not produce any photoelectric effect because the range of wavelength in white light is (400 – 700)nm. The energy corresponding to this range of wavelength is small in comparison to the work function of any metal in general.
The correct answer is: No photo-current observed
Choose the correct statement for a free particle with ψ(x) = Aeikx- a)Energy eigenvalue is
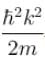 and momentum is
and momentum is 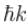
- b)

- c)
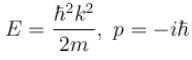
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Choose the correct statement for a free particle with ψ(x) = Aeikx
a)
Energy eigenvalue is 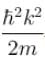 and momentum is
and momentum is 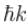
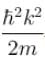 and momentum is
and momentum is 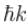
b)

c)
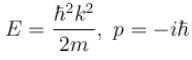
d)
None of the above

|
Pie Academy answered |
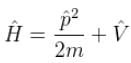
V = 0 for a free particle
∴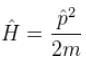
Schrödinger equation.

∴
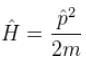
Schrödinger equation.


∴ Aeikx and Be–ikx (General solution)
∴ are solution to the free particle
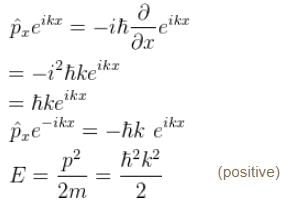
The correct answer is: Energy eigenvalue is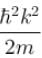 and momentum is
and momentum is 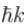
∴ are solution to the free particle
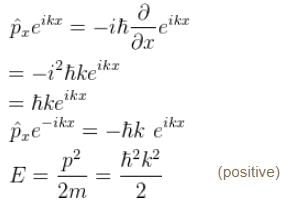
The correct answer is: Energy eigenvalue is
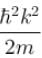 and momentum is
and momentum is 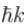
The phase velocity of ripples on a liquid surface is 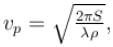 where S is the surface tension and ρ the density of the liquid. The group velocity of the ripples is given by :
where S is the surface tension and ρ the density of the liquid. The group velocity of the ripples is given by :- a)
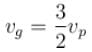
- b)
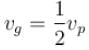
- c)
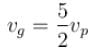
- d)
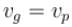
Correct answer is option 'A'. Can you explain this answer?
The phase velocity of ripples on a liquid surface is 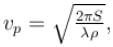 where S is the surface tension and ρ the density of the liquid. The group velocity of the ripples is given by :
where S is the surface tension and ρ the density of the liquid. The group velocity of the ripples is given by :
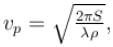 where S is the surface tension and ρ the density of the liquid. The group velocity of the ripples is given by :
where S is the surface tension and ρ the density of the liquid. The group velocity of the ripples is given by :a)
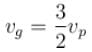
b)
c)
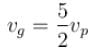
d)
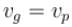

|
Pie Academy answered |
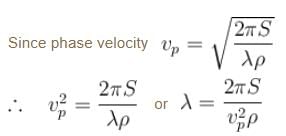
As wave number,
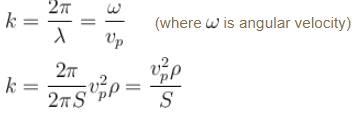
We know that group velocity given by
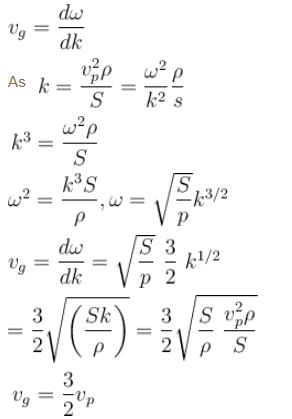
The correct answer is:
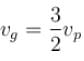
If de-Broglie wavelength of an electron is 7.3Å. Then the velocity of electron is. Given, me = 9.1 × 10–31 kg and h = 6.6 × 10–34 Js.
- a)7.5 × 104 m/s
- b)8.67 × 104 m/s
- c)9.0 × 104 m/s
- d)9.93 × 104 m/s
Correct answer is option 'D'. Can you explain this answer?
If de-Broglie wavelength of an electron is 7.3Å. Then the velocity of electron is. Given, me = 9.1 × 10–31 kg and h = 6.6 × 10–34 Js.
a)
7.5 × 104 m/s
b)
8.67 × 104 m/s
c)
9.0 × 104 m/s
d)
9.93 × 104 m/s

|
Pie Academy answered |
Λ = h/mv
v = h/mΛ
v = (6.6*10-34)/(9.1 × 10–31 * 7.3 * 10-10)
= 9.93 × 104 m/s
The correct answer is: 9.93 × 104 m/s
= 9.93 × 104 m/s
The correct answer is: 9.93 × 104 m/s
When light of intensity 1 W/m2 and wavelength 5 × 10–7 m is incident on a surface, it is completely absorbed by the surface. If 100 photons emit one electron and area of the surface is 1 cm2, then the photoelectric current (in µA) will be.
Correct answer is '0.4'. Can you explain this answer?
When light of intensity 1 W/m2 and wavelength 5 × 10–7 m is incident on a surface, it is completely absorbed by the surface. If 100 photons emit one electron and area of the surface is 1 cm2, then the photoelectric current (in µA) will be.
|
|
Vedika Singh answered |
Number of photon/second 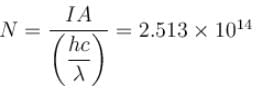
Number of photon electron emitted/second by 1 photon = N/100
Photo electric current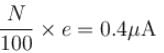
The correct answer is: 0.4
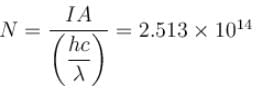
Number of photon electron emitted/second by 1 photon = N/100
Photo electric current
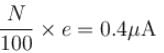
The correct answer is: 0.4
A particle of mass m has the wave function ψ(x, t) = eiωt (α cos kx + β sin kx) where α, β are complex constants and ω and k are real constants. The probability current density is equal to :- a)
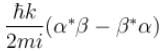
- b)0
- c)
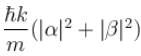
- d)

Correct answer is option 'A'. Can you explain this answer?
A particle of mass m has the wave function ψ(x, t) = eiωt (α cos kx + β sin kx) where α, β are complex constants and ω and k are real constants. The probability current density is equal to :
a)
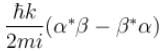
b)
0
c)
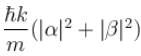
d)


|
Pie Academy answered |
Probability current density 
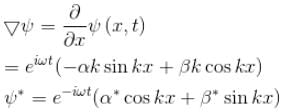

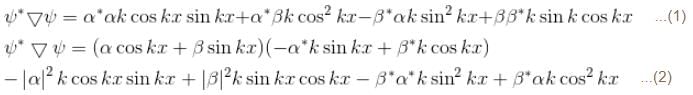
subtract (2) and (1)
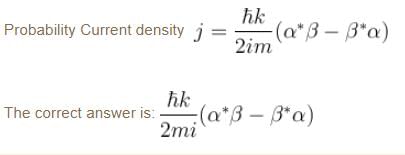

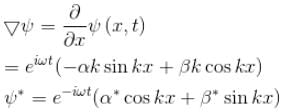

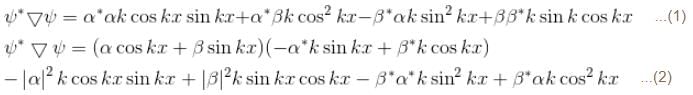
subtract (2) and (1)
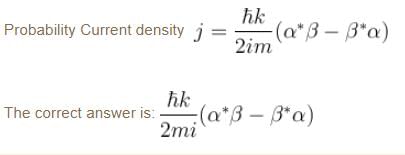
In the photoelectric, if we use a monochromatic light, the I-V curve is as shown. If work function of the metal is 2eV, estimate the power of light used. (Assume efficiency of photo emission = 10–3 % i.e. number of photoelectrons emitted are 10–3 % of number of photons incident on metal.)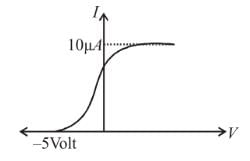
- a)10mW
- b)7mW
- c)2mW
- d)5mW
Correct answer is option 'B'. Can you explain this answer?
In the photoelectric, if we use a monochromatic light, the I-V curve is as shown. If work function of the metal is 2eV, estimate the power of light used. (Assume efficiency of photo emission = 10–3 % i.e. number of photoelectrons emitted are 10–3 % of number of photons incident on metal.)

a)
10mW
b)
7mW
c)
2mW
d)
5mW
|
|
Jayant Mishra answered |
The energy of incident photons is given by

(Vs is stopping potential and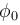 is work function)
is work function)
Saturation current =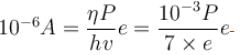 (η is photo emission efficiency)
(η is photo emission efficiency)
∴ P = 7mW
The correct answer is: 7mW

(Vs is stopping potential and
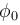 is work function)
is work function)Saturation current =
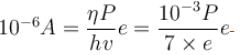 (η is photo emission efficiency)
(η is photo emission efficiency)∴ P = 7mW
The correct answer is: 7mW
If we replaced the electron in a hydrogen atom with muon, (mass of a neon is 207 times the mass of an electron) then what will be the energy levels En of this new hydrogen atom in terms of the binding energy E0 (of the ordinary hydrogen), mass of the proton mp and the principal quantum number, n? (mµ = 207 me)- a)
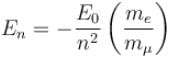
- b)
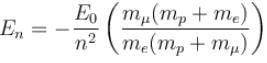
- c)
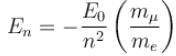
- d)
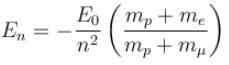
Correct answer is option 'B'. Can you explain this answer?
If we replaced the electron in a hydrogen atom with muon, (mass of a neon is 207 times the mass of an electron) then what will be the energy levels En of this new hydrogen atom in terms of the binding energy E0 (of the ordinary hydrogen), mass of the proton mp and the principal quantum number, n? (mµ = 207 me)
a)
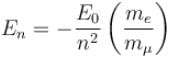
b)
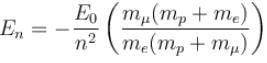
c)
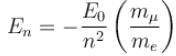
d)
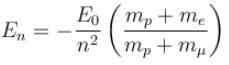

|
Pie Academy answered |
In case the electron is replaced by the muon, we will have to consider the reduced mass of the proton- muon system.
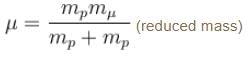
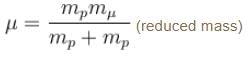
The Rydberg constant is changed by a factor of.
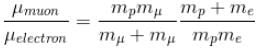
The Rydbergs constant is proportional to the reduced mass of the system in consideration.
R1 = Rydbergs constant for e– and p system is proportional to reduced mass of e– and p
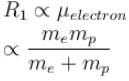
R2 = Rydbergs constant for µ and p system
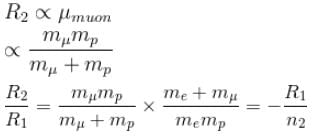
∴ New energy levels are
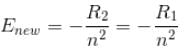
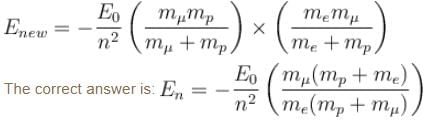
In the figure six lines of emission spectrum are shown. Which of them will be absent in the absorption spectrum.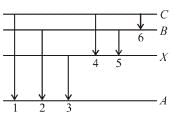
- a)4, 5, 6
- b)4, 6
- c)1, 2, 3, 4, 6
- d)1, 2, 3
Correct answer is option 'D'. Can you explain this answer?
In the figure six lines of emission spectrum are shown. Which of them will be absent in the absorption spectrum.

a)
4, 5, 6
b)
4, 6
c)
1, 2, 3, 4, 6
d)
1, 2, 3

|
Pie Academy answered |
The photon’s with energies equal to that required for upward transition A → X, A → B and A → C would be absorbed, these photons shall be absent from absorption spectrum.
The correct answer is: 1, 2, 3
In the photoelectric effect experiment we use a source of light whose frequency can be varied. If we gradually increase the frequency of the incident light then :- a)Photoelectric effect observed after some time with the maximum kinetic energy (KE) of electron varying (increasing with time)
- b)No photoelectric effect observed
- c)Photoelectric effect observed (after some time) with photocurrent remaining constant.
- d)Photoelectric effect observed with photocurrent increasing with time.
Correct answer is option 'A,C'. Can you explain this answer?
In the photoelectric effect experiment we use a source of light whose frequency can be varied. If we gradually increase the frequency of the incident light then :
a)
Photoelectric effect observed after some time with the maximum kinetic energy (KE) of electron varying (increasing with time)
b)
No photoelectric effect observed
c)
Photoelectric effect observed (after some time) with photocurrent remaining constant.
d)
Photoelectric effect observed with photocurrent increasing with time.
|
|
Jayant Mishra answered |
No photocurrent till the incident frequency reaches the minimum frequency required to overcome the work function of the metal φ
i.e. hvin = φ
After that, any increase in the frequency will not increase the photocurrent because the incident light has the same intensity.
Whereas KEmax increase linearly with the frequency of the incident radiation.
The correct answers are: Photoelectric effect observed (after some time) with photocurrent remaining constant., Photoelectric effect observed after some time with the maximum kinetic energy (KE) of electron varying (increasing with time)
Which of the following is true about the absorption and emission of energy by an atom?- a)At low temperatures, the lines in the absorption spectrum of an atom coincide with the lines in its emission spectrum that represent transitions to the ground slate
- b)An atom can emit photons of only certain specific energies.
- c)An atom can emit photons of any energy.
- d)An atom can only absorb photons that have certain specific energies.
Correct answer is option 'A,B,D'. Can you explain this answer?
Which of the following is true about the absorption and emission of energy by an atom?
a)
At low temperatures, the lines in the absorption spectrum of an atom coincide with the lines in its emission spectrum that represent transitions to the ground slate
b)
An atom can emit photons of only certain specific energies.
c)
An atom can emit photons of any energy.
d)
An atom can only absorb photons that have certain specific energies.
|
|
Jayant Mishra answered |
E = hv = Ef – Ein
The frequency can only corresponds to the difference in two energy states.
The correct answers are: An atom can only absorb photons that have certain specific energies., An atom can emit photons of only certain specific energies., At low temperatures, the lines in the absorption spectrum of an atom coincide with the lines in its emission spectrum that represent transitions to the ground slate
The correct answers are: An atom can only absorb photons that have certain specific energies., An atom can emit photons of only certain specific energies., At low temperatures, the lines in the absorption spectrum of an atom coincide with the lines in its emission spectrum that represent transitions to the ground slate
Consider a set of wave functions ψi(x). Which of the following condition guarantees that the functions are normalized and mutually orthogonal? (i, j take values from 1 to n)- a)
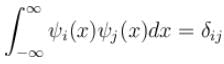
- b)
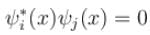
- c)
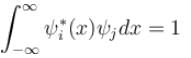
- d)
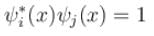
Correct answer is option 'A'. Can you explain this answer?
Consider a set of wave functions ψi(x). Which of the following condition guarantees that the functions are normalized and mutually orthogonal? (i, j take values from 1 to n)
a)
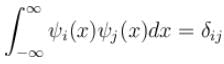
b)
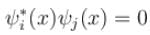
c)
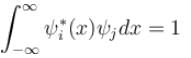
d)
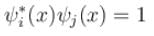
|
|
Jayant Mishra answered |
For the states to be orthogonal
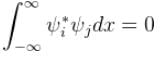
and for them to be normalised,
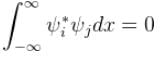
and for them to be normalised,
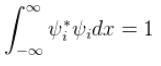
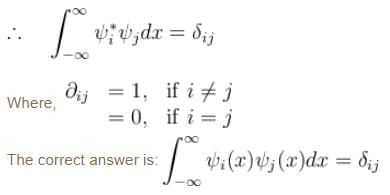
In a photoelectric effect set up two metals with work function φ1 and φ2 are used. For the same value of incident frequency, the stopping potential for metal 1 is twice that for metal 2. If the incident energy is 5eV and the φ1 = 1.5eV. Find φ2. (in eV)
Correct answer is '6.5'. Can you explain this answer?
In a photoelectric effect set up two metals with work function φ1 and φ2 are used. For the same value of incident frequency, the stopping potential for metal 1 is twice that for metal 2. If the incident energy is 5eV and the φ1 = 1.5eV. Find φ2. (in eV)

|
Pie Academy answered |
Solution=
eVs1=hv- Φ1
eVs2=hv- Φ2
eVs1= eVs2
eVs1/eVs2 = hv- Φ2/ hv- Φ1=1/2
2hv-2Φ2 = 1hv- Φ1
2Φ2 = hv+ Φ1
2Φ2 = 5+1.5
2Φ2 = 6.5eV
Φ2 = 3.25eV
The correct answer would be 3.25eV
The intensity of scattered monochromatic beam of X-rays is plotted as a function of wavelength. There are 2 peaks observed. The distance between the 2 peaks is 0.024 Å. Find the angle (in degrees) at which the X-rays are scattered.
Correct answer is '90'. Can you explain this answer?
The intensity of scattered monochromatic beam of X-rays is plotted as a function of wavelength. There are 2 peaks observed. The distance between the 2 peaks is 0.024 Å. Find the angle (in degrees) at which the X-rays are scattered.
|
|
Manish Bajaj answered |
To find the distance between the two peaks, we need to determine the wavelength difference between them.
The distance between the two peaks is given as 0.024. This distance can be represented as the wavelength difference Δλ between the two peaks.
Therefore, Δλ = 0.024.
Since the intensity of the scattered X-ray beam is plotted as a function of wavelength, we can assume that the x-axis of the graph represents wavelength (λ). The distance between the two peaks can be represented as a difference in wavelength.
So, the distance between the two peaks is 0.024 units of wavelength (Δλ = 0.024).
The distance between the two peaks is given as 0.024. This distance can be represented as the wavelength difference Δλ between the two peaks.
Therefore, Δλ = 0.024.
Since the intensity of the scattered X-ray beam is plotted as a function of wavelength, we can assume that the x-axis of the graph represents wavelength (λ). The distance between the two peaks can be represented as a difference in wavelength.
So, the distance between the two peaks is 0.024 units of wavelength (Δλ = 0.024).
Calculate the ground state energy of a Helium atom, using the uncertainty principle. (in eV)
Correct answer is '-10.34'. Can you explain this answer?
Calculate the ground state energy of a Helium atom, using the uncertainty principle. (in eV)

|
Pie Academy answered |
For a helium atom
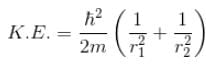
Because momentum of electron 1,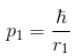
and momentum of electron 2,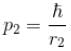
p1, p2 is the spread in momentum corresponding to electron 1 & 2 respectively.
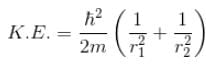
Because momentum of electron 1,
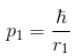
and momentum of electron 2,
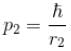
p1, p2 is the spread in momentum corresponding to electron 1 & 2 respectively.
r1, r2 is the localization of electron 1 & 2 respectively.
Total energy, E = K.E + Interaction energy of e1 and e2 + Interaction energy between nucleus and electrons
Interaction Energy between 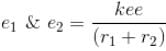
Since the separation between r1 and r2 is of the order r1 + r2 and k = 1 in natural system of units.
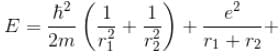 Interaction between nucleus and electron
Interaction between nucleus and electron
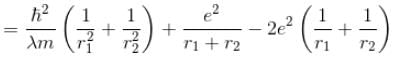
For the ground state energy
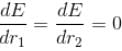 since in the ground state, Energy is minimum.
since in the ground state, Energy is minimum.
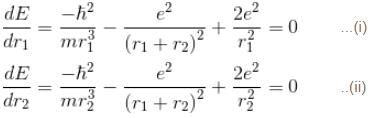
solving these two equations, we get
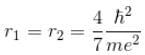
substituting r1 and r2 in the expression for E
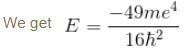
Emin = –10.34 eV
Put m = 9.1 × 10–31 kg
e = 1.6 × 10–19C
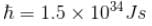
The correct answer is: -10.34
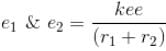
Since the separation between r1 and r2 is of the order r1 + r2 and k = 1 in natural system of units.
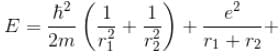 Interaction between nucleus and electron
Interaction between nucleus and electron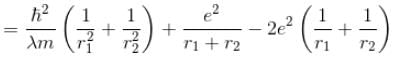
For the ground state energy
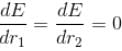 since in the ground state, Energy is minimum.
since in the ground state, Energy is minimum.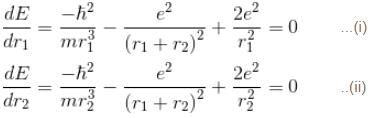
solving these two equations, we get
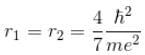
substituting r1 and r2 in the expression for E
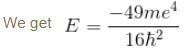
Emin = –10.34 eV
Put m = 9.1 × 10–31 kg
e = 1.6 × 10–19C
The correct answer is: -10.34
Gamma ray photons of energy 1.02 MeV are scattered from electrons which are initially at rest. Find the angle for symmetric scattering at this energy (in degrees)
Correct answer is '41.4'. Can you explain this answer?
Gamma ray photons of energy 1.02 MeV are scattered from electrons which are initially at rest. Find the angle for symmetric scattering at this energy (in degrees)
|
|
Vedika Singh answered |
For symmetric scattering θ = φ
From the relation between θ & φ
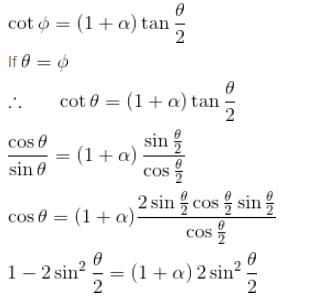
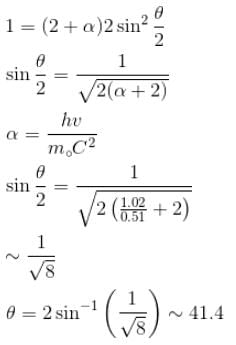
The correct answer is: 41.4
From the relation between θ & φ
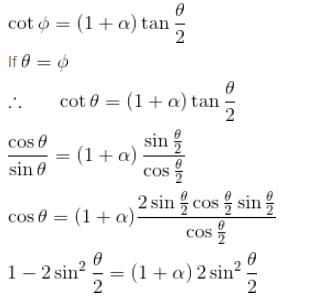
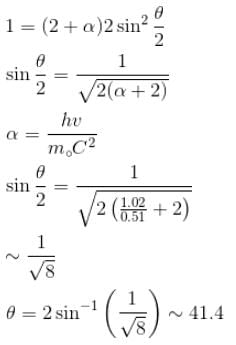
The correct answer is: 41.4
The energy of an electron moving in one dimension in an infinitely high potential box of width 1Å.
Correct answer is '37.62'. Can you explain this answer?
The energy of an electron moving in one dimension in an infinitely high potential box of width 1Å.
|
|
Vedika Singh answered |
The eigenvalue of energy
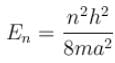
when the particle is in the least energy state (n = 1), the energy
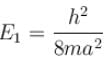 with h = 6.62 × 10–34 Js, m = 9.1 × 10–31 kg and a = 1 × 10–10 m
with h = 6.62 × 10–34 Js, m = 9.1 × 10–31 kg and a = 1 × 10–10 mAfter calculating,
The correct answer is: 37.62 eV
In X-rays production an electron accelerated with voltage V strikes a metal target. For which of the following voltages X-rays of minimum wavelength will be produced :- a)40 kV
- b)30 kV
- c)10 kV
- d)20 kV
Correct answer is option 'A'. Can you explain this answer?
In X-rays production an electron accelerated with voltage V strikes a metal target. For which of the following voltages X-rays of minimum wavelength will be produced :
a)
40 kV
b)
30 kV
c)
10 kV
d)
20 kV
|
|
Jayant Mishra answered |
λ will be minimum for maximum accelerated voltage 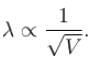
The correct answer is: 40 kV
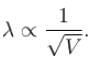
The correct answer is: 40 kV
For what value of an electron’s speed will its de-Broglie wavelength be same as the compton wavelength? (in terms of c)
Correct answer is '0.707'. Can you explain this answer?
For what value of an electron’s speed will its de-Broglie wavelength be same as the compton wavelength? (in terms of c)
|
|
Jayant Mishra answered |
de Broglie wavelength
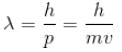
Compton wavelength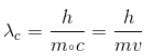
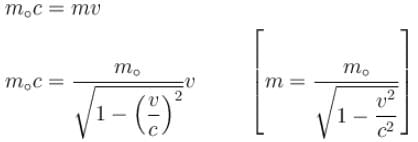
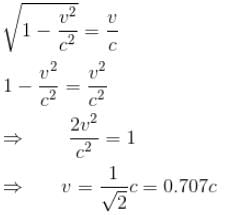
The correct answer is: 0.707
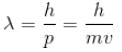
Compton wavelength
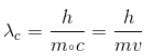
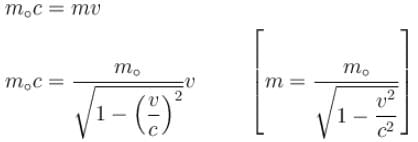
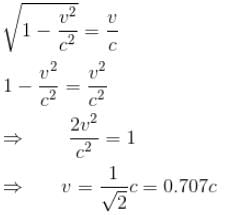
The correct answer is: 0.707
In a hydrogen atom following Bohr's postulates, the product of linear momentum and angular momentum is proportional to (n)x where n is the orbit number. Then x is
Correct answer is '0'. Can you explain this answer?
In a hydrogen atom following Bohr's postulates, the product of linear momentum and angular momentum is proportional to (n)x where n is the orbit number. Then x is

|
Pie Academy answered |
Linear momentum ⇒ 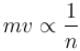
Angular momentum ⇒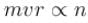
∴ Product of linear momentum and angular momentum ∝ n0.
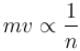
Angular momentum ⇒
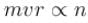
∴ Product of linear momentum and angular momentum ∝ n0.
The correct answer is: 0
The energy E and momentum p of photon is given by E = hv and p = h/λ, the velocity of photon will be :- a)Ep
- b)[E/p]2
- c)E/p
- d)[E/p]1/2
Correct answer is option 'C'. Can you explain this answer?
The energy E and momentum p of photon is given by E = hv and p = h/λ, the velocity of photon will be :
a)
Ep
b)
[E/p]2
c)
E/p
d)
[E/p]1/2

|
Bhavana Dasgupta answered |
E = hc/λ put c = v
and p = h/λ
E = pv
⇒ v = E/p
and p = h/λ
E = pv
⇒ v = E/p
The correct answer is: E/p
A singly ionized Helium atom in n = 4 state emits a photon of wavelength 470nm. If Ef is the final state energy and nf is the principal quantum number of the final state then :- a)nf = 2
- b)nf = 3
- c)Ef = –14 eV
- d)Ef = –6.0 eV
Correct answer is option 'B,D'. Can you explain this answer?
A singly ionized Helium atom in n = 4 state emits a photon of wavelength 470nm. If Ef is the final state energy and nf is the principal quantum number of the final state then :
a)
nf = 2
b)
nf = 3
c)
Ef = –14 eV
d)
Ef = –6.0 eV
|
|
Vedika Singh answered |
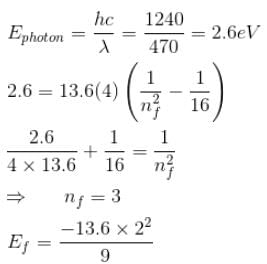
= –6.0 eV
The correct answers are: Ef = –6.0 eV, nf = 3
In the spectrum of hydrogen, what is the ratio of the longest wavelength in the Lymann series (nf = 1) to the longest is Balmer series (nf = 2)?
Correct answer is '656.3'. Can you explain this answer?
In the spectrum of hydrogen, what is the ratio of the longest wavelength in the Lymann series (nf = 1) to the longest is Balmer series (nf = 2)?
|
|
Jayant Mishra answered |
In 1885, when Johann Balmer observed a spectral series in the visible spectrum of hydrogen, he made the following observations: The longest wavelength is 656.3 nm. The second longest wavelength is 486.1 nm.
In a hydrogen atom, the ratio of the wavelengths of lyman-α radiation (in n = 2 to n =1) to Balmer-α radiation (n = 3 → n = 2) is- a)4/3
- b) 5/27
- c)5/48
- d)27/5
Correct answer is option 'B'. Can you explain this answer?
In a hydrogen atom, the ratio of the wavelengths of lyman-α radiation (in n = 2 to n =1) to Balmer-α radiation (n = 3 → n = 2) is
a)
4/3
b)
5/27
c)
5/48
d)
27/5
|
|
Vedika Singh answered |
Solution:
Rydberg formula:
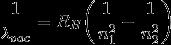
λvac = the wavelength of the light emitted in vacuum
RH = Rydberg constant for Hydrogen
n1 and n2 are integers such that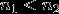
For Lyman-radiation (n = 2 → n = 1):
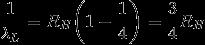
For Balmer-radiation (n = 3 → n = 2):
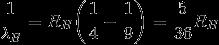
The Ratio:
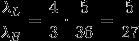
Answer: B
Rydberg formula:
λvac = the wavelength of the light emitted in vacuum
RH = Rydberg constant for Hydrogen
n1 and n2 are integers such that
For Lyman-radiation (n = 2 → n = 1):
For Balmer-radiation (n = 3 → n = 2):
The Ratio:
Answer: B
The wavelength of a photon and the de-Broglie wavelength of an electron and uranium atom are identical. Which one of them will have highest kinetic energy- a)Electron
- b)U-atom
- c)Nothing can be predicted
- d)Photon
Correct answer is option 'D'. Can you explain this answer?
The wavelength of a photon and the de-Broglie wavelength of an electron and uranium atom are identical. Which one of them will have highest kinetic energy
a)
Electron
b)
U-atom
c)
Nothing can be predicted
d)
Photon

|
Tarun Singh answered |
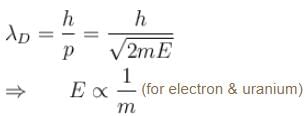
for photon & electron

The correct answer is: Photon
Which of the following values of A will properly normalize the wave function of a rigid dumbbell rotating about its centre having φ dependence as ψ(φ) = Aeimφ where m is a quantum number.- a)1/2π
- b)
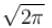
- c)
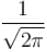
- d)√π
Correct answer is option 'C'. Can you explain this answer?
Which of the following values of A will properly normalize the wave function of a rigid dumbbell rotating about its centre having φ dependence as ψ(φ) = Aeimφ where m is a quantum number.
a)
1/2π
b)
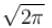
c)
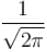
d)
√π

|
Pie Academy answered |
For normalization
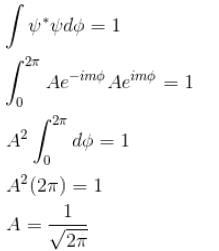
The correct answer is: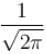
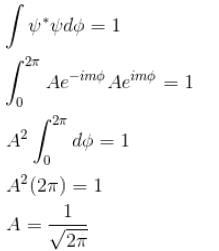
The correct answer is:
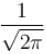
The ground state energy of an electron confined to a box 1Å wide is.- a)6.016 × 10–20 J
- b)2.016 × 10–18 J
- c)5.02 × 10–18 J
- d)6.016 × 10–18 J
Correct answer is option 'D'. Can you explain this answer?
The ground state energy of an electron confined to a box 1Å wide is.
a)
6.016 × 10–20 J
b)
2.016 × 10–18 J
c)
5.02 × 10–18 J
d)
6.016 × 10–18 J
|
|
Jayant Mishra answered |
We have, m = 9.1 × 10–31 kg, a = 1 × 10–10 m
Since energy of electron in nth state is given by
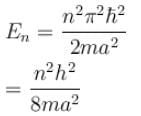
For ground state,
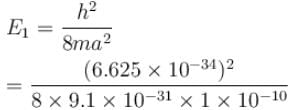
Since energy of electron in nth state is given by
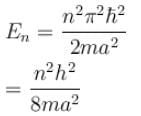
For ground state,
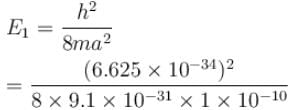
E1 = 6.016 × 10–18 J
The correct answer is: 6.016 × 10–18 J
The correct answer is: 6.016 × 10–18 J
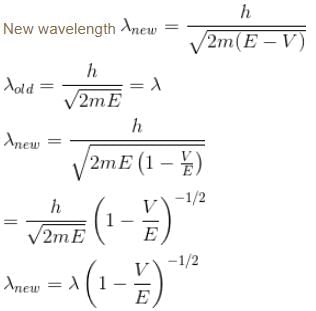
A particle with initial K.E = E and de-Broglie wavelength λ enters a region in which it has potential energy V. What is the particle’s new de-Broglie wavelength?- a)
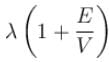
- b)
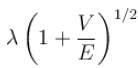
- c)
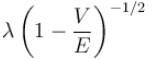
- d)
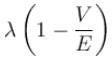
Correct answer is option 'C'. Can you explain this answer?
A particle with initial K.E = E and de-Broglie wavelength λ enters a region in which it has potential energy V. What is the particle’s new de-Broglie wavelength?
a)
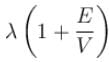
b)
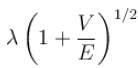
c)
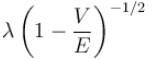
d)
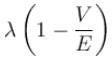
|
|
Jayant Mishra answered |
In the initial case (free particle)
V = 0
Total energy = E = kinetic energy
Since the total energy remains conserved
∴ E = K.Enew + V
K.E. (new) = E – V
Now, new wavelength =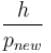
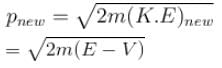
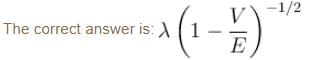
V = 0
Total energy = E = kinetic energy
Since the total energy remains conserved
∴ E = K.Enew + V
K.E. (new) = E – V
Now, new wavelength =
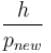
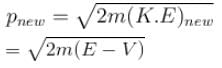

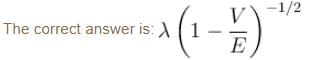
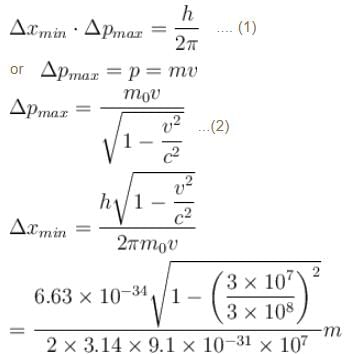
Smallest possible uncertainty in position of the electron moving with velocity 3 × 107 m/s. Given, h = 6.63 × 10–34 Js, m0 = 9.1 × 10–31 kg.- a)1.8 × 10–12 m
- b)2.8 × 10–12 m
- c)3.8 × 10–12 m
- d)5.4 × 10–12 m
Correct answer is option 'C'. Can you explain this answer?
Smallest possible uncertainty in position of the electron moving with velocity 3 × 107 m/s. Given, h = 6.63 × 10–34 Js, m0 = 9.1 × 10–31 kg.
a)
1.8 × 10–12 m
b)
2.8 × 10–12 m
c)
3.8 × 10–12 m
d)
5.4 × 10–12 m
|
|
Vedika Singh answered |
Given, v = 3 × 107 m/s
Let Δxmin be the minimum uncertainty in position of the electron and Δp the maximum uncertainty in the momentum of the electron.
Let Δxmin be the minimum uncertainty in position of the electron and Δp the maximum uncertainty in the momentum of the electron.
Thus, we have,
= 0.03867 × 0.9949 × 10–10 m
= 3.8 × 10–12 m
The correct answer is: 3.8 × 10–12 m

= 0.03867 × 0.9949 × 10–10 m
= 3.8 × 10–12 m
The correct answer is: 3.8 × 10–12 m
In compton effect,- a)The scattered radiation contains X-rays of a single frequency
- b)The scattered radiation contains X-rays of wavelength same as that of incident wavelength and larger than the incident wavelength
- c)The scattered radiation contains X rays of a single wavelength.
- d)The scattered radiation contains X-rays of the incident frequency and frequency smaller than the incident frequency (vin)
Correct answer is option 'B,D'. Can you explain this answer?
In compton effect,
a)
The scattered radiation contains X-rays of a single frequency
b)
The scattered radiation contains X-rays of wavelength same as that of incident wavelength and larger than the incident wavelength
c)
The scattered radiation contains X rays of a single wavelength.
d)
The scattered radiation contains X-rays of the incident frequency and frequency smaller than the incident frequency (vin)
|
|
Jayant Mishra answered |
Refer the theory of compton effect λ
The correct answers are: The scattered radiation contains X-rays of the incident frequency and frequency smaller than the incident frequency (vin), The scattered radiation contains X-rays of wavelength same as that of incident wavelength and larger than the incident wavelength
For the photoelectric effect, stopping potential v/s incident frequency is plotted. Choose the correct statements.- a)slope
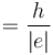 and y intercept
and y intercept 
- b)graph is a straight line with positive slope and positive y intercept
- c)the graph is a straight line with a positive slope and negative y intercept.
- d)slope
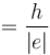 and y intercept
and y intercept 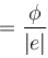
Correct answer is option 'A,C'. Can you explain this answer?
For the photoelectric effect, stopping potential v/s incident frequency is plotted. Choose the correct statements.
a)
slope 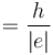 and y intercept
and y intercept 
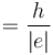 and y intercept
and y intercept 
b)
graph is a straight line with positive slope and positive y intercept
c)
the graph is a straight line with a positive slope and negative y intercept.
d)
slope 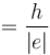 and y intercept
and y intercept 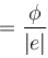
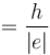 and y intercept
and y intercept 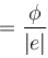
|
|
Vedika Singh answered |
For photoelectric effect,
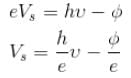
Compare with y = mx + c
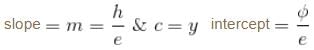
The correct answers are: the graph is a straight line with a positive slope and negative y intercept., slope

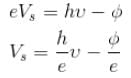
Compare with y = mx + c
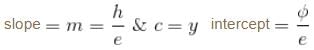
The correct answers are: the graph is a straight line with a positive slope and negative y intercept., slope

The wavelength of Ln line in X-rays spectrum of 78Pt is 1.32 Å. The wavelength of Ln line in X-rays spectrum of another unknown element is 4.17Å. If screening constant for La line is 7.4, then atomic number of the unknown element is :
Correct answer is '47'. Can you explain this answer?
The wavelength of Ln line in X-rays spectrum of 78Pt is 1.32 Å. The wavelength of Ln line in X-rays spectrum of another unknown element is 4.17Å. If screening constant for La line is 7.4, then atomic number of the unknown element is :
|
|
Jayant Mishra answered |
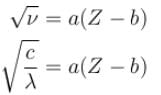
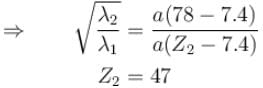
The correct answer is: 47
In case of an one electron Bohr atom, pn is the linear momentum of the electron in the nth orbit and En is its total energy. Which of the following is true? Here rn is the radius of the nth orbit and m is the mass.- a)
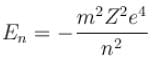
- b)
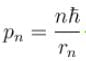
- c)
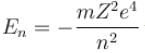
- d)
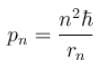
Correct answer is option 'B,C'. Can you explain this answer?
In case of an one electron Bohr atom, pn is the linear momentum of the electron in the nth orbit and En is its total energy. Which of the following is true? Here rn is the radius of the nth orbit and m is the mass.
a)
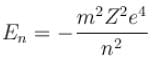
b)
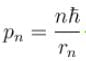
c)
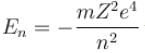
d)
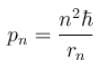
|
|
Jayant Mishra answered |
From the quantization of orbital angular momentum,
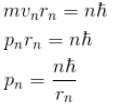
For energy,
Centrifugal force = Coulomb attraction
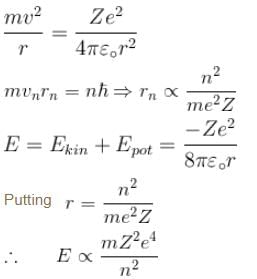
The correct answers are: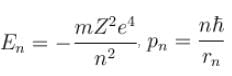
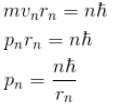
For energy,
Centrifugal force = Coulomb attraction
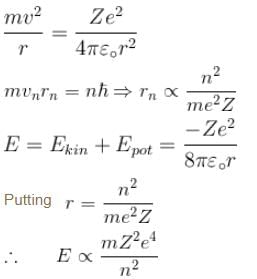
The correct answers are:
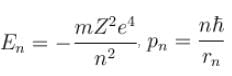
Chapter doubts & questions for The Quantum Model of the Atom - Physics 2025 is part of Grade 9 exam preparation. The chapters have been prepared according to the Grade 9 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 9 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of The Quantum Model of the Atom - Physics in English & Hindi are available as part of Grade 9 exam.
Download more important topics, notes, lectures and mock test series for Grade 9 Exam by signing up for free.
Physics
307 videos|482 docs|202 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily

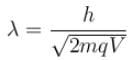
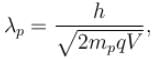
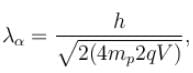
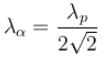
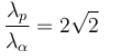
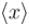 of the particle's position is.
of the particle's position is.
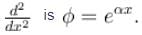 The corresponding eigenvalue is.
The corresponding eigenvalue is.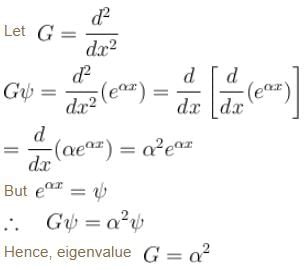
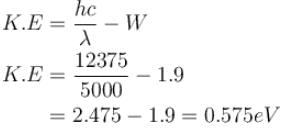
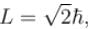 which of the following can be the possible values of a measurement of Lz. (z component of L)?
which of the following can be the possible values of a measurement of Lz. (z component of L)?