All Exams >
Mechanical Engineering >
6 Months Preparation for GATE Mechanical >
All Questions
All questions of Availability & Irreversibility for Mechanical Engineering Exam
The pressure, temperature and velocity of air flowing in pipe are 5 bar, 500 K and 50 m/s, respectively. The specific heats of air at a constant pressure and at constant volume are 1.005 kJ/kgK and 0.718 kJ/kgK, respectively.Neglect potential energy. If the pressure and temperature of the surroundings are 1 bar and 300 K, respectively, the available energy in kJ/ kg of the air stream is- a)170
- b)187
- c)191
- d)213
Correct answer is option 'B'. Can you explain this answer?
The pressure, temperature and velocity of air flowing in pipe are 5 bar, 500 K and 50 m/s, respectively. The specific heats of air at a constant pressure and at constant volume are 1.005 kJ/kgK and 0.718 kJ/kgK, respectively.Neglect potential energy. If the pressure and temperature of the surroundings are 1 bar and 300 K, respectively, the available energy in kJ/ kg of the air stream is
a)
170
b)
187
c)
191
d)
213
|
|
Zoya Sharma answered |
For flow stream,
A.E. =(h2 – h1) + K.E. – T0 (S2 – S1)
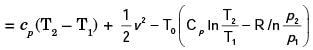
= 187 kJ/kg
A.E. =(h2 – h1) + K.E. – T0 (S2 – S1)
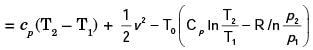
= 187 kJ/kg
A heat engine receives heat from a source at 1500 K at a rate of 600 kW and rejects the waste heat to a medium at 350 K. If the power output from the heat engine is 250 kW, the irreversibility rate for the process is- a)125 kW
- b)150 kW
- c)180 kW
- d)210 kW
Correct answer is option 'D'. Can you explain this answer?
A heat engine receives heat from a source at 1500 K at a rate of 600 kW and rejects the waste heat to a medium at 350 K. If the power output from the heat engine is 250 kW, the irreversibility rate for the process is
a)
125 kW
b)
150 kW
c)
180 kW
d)
210 kW
|
|
Rashi Chauhan answered |
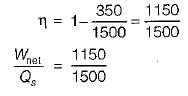
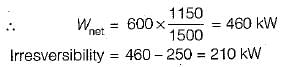
A heat reservoir at 900 K is brought into contact with the ambient at 300 K for a short time. During this period 9000 kJ of heat is lost by the heat reservoir. The total loss in availability due to this process is- a)18000 kJ
- b)9000 kJ
- c)6000 kJ
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
A heat reservoir at 900 K is brought into contact with the ambient at 300 K for a short time. During this period 9000 kJ of heat is lost by the heat reservoir. The total loss in availability due to this process is
a)
18000 kJ
b)
9000 kJ
c)
6000 kJ
d)
None of these
|
|
Zoya Sharma answered |
Entropy change for hot reservoir
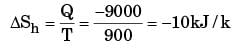
Energy gain in cold reservoir
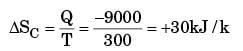
Loss in availability = T0 [ ΔSc + ΔSh ]
⇒ 300(30 – 10)
= 300(20)
= 6000 kJ
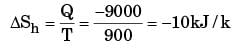
Energy gain in cold reservoir
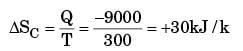
Loss in availability = T0 [ ΔSc + ΔSh ]
⇒ 300(30 – 10)
= 300(20)
= 6000 kJ
Availability of a system at any given state is- a)a property of the system
- b)the maxim um wo r k obtain able as th e system goes to dead state
- c)the total energy of the system
- d)the maximum useful work obtainable as the system goes to dead state
Correct answer is option 'D'. Can you explain this answer?
Availability of a system at any given state is
a)
a property of the system
b)
the maxim um wo r k obtain able as th e system goes to dead state
c)
the total energy of the system
d)
the maximum useful work obtainable as the system goes to dead state
|
|
Zoya Sharma answered |
Availability of system of any given state is when no maximum useful work obtainable as the system goes to dead state.
The maximum theoretical work obtainable, when a system interacts to equilibrium with a reference environment, is called- a)Entropy
- b)Enthalpy
- c)Exergy
- d)Rothalpy
Correct answer is option 'C'. Can you explain this answer?
The maximum theoretical work obtainable, when a system interacts to equilibrium with a reference environment, is called
a)
Entropy
b)
Enthalpy
c)
Exergy
d)
Rothalpy
|
|
Sanvi Kapoor answered |
Exergy (or) Available Energy :
The maximum portion of energy which could be converted into useful work by ideal processes which reduce the system to dead state (a state in equilibrium with the earth and its atmosphere).
The maximum portion of energy which could be converted into useful work by ideal processes which reduce the system to dead state (a state in equilibrium with the earth and its atmosphere).
Availability of a system at any given state is- a)a property of the system
- b)the total energy of the system
- c)the maximum work obtainable as system goes to dead state
- d)the maximum useful work obtainable as the system goes to dead state
Correct answer is option 'D'. Can you explain this answer?
Availability of a system at any given state is
a)
a property of the system
b)
the total energy of the system
c)
the maximum work obtainable as system goes to dead state
d)
the maximum useful work obtainable as the system goes to dead state
|
|
Sagarika Mukherjee answered |
The Availability of a given system is defined as the maximum useful work (total work minus Pdv work) that is obtainable in a process in which the system comes to equilibrium with its surroundings.
A steel billet of 2000 kg mass is to be cooled from 1250 K to 450 K. The heat released during this process is to be used as a source of energy. The ambient temperature is 303 K and specific heat of steel is 0.5 kJ/kgK. The available energy of this billet is- a)490.44 MJ
- b)30.95 MJ
- c)10.35 MJ
- d)0.10 MJ
Correct answer is option 'A'. Can you explain this answer?
A steel billet of 2000 kg mass is to be cooled from 1250 K to 450 K. The heat released during this process is to be used as a source of energy. The ambient temperature is 303 K and specific heat of steel is 0.5 kJ/kgK. The available energy of this billet is
a)
490.44 MJ
b)
30.95 MJ
c)
10.35 MJ
d)
0.10 MJ
|
|
Zoya Sharma answered |
Heat lost by steel = (0.5)(2000)(450 – 1250)
Gained = 800MJ
ΔSLost by steel = (2000) (0.5)) ln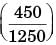
⇒ –1.021 MJ
(ΔS) gained = 1.021MJ
AE (W) = a – T0dS
= 800 – (303) (1.021)
= 490.7 MJ
Gained = 800MJ
ΔSLost by steel = (2000) (0.5)) ln
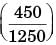
⇒ –1.021 MJ
(ΔS) gained = 1.021MJ
AE (W) = a – T0dS
= 800 – (303) (1.021)
= 490.7 MJ
Which of the following thermodynamic relation is Keenan function?
- a)U + P V
- b)H - T0 S
- c)E - T0S
- d)U - T0S
Correct answer is option 'B'. Can you explain this answer?
Which of the following thermodynamic relation is Keenan function?
a)
U + P V
b)
H - T0 S
c)
E - T0S
d)
U - T0S

|
Constructing Careers answered |
Explanation:
- The Keenan function is given by the thermodynamic relation: H - T0 S
- This equation represents the enthalpy (H) minus the product of the temperature (T0) and the entropy (S)
- The Keenan function is often used in thermodynamics to analyze and calculate the energy changes in a system
- It is a useful tool for studying the relationships between heat, work, and energy transfer in various processes.
- The Keenan function is given by the thermodynamic relation: H - T0 S
- This equation represents the enthalpy (H) minus the product of the temperature (T0) and the entropy (S)
- The Keenan function is often used in thermodynamics to analyze and calculate the energy changes in a system
- It is a useful tool for studying the relationships between heat, work, and energy transfer in various processes.
In which one of the following processes is there an increase in entropy with no degradation of energy- a)Polytropic expansion
- b)Isothermal expansion
- c)Isochoric heat addition
- d)Isobaric heat addition
Correct answer is option 'B'. Can you explain this answer?
In which one of the following processes is there an increase in entropy with no degradation of energy
a)
Polytropic expansion
b)
Isothermal expansion
c)
Isochoric heat addition
d)
Isobaric heat addition
|
|
Anshu Patel answered |
Explanation:
Entropy is a thermodynamic property that measures the degree of disorder or randomness of a system. The second law of thermodynamics states that the entropy of an isolated system always increases over time, or at best remains constant.
Entropy and Energy Degradation:
The term "energy degradation" refers to the process by which energy is converted from a more usable form to a less usable form, such as from mechanical energy to heat energy. This process always results in an increase in entropy. Therefore, any process that involves energy degradation will also involve an increase in entropy.
Isothermal Expansion:
Isothermal expansion is a process in which a gas expands at a constant temperature. During this process, the gas does work on its surroundings, but there is no energy degradation. The reason for this is that the work done by the gas is reversible, meaning that it could be undone without any loss of energy.
In contrast, a polytropic expansion involves a change in temperature, which means that energy is being added or removed from the system. This process always results in an increase in entropy. Similarly, isochoric heat addition and isobaric heat addition involve energy being added to the system, which also leads to an increase in entropy.
Conclusion:
In conclusion, the only process in which there is an increase in entropy with no degradation of energy is isothermal expansion. This is because the work done by the gas is reversible and can be undone without any loss of energy.
Entropy is a thermodynamic property that measures the degree of disorder or randomness of a system. The second law of thermodynamics states that the entropy of an isolated system always increases over time, or at best remains constant.
Entropy and Energy Degradation:
The term "energy degradation" refers to the process by which energy is converted from a more usable form to a less usable form, such as from mechanical energy to heat energy. This process always results in an increase in entropy. Therefore, any process that involves energy degradation will also involve an increase in entropy.
Isothermal Expansion:
Isothermal expansion is a process in which a gas expands at a constant temperature. During this process, the gas does work on its surroundings, but there is no energy degradation. The reason for this is that the work done by the gas is reversible, meaning that it could be undone without any loss of energy.
In contrast, a polytropic expansion involves a change in temperature, which means that energy is being added or removed from the system. This process always results in an increase in entropy. Similarly, isochoric heat addition and isobaric heat addition involve energy being added to the system, which also leads to an increase in entropy.
Conclusion:
In conclusion, the only process in which there is an increase in entropy with no degradation of energy is isothermal expansion. This is because the work done by the gas is reversible and can be undone without any loss of energy.
The main cause for the irreversibility is- a)mechanical and fluid friction
- b)unrestricted expansion
- c)heat transfer with a finite temperature difference
- d)all of the above
Correct answer is option 'D'. Can you explain this answer?
The main cause for the irreversibility is
a)
mechanical and fluid friction
b)
unrestricted expansion
c)
heat transfer with a finite temperature difference
d)
all of the above
|
|
Kiran Basu answered |
Irreversibility in Thermodynamics
Irreversibility in thermodynamics refers to the phenomenon where a process or a system cannot be reversed to its original state with the same efficiency. The main causes for irreversibility are as follows:
Mechanical and Fluid Friction
Mechanical and fluid friction occur when there is resistance to motion or flow within a system. This resistance leads to energy losses in the form of heat, which cannot be completely recovered. Examples include friction in moving parts of machines or fluid flow through pipes.
Unrestricted Expansion
Unrestricted expansion refers to a process where a system expands freely without any external constraints. During this process, the system loses the ability to do work, leading to irreversibility. An example is the sudden release of compressed gas into a vacuum.
Heat Transfer with a Finite Temperature Difference
When heat is transferred between two bodies at different temperatures, there is always some irreversibility due to the temperature difference. This irreversibility is caused by the entropy generation in the system, leading to a decrease in overall efficiency.
Conclusion
In summary, the irreversibility in thermodynamic processes is mainly caused by mechanical and fluid friction, unrestricted expansion, and heat transfer with a finite temperature difference. These factors result in energy losses and an overall decrease in efficiency, making the process irreversible.
Irreversibility in thermodynamics refers to the phenomenon where a process or a system cannot be reversed to its original state with the same efficiency. The main causes for irreversibility are as follows:
Mechanical and Fluid Friction
Mechanical and fluid friction occur when there is resistance to motion or flow within a system. This resistance leads to energy losses in the form of heat, which cannot be completely recovered. Examples include friction in moving parts of machines or fluid flow through pipes.
Unrestricted Expansion
Unrestricted expansion refers to a process where a system expands freely without any external constraints. During this process, the system loses the ability to do work, leading to irreversibility. An example is the sudden release of compressed gas into a vacuum.
Heat Transfer with a Finite Temperature Difference
When heat is transferred between two bodies at different temperatures, there is always some irreversibility due to the temperature difference. This irreversibility is caused by the entropy generation in the system, leading to a decrease in overall efficiency.
Conclusion
In summary, the irreversibility in thermodynamic processes is mainly caused by mechanical and fluid friction, unrestricted expansion, and heat transfer with a finite temperature difference. These factors result in energy losses and an overall decrease in efficiency, making the process irreversible.
The exergy of an isolated system in a process- a)can never increase
- b)can never decrease
- c)always remains constant
- d)is always positive
Correct answer is option 'A'. Can you explain this answer?
The exergy of an isolated system in a process
a)
can never increase
b)
can never decrease
c)
always remains constant
d)
is always positive
|
|
Arshiya Dey answered |
Exergy of an Isolated System in a Process:
Exergy is defined as the maximum useful work that can be obtained from a system when it is brought into equilibrium with a reference environment. It is a measure of the quality of energy, and it reflects the availability of energy to do useful work. The exergy of an isolated system in a process can never increase because of the second law of thermodynamics.
Second Law of Thermodynamics:
The second law of thermodynamics states that the total entropy of an isolated system always increases over time, or remains constant in ideal cases where the system is in a state of equilibrium. Entropy is a measure of the disorder or randomness of a system, and it increases as energy is converted from one form to another. When energy is converted from a higher quality form to a lower quality form, the exergy decreases.
Exergy Destruction:
Exergy destruction is a measure of the irreversibility of a process, and it is defined as the difference between the initial exergy of a system and the final exergy of the system after the process. Exergy destruction occurs when energy is converted from a higher quality form to a lower quality form, such as from mechanical energy to thermal energy. This irreversibility is due to the increase in entropy during the process.
Conclusion:
In conclusion, exergy is a measure of the quality of energy, and it reflects the availability of energy to do useful work. The exergy of an isolated system in a process can never increase because of the second law of thermodynamics. Exergy destruction is a measure of the irreversibility of a process, and it occurs when energy is converted from a higher quality form to a lower quality form.
Exergy is defined as the maximum useful work that can be obtained from a system when it is brought into equilibrium with a reference environment. It is a measure of the quality of energy, and it reflects the availability of energy to do useful work. The exergy of an isolated system in a process can never increase because of the second law of thermodynamics.
Second Law of Thermodynamics:
The second law of thermodynamics states that the total entropy of an isolated system always increases over time, or remains constant in ideal cases where the system is in a state of equilibrium. Entropy is a measure of the disorder or randomness of a system, and it increases as energy is converted from one form to another. When energy is converted from a higher quality form to a lower quality form, the exergy decreases.
Exergy Destruction:
Exergy destruction is a measure of the irreversibility of a process, and it is defined as the difference between the initial exergy of a system and the final exergy of the system after the process. Exergy destruction occurs when energy is converted from a higher quality form to a lower quality form, such as from mechanical energy to thermal energy. This irreversibility is due to the increase in entropy during the process.
Conclusion:
In conclusion, exergy is a measure of the quality of energy, and it reflects the availability of energy to do useful work. The exergy of an isolated system in a process can never increase because of the second law of thermodynamics. Exergy destruction is a measure of the irreversibility of a process, and it occurs when energy is converted from a higher quality form to a lower quality form.
Considering the relationship TdS = dU + pdV between the entropy (S), internal energy (U), pressure (p), temperature (T) and volume (V), which of the following statements is correct?
[2003]
- a)It is applicable only for a reversible process
- b)For an irreversible process, TdS > dU+ pdV
- c)It is valid only for an ideal gas
- d)It is equivalent to 1st law, for a reversible process
Correct answer is option 'D'. Can you explain this answer?
Considering the relationship TdS = dU + pdV between the entropy (S), internal energy (U), pressure (p), temperature (T) and volume (V), which of the following statements is correct?
[2003]
a)
It is applicable only for a reversible process
b)
For an irreversible process, TdS > dU+ pdV
c)
It is valid only for an ideal gas
d)
It is equivalent to 1st law, for a reversible process
|
|
Sanvi Kapoor answered |
TdS=dU+pdV
This equation holds for for any process reversible or irreversible, undergone by a closed system, since it is a relation among properties which are independent of path.
This equation holds for for any process reversible or irreversible, undergone by a closed system, since it is a relation among properties which are independent of path.
Chapter doubts & questions for Availability & Irreversibility - 6 Months Preparation for GATE Mechanical 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Availability & Irreversibility - 6 Months Preparation for GATE Mechanical in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
6 Months Preparation for GATE Mechanical
499 videos|1037 docs|710 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup


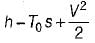
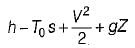
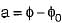 where φ is the availability function of the
where φ is the availability function of the







