All Exams >
NEET >
NCERT on your Fingertips 2025-2026 Edition >
All Questions
All questions of MCQ Corner for NEET Exam
Fermentation of Glucose to alcohol undergoes in presence of :- a)ZnO
- b)Cr2O3
- c)Invertase
- d)Zymase
Correct answer is option 'D'. Can you explain this answer?
Fermentation of Glucose to alcohol undergoes in presence of :
a)
ZnO
b)
Cr2O3
c)
Invertase
d)
Zymase
|
|
Lavanya Menon answered |
The correct answer is option D
Zymase is an enzyme complex that catalyzes the fermentation of sugar into ethanol and carbon dioxide. It occurs naturally in yeasts. Zymase activity varies among yeast strains. Zymase is also the brand name of the drug pancrelipase.
Zymase is an enzyme complex that catalyzes the fermentation of sugar into ethanol and carbon dioxide. It occurs naturally in yeasts. Zymase activity varies among yeast strains. Zymase is also the brand name of the drug pancrelipase.
C2H5OH and CH3OCH3 are:- a)Position isomers
- b)Functional isomers
- c)Chain isomer
- d)Metamers
Correct answer is option 'B'. Can you explain this answer?
C2H5OH and CH3OCH3 are:
a)
Position isomers
b)
Functional isomers
c)
Chain isomer
d)
Metamers
|
|
Vivek Rana answered |
Alkyl alcohol and ether having same molecular formula ( Here C2H6O) are functional isomers of each other. As both having different functional group first one has -OH & second one has -O- functional group in the same carbon chain respectively .
Which alkene is obtained on dehydration of the following alcohol in the presence of H2SO4 ?
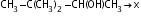
- a)
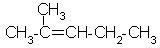
- b)
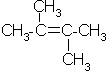
- c)
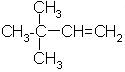
- d) None of the above
Correct answer is option 'B'. Can you explain this answer?
Which alkene is obtained on dehydration of the following alcohol in the presence of H2SO4 ?
a)
b)
c)
d)
None of the above

|
Mohit Rajpoot answered |
Carbonium ion formed in 1st step undergoes rearrangement to give tert. carbonium ion since tert-carbonium ions are more stable than secondary one.
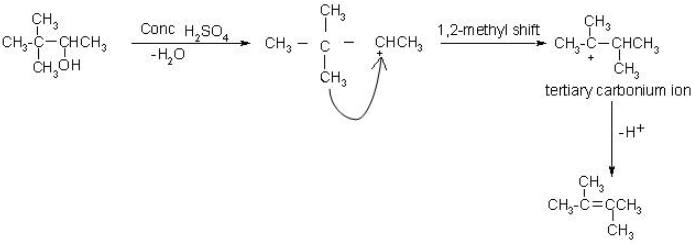
Phenol is acidic due to resonance stabilization of its conjugate base :- a)O-H
- b)Benzyl alcohol
- c)Alkoxide ion
- d)Phenoxide ion
Correct answer is option 'D'. Can you explain this answer?
Phenol is acidic due to resonance stabilization of its conjugate base :
a)
O-H
b)
Benzyl alcohol
c)
Alkoxide ion
d)
Phenoxide ion
|
|
Rishika Patel answered |
Polarity of Phenol
Phenol, also known as benzenol or carbolic acid, is an aromatic compound that consists of a benzene ring bonded to a hydroxyl group (-OH). The presence of the hydroxyl group makes phenol a polar molecule because oxygen is more electronegative than carbon and hydrogen.
Acidity of Phenol
Phenol is considered an acid because it can donate a proton (H+) and form a conjugate base. The acidity of phenol is attributed to resonance stabilization of its conjugate base, the phenoxide ion.
Resonance Stabilization of Phenoxide Ion
When phenol loses a proton, it forms the phenoxide ion (C6H5O-). The phenoxide ion is resonance stabilized, which means that the negative charge can be delocalized over the entire ring system through resonance structures.
Resonance Structures of Phenoxide Ion
The resonance structures of the phenoxide ion can be represented as follows:
1. In the first resonance structure, the lone pair on the oxygen atom forms a double bond with carbon, while the negative charge is located on the oxygen atom.
2. In the second resonance structure, the lone pair on the oxygen atom forms a double bond with one of the carbon atoms in the ring, while the negative charge is located on that carbon atom.
3. In the third resonance structure, the lone pair on the oxygen atom forms a double bond with another carbon atom in the ring, while the negative charge is located on that carbon atom.
The resonance structures demonstrate that the negative charge of the phenoxide ion is delocalized over the entire ring system, resulting in stability.
Comparison with Other Options
a) O-H: The O-H bond in phenol is the source of acidity, but it does not explain the resonance stabilization of the conjugate base.
b) Benzyl alcohol: While benzyl alcohol also has an -OH group, it lacks the aromatic ring required for resonance stabilization of the conjugate base.
c) Alkoxide ion: Alkoxide ions are formed when an alcohol loses a proton, but they do not have the same resonance stabilization as the phenoxide ion.
Conclusion
The correct answer is option 'D' (Phenoxide ion) because the resonance stabilization of the phenoxide ion is responsible for the acidity of phenol. The delocalization of the negative charge over the aromatic ring system increases the stability of the conjugate base, making phenol acidic.
Phenol, also known as benzenol or carbolic acid, is an aromatic compound that consists of a benzene ring bonded to a hydroxyl group (-OH). The presence of the hydroxyl group makes phenol a polar molecule because oxygen is more electronegative than carbon and hydrogen.
Acidity of Phenol
Phenol is considered an acid because it can donate a proton (H+) and form a conjugate base. The acidity of phenol is attributed to resonance stabilization of its conjugate base, the phenoxide ion.
Resonance Stabilization of Phenoxide Ion
When phenol loses a proton, it forms the phenoxide ion (C6H5O-). The phenoxide ion is resonance stabilized, which means that the negative charge can be delocalized over the entire ring system through resonance structures.
Resonance Structures of Phenoxide Ion
The resonance structures of the phenoxide ion can be represented as follows:
1. In the first resonance structure, the lone pair on the oxygen atom forms a double bond with carbon, while the negative charge is located on the oxygen atom.
2. In the second resonance structure, the lone pair on the oxygen atom forms a double bond with one of the carbon atoms in the ring, while the negative charge is located on that carbon atom.
3. In the third resonance structure, the lone pair on the oxygen atom forms a double bond with another carbon atom in the ring, while the negative charge is located on that carbon atom.
The resonance structures demonstrate that the negative charge of the phenoxide ion is delocalized over the entire ring system, resulting in stability.
Comparison with Other Options
a) O-H: The O-H bond in phenol is the source of acidity, but it does not explain the resonance stabilization of the conjugate base.
b) Benzyl alcohol: While benzyl alcohol also has an -OH group, it lacks the aromatic ring required for resonance stabilization of the conjugate base.
c) Alkoxide ion: Alkoxide ions are formed when an alcohol loses a proton, but they do not have the same resonance stabilization as the phenoxide ion.
Conclusion
The correct answer is option 'D' (Phenoxide ion) because the resonance stabilization of the phenoxide ion is responsible for the acidity of phenol. The delocalization of the negative charge over the aromatic ring system increases the stability of the conjugate base, making phenol acidic.
The process of converting alkyl halides into alcohols involves_____________.- a)rearrangement reaction
- b)substitution reaction
- c)dehydrohalogenation reaction
- d)addition reaction
Correct answer is option 'B'. Can you explain this answer?
The process of converting alkyl halides into alcohols involves_____________.
a)
rearrangement reaction
b)
substitution reaction
c)
dehydrohalogenation reaction
d)
addition reaction
|
|
Gaurav Kumar answered |
Converstion of alcohol to alkyl halide is via substitution reaction.
Ethers are :- a)Basic
- b)Amphoteric substances
- c)Acidic
- d)Neutral
Correct answer is option 'A'. Can you explain this answer?
Ethers are :
a)
Basic
b)
Amphoteric substances
c)
Acidic
d)
Neutral
|
|
Sandeep Kumar Chaudhary answered |
O
HAS LONE PAIR IN ETHER SO IT CAN ACCEPT H+.
SO IT IS BEHAVES AS BASE
HAS LONE PAIR IN ETHER SO IT CAN ACCEPT H+.
SO IT IS BEHAVES AS BASE
When aryl halide is heated with 6-8% NaOH solution ,at 623 K under 300 atm pressure we get:- a)Phenol
- b)Benzyl ether
- c)Ethyl alcohol
- d)Cumene
Correct answer is option 'A'. Can you explain this answer?
When aryl halide is heated with 6-8% NaOH solution ,at 623 K under 300 atm pressure we get:
a)
Phenol
b)
Benzyl ether
c)
Ethyl alcohol
d)
Cumene

|
Divey Sethi answered |
The correct answer is Option A.
The aryl halide is heated with 6-8%. NaOH solution at 623K under 300 atm pressure 1) it gives sodium phenoxide 2) sodium phenoxide treated with HCl(acidification) then it gives phenol.
The aryl halide is heated with 6-8%. NaOH solution at 623K under 300 atm pressure 1) it gives sodium phenoxide 2) sodium phenoxide treated with HCl(acidification) then it gives phenol.
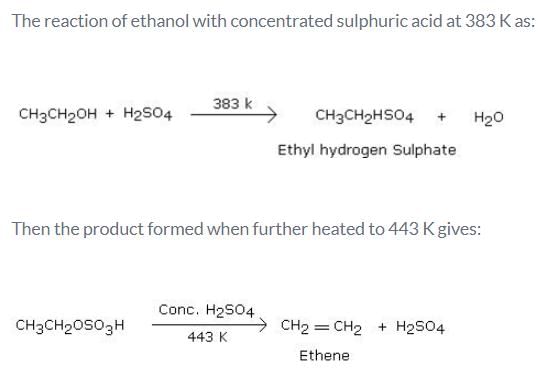
What is the product formed when excess of ethanol reacts with concentrated sulphuric acid at 383 K and then the temperature of reaction mixture is increased to 443 K?- a)Ethene
- b)Ethyl hydrogen sulphate
- c)Ethyl chloride
- d)Ethoxy ethane
Correct answer is 'A'. Can you explain this answer?
What is the product formed when excess of ethanol reacts with concentrated sulphuric acid at 383 K and then the temperature of reaction mixture is increased to 443 K?
a)
Ethene
b)
Ethyl hydrogen sulphate
c)
Ethyl chloride
d)
Ethoxy ethane

|
EduRev Support answered |
Nitration of anisole gives majorly:- a)Nitroanisole
- b)para-Nitroanisole
- c)ortho-Nitroanisole
- d)meta-Nitroanisole
Correct answer is option 'B'. Can you explain this answer?
Nitration of anisole gives majorly:
a)
Nitroanisole
b)
para-Nitroanisole
c)
ortho-Nitroanisole
d)
meta-Nitroanisole

|
Prasenjit Malik answered |
When anisole is nitrated with a mixture of conc HNO3 and H2SO4 it gives a mixture of ortho-Nitroanisole and para-Nitroanisole (major) products.


Identify the alcohol or phenol from the following which is most soluble in water.
- a)CH3– OH
- b)CH(CH3)2 CH2O-H
- c)CH3(CH2)2CH2OH
- d)C6H5OH
Correct answer is option 'A'. Can you explain this answer?
Identify the alcohol or phenol from the following which is most soluble in water.
a)
CH3– OH
b)
CH(CH3)2 CH2O-H
c)
CH3(CH2)2CH2OH
d)
C6H5OH

|
Aryan answered |
As...the ...linear chain in alcohol increase ...the solubility decrease .....due to ....staeric + vanderwall force inreacse ...
Identify the alcohol or phenol having a stronger acidic nature from the following.- a)CH3CH2OH
- b)C6H5OH
- c)CH3CHOHCH2CH3
- d)CH3CH2CH2CH2OH
Correct answer is option 'B'. Can you explain this answer?
Identify the alcohol or phenol having a stronger acidic nature from the following.
a)
CH3CH2OH
b)
C6H5OH
c)
CH3CHOHCH2CH3
d)
CH3CH2CH2CH2OH

|
Mohit Rajpoot answered |
- Phenol (C₆H₅OH) is more acidic than typical alcohols.
- This is due to the resonance stabilization of the phenoxide ion formed when phenol loses a hydrogen ion (H⁺).
- The negative charge on the oxygen atom in phenoxide ion is delocalized over the aromatic ring, increasing stability and acidity.
- In contrast, alcohols like ethanol (CH₃CH₂OH) do not have such resonance stabilization, making them less acidic.
- Therefore, phenol is the strongest acid among the given options.
- This is due to the resonance stabilization of the phenoxide ion formed when phenol loses a hydrogen ion (H⁺).
- The negative charge on the oxygen atom in phenoxide ion is delocalized over the aromatic ring, increasing stability and acidity.
- In contrast, alcohols like ethanol (CH₃CH₂OH) do not have such resonance stabilization, making them less acidic.
- Therefore, phenol is the strongest acid among the given options.
Which of the following statement is correct?- a)The -OH group in phenol is meta directing.
- b)The electron releasing groups increase the acidic character of phenols.
- c)Phenols are less acidic than aromatic alcohols.
- d)Boiling point of phenol is higher than that of toluene of comparable molecular mass.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is correct?
a)
The -OH group in phenol is meta directing.
b)
The electron releasing groups increase the acidic character of phenols.
c)
Phenols are less acidic than aromatic alcohols.
d)
Boiling point of phenol is higher than that of toluene of comparable molecular mass.

|
Divey Sethi answered |
The correct answer is Option D.
Phenols have higher boiling point than toluene due to the presence of intermolecular hydrogen bonding in phenols. The formation of hydrogen bonds increases the intermolecular force of attraction between the phenol molecules and thereby increases its boiling point.
Can you explain the answer of this question below:The acidity of phenols is due to
- A:
Oxidation process
- B:
Resonance stabilization of its ions.
- C:
Hybridisation
- D:
Presence of O-H group
The answer is b.
The acidity of phenols is due to
Oxidation process
Resonance stabilization of its ions.
Hybridisation
Presence of O-H group
|
|
Rahul Bansal answered |
The acidity of phenols is due to its ability to lose hydrogen ion to form phenoxide ions. In a phenol molecule, the sp2hybridised carbon atom of benzene ring attached directly to the hydroxyl group acts as an electron withdrawing group. This sp2 hybridized carbon atom of benzene ring attached directly to the hydroxyl group has higher electronegativity in comparison to hydroxyl group. Due to the higher electronegativity of this carbon atom in comparison to the hydroxyl group attached, electron density decreases on oxygen atom. The decrease in electron density increases the polarity of O-H bond and results in the increase in ionization of phenols. Thus, the phenoxide ion is formed. The phenoxide ion formed is stabilized by the delocalization of negative charge due to the resonance in benzene ring. Phenoxide ion has greater stability than phenols, as in case of phenol charge separation takes place during resonance.The resonance structures of phenoxide ions explain the delocalization of negative charge. In case of substituted phenols, acidity of phenols increases in the presence of electron withdrawing group. This is due to the stability of the phenoxide ion generated. The acidity of phenols further increases if these groups are attached at ortho and para positions. This is due to the fact that the negative charge in phenoxide ion is mainly delocalized at ortho and para positions of the attached benzene ring. On the other hand, the acidity of phenols decreases in presence of electron donating groups as they prohibit the formation of phenoxide ion.
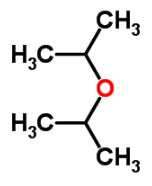
What is the IUPAC name of di-isopropyl ethera)Salicyaldehydeb)Ethyl acetatec)Iso propoxy propaned)Ethyl methyl etherCorrect answer is option 'C'. Can you explain this answer?
|
|
Shreya Gupta answered |
2-Iso propoxy propane
- Molecular Formula: C6H14O
- Average mass: 102.174797 Da
- Monoisotopic mass: 102.104462 Da

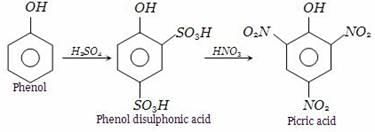
We can obtain picric acid from phenol by:- a)Sulphonation of phenol
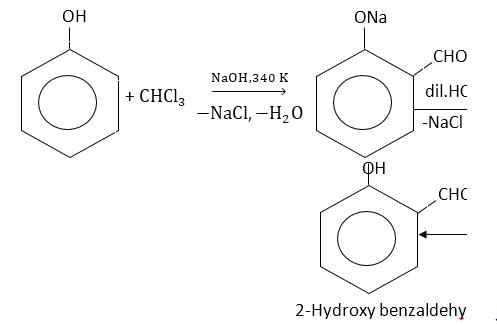
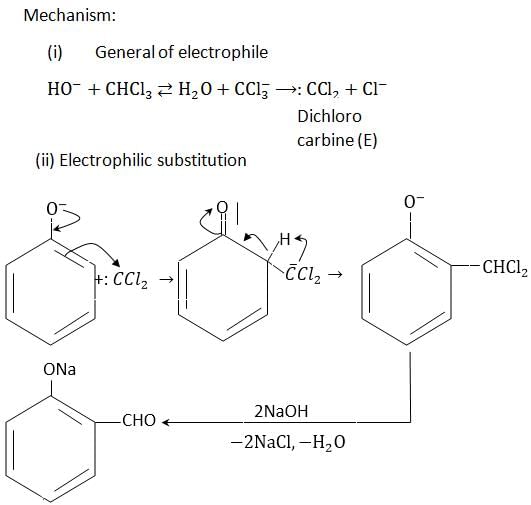
- b)By Reimer Tiemann reaction
- c)Nitration of phenol
- d)Halogenation of phenol
Correct answer is option 'C'. Can you explain this answer?
We can obtain picric acid from phenol by:
a)
Sulphonation of phenol
b)
By Reimer Tiemann reaction
c)
Nitration of phenol
d)
Halogenation of phenol
|
|
Shreya Gupta answered |
Phenol heated with sulphuric acid gives phenol disulphonic acid, which further on reaction with nitric acid forms picric acid (2,4,6-trinitrophenol).
IUPAC name of m-cresol is ___________.- a)3-methylphenol
- b)3-chlorophenol
- c) 3-methoxyphenol
- d)benzene-1,3-diol
Correct answer is option 'A'. Can you explain this answer?
IUPAC name of m-cresol is ___________.
a)
3-methylphenol
b)
3-chlorophenol
c)
3-methoxyphenol
d)
benzene-1,3-diol
|
|
Preeti Iyer answered |
Meta-Cresol, also 3-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless, viscous liquid that is used as an intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and o-cresol.
Which catalyst is used in Fischer-Speier esterification?
- a)Concentrated H2SO4
- b)Dry HCl gas
- c)Concentrated HNO3
- d)Pyridine
Correct answer is option 'A'. Can you explain this answer?
Which catalyst is used in Fischer-Speier esterification?
a)
Concentrated H2SO4
b)
Dry HCl gas
c)
Concentrated HNO3
d)
Pyridine
|
|
Pooja Mehta answered |
Esterification is a relatively slow process at room temperature and does not proceed to completion. Concentrated sulfuric acid is used as a catalyst, and has a dual role: Speeds up the reaction. Acts as a dehydrating agent, forcing the equilibrium to the right and resulting in a greater yield of ester.
Williamsons synthesis is an example of :- a)Electrophilic substitution
- b)Electrophilic addition
- c)Nucleophilic substitution reaction
- d)Nucleophilic addition
Correct answer is option 'C'. Can you explain this answer?
Williamsons synthesis is an example of :
a)
Electrophilic substitution
b)
Electrophilic addition
c)
Nucleophilic substitution reaction
d)
Nucleophilic addition
|
|
Shreya Gupta answered |
The Williamson ether synthesis is an organic reaction used to convert an alcohol and an alkyl halide to an ether using a base such as NaOH. The mechanism begins with the base abstracting the proton from the alcohol to form an alkoxide intermediate. The alkoxide then attacks the alkyl halide in a nucleophilic substi-tution reaction (SN2), which results in the formation of the final ether product and a metal halide by-product.
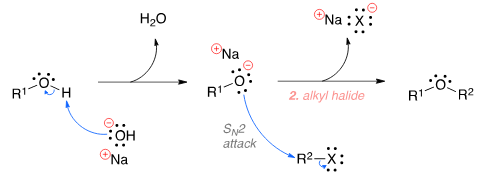

Phenols do not respond to which of these tests?- a)Schiff’s reagent test
- b)FeCl3 test
- c)Br2 water test
- d)Litmus test
Correct answer is option 'A'. Can you explain this answer?
Phenols do not respond to which of these tests?
a)
Schiff’s reagent test
b)
FeCl3 test
c)
Br2 water test
d)
Litmus test
|
|
Rajeev Saxena answered |
Phenols respond to all the above mentioned tests except Schiff’s reagent test, which is shown by aldehydes.
Phenol on treatment with Phthalic anhydride gives:- a)Phenolphthalein
- b)Salicylaldehyde
- c)Phthalic acid
- d)Salicylic acid
Correct answer is option 'A'. Can you explain this answer?
Phenol on treatment with Phthalic anhydride gives:
a)
Phenolphthalein
b)
Salicylaldehyde
c)
Phthalic acid
d)
Salicylic acid

|
Infinity Academy answered |
The correct answer is option A
Phenolphthalein gives pink colour with alkali.
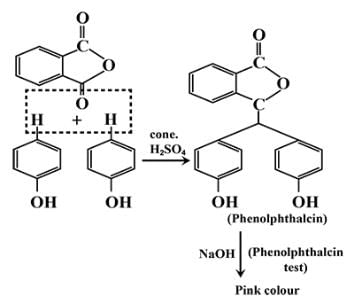
Phenolphthalein gives pink colour with alkali.

Phenol can be distinguished from ethanol by the reactions with _________.
- a)Br2/water
- b)Na
- c)Neutral FeCl3
- d)A and C
Correct answer is option 'D'. Can you explain this answer?
Phenol can be distinguished from ethanol by the reactions with _________.
a)
Br2/water
b)
Na
c)
Neutral FeCl3
d)
A and C

|
Nisha Banerjee answered |
- Phenol can be distinguished from ethanol using Br2/water and Neutral FeCl3.
- Br2/water: Phenol reacts with bromine water to give a white precipitate of 2,4,6-tribromophenol, while ethanol does not react.
- Neutral FeCl3: Phenol forms a violet complex with neutral ferric chloride, whereas ethanol shows no color change.
- Therefore, options A and C are correct for distinguishing phenol from ethanol.
Ethers may be used as solvents because they react only with which of the following reactants?- a)Acids
- b)Bases
- c)Oxidising agent
- d)Reducing agents
Correct answer is option 'A'. Can you explain this answer?
Ethers may be used as solvents because they react only with which of the following reactants?
a)
Acids
b)
Bases
c)
Oxidising agent
d)
Reducing agents
|
|
Geetika Shah answered |
Ethers resist the attack of nucleophiles and bases. However, they are very good solvents in many organic reactions due to their ability to solvate cations by donating the electron pair from oxygen atom. Ethers are generally less reactive and react only with acids.
Isopropyl alcohol on oxidation forms:- a)Acetone
- b)Ether
- c)Acetaldehyde
- d)Methane
Correct answer is option 'A'. Can you explain this answer?
Isopropyl alcohol on oxidation forms:
a)
Acetone
b)
Ether
c)
Acetaldehyde
d)
Methane
|
|
Rahul Bansal answered |
The oxidation of isopropyl alcohol by potassium dichromate (K 2Cr 2O 7) gives acetone, the simplest ketone: Unlike aldehydes, ketones are relatively resistant to further oxidation, so no special precautions are required to isolate them as they form.
To prepare tert-butyl ethyl ether, the reagents required are: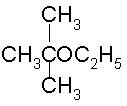
- a)Sodium ethoxide and tert-butyl bromide
- b)Sodium tert butoxide and ethyl bromide
- c)Dimethyl ketone, ethylbromide and sodium
- d)Sodium propoxide and propyl bromide
Correct answer is option 'B'. Can you explain this answer?
To prepare tert-butyl ethyl ether, the reagents required are:
a)
Sodium ethoxide and tert-butyl bromide
b)
Sodium tert butoxide and ethyl bromide
c)
Dimethyl ketone, ethylbromide and sodium
d)
Sodium propoxide and propyl bromide

|
Ayush Joshi answered |
Because 3degree haloalkanes like tert-butyl bromide (option a) give alkenes and not ethers when treated with a strong base like sodium ethoxide. So the exact answer is (b).
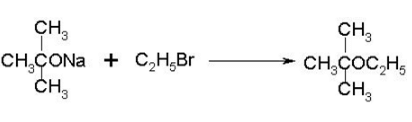
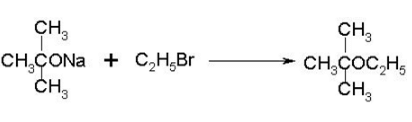
Which of the following compounds will react with sodium hydroxide solution in water?- a)C6H5OH
- b)C6H5CH2OH
- c)(CH3)3COH
- d)C2H5OH
Correct answer is option 'A'. Can you explain this answer?
Which of the following compounds will react with sodium hydroxide solution in water?
a)
C6H5OH
b)
C6H5CH2OH
c)
(CH3)3COH
d)
C2H5OH
|
|
Vijay Bansal answered |
When phenol reacts with sodium hydroxide solution it gives a colourless solution containing sodium phenoxide.
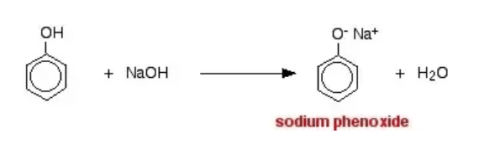
In this reaction, the hydrogen ion has been removed by the strongly basic hydroxide ion in the sodium hydroxide solution.
How many alcohols with molecular formula C4H10O are chiral in nature?a)2b)1c)4d)3Correct answer is option 'B'. Can you explain this answer?
|
|
Mansi Nair answered |

Here, again carbon is not chiral in nature.
So, only one alcohol is chiral in nature
So, only one alcohol is chiral in nature
An organic compound X is oxidised by using acidified K2Cr2O7. The product obtained reacts with Phenyl hydrazine but does not answer silver mirror test. The possible structure of X is- a)CH3CH2OH
- b)CH3CHO
- c)(CH3)2CHOH
- d)None of the these
Correct answer is option 'C'. Can you explain this answer?
An organic compound X is oxidised by using acidified K2Cr2O7. The product obtained reacts with Phenyl hydrazine but does not answer silver mirror test. The possible structure of X is
a)
CH3CH2OH
b)
CH3CHO
c)
(CH3)2CHOH
d)
None of the these
|
|
Baby Ghosh answered |
Yup...it will be propan-2-ol..by oxidation it..it will convert into acetone and it reacts with phenyl hydrazine,it produces acetone phenyl hydrazone and there is no aldehyde grp in acetone. so it doesn't react in silver mirror test means with tollen's reagent..
One Integer Value Correct TypeDirection (Q. Nos. 19-22) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).In the following reaction,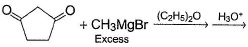 Q. How many different diols are formed as a result of nucleophilic addition reaction?
Q. How many different diols are formed as a result of nucleophilic addition reaction?
Correct answer is '3'. Can you explain this answer?
One Integer Value Correct Type
Direction (Q. Nos. 19-22) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
In the following reaction,
Q.
How many different diols are formed as a result of nucleophilic addition reaction?
|
|
Ritu Singh answered |
What is the correct structure for the major compound produced by the following reaction sequence?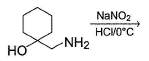
- a)

- b)

- c)

- d)

Correct answer is option 'B'. Can you explain this answer?
What is the correct structure for the major compound produced by the following reaction sequence?
a)
b)
c)
d)

|
Gunjan Lakhani answered |
Reaction involves rearrangement of carbocation
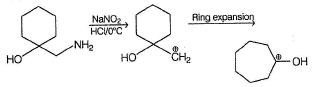
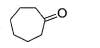
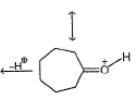


One of the following alcohol is a poison and Ingestion of even small quantities can cause blindness and large quantities causes even death.
- a)methanol
- b)propanol
- c)ethanol
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
One of the following alcohol is a poison and Ingestion of even small quantities can cause blindness and large quantities causes even death.
a)
methanol
b)
propanol
c)
ethanol
d)
None of the above

|
Anoushka Chopra answered |
- Correct Answer: Methanol (A)
- Reason:
- Methanol, also known as wood alcohol, is highly toxic.
- Even small quantities can cause severe poisoning.
- Metabolism of methanol in the body produces formaldehyde and formic acid, which are toxic.
- Symptoms include visual disturbances that may lead to blindness.
- Larger doses can lead to acidosis, nervous system damage, and death.
- Safety Note:
- Methanol is not safe for consumption and should be handled with caution.
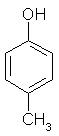
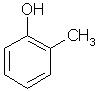
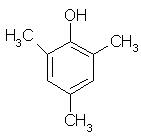
Which of the following is obtained as a major product in Friedel-Craft’s alkylation of phenol ?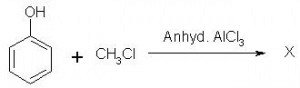
- a)
- b)
- c)
- d)
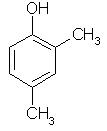
Correct answer is option 'A'. Can you explain this answer?
Which of the following is obtained as a major product in Friedel-Craft’s alkylation of phenol ?
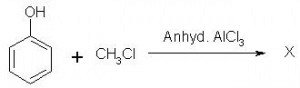
a)
b)
c)

d)

|
Sushil Kumar answered |
The correct answer is option A
P-methyl phenol is the major product.
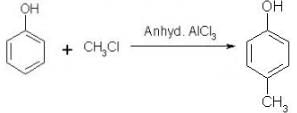
P-methyl phenol is the major product.

Lucas test is associated with
- a)Phenols
- b)Aldehydes
- c)Carboxylic acid
- d)Alcohols
Correct answer is option 'D'. Can you explain this answer?
Lucas test is associated with
a)
Phenols
b)
Aldehydes
c)
Carboxylic acid
d)
Alcohols

|
Mihir Patel answered |
Lucas Test and Alcohols
The Lucas test is a chemical test used to distinguish between primary, secondary, and tertiary alcohols. It is based on the observation of the rate at which an alcohol reacts with Lucas reagent, which is a mixture of concentrated hydrochloric acid and zinc chloride.
Principle of the Lucas Test
- Tertiary alcohols react almost immediately with Lucas reagent to form an alkyl chloride, which is seen as a cloudy solution due to the low solubility of the resulting product.
- Secondary alcohols react within a few minutes to form a cloudy solution.
- Primary alcohols do not react with Lucas reagent at room temperature and therefore do not produce a cloudy solution.
Significance of the Lucas Test
- The Lucas test is a quick and simple method to identify the type of alcohol present in a given compound.
- It is especially useful in organic chemistry labs for qualitative analysis of alcohols.
Application of the Lucas Test
- The Lucas test is commonly used in organic chemistry laboratories to differentiate between primary, secondary, and tertiary alcohols.
- It helps in the identification and characterization of unknown compounds containing alcohol functional groups.
In conclusion, the Lucas test is primarily associated with alcohols as it is a specific test used to classify alcohols based on their reactivity with Lucas reagent.
The Lucas test is a chemical test used to distinguish between primary, secondary, and tertiary alcohols. It is based on the observation of the rate at which an alcohol reacts with Lucas reagent, which is a mixture of concentrated hydrochloric acid and zinc chloride.
Principle of the Lucas Test
- Tertiary alcohols react almost immediately with Lucas reagent to form an alkyl chloride, which is seen as a cloudy solution due to the low solubility of the resulting product.
- Secondary alcohols react within a few minutes to form a cloudy solution.
- Primary alcohols do not react with Lucas reagent at room temperature and therefore do not produce a cloudy solution.
Significance of the Lucas Test
- The Lucas test is a quick and simple method to identify the type of alcohol present in a given compound.
- It is especially useful in organic chemistry labs for qualitative analysis of alcohols.
Application of the Lucas Test
- The Lucas test is commonly used in organic chemistry laboratories to differentiate between primary, secondary, and tertiary alcohols.
- It helps in the identification and characterization of unknown compounds containing alcohol functional groups.
In conclusion, the Lucas test is primarily associated with alcohols as it is a specific test used to classify alcohols based on their reactivity with Lucas reagent.
What is the product of the reaction of methyl cyclohexene with B2H6 in THF followed by the oxidation with alkaline H2O2?

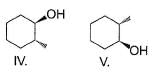
- a)Both I and IV
- b)Only II
- c)Both III and IV
- d)Both II and II
Correct answer is option 'D'. Can you explain this answer?
What is the product of the reaction of methyl cyclohexene with B2H6 in THF followed by the oxidation with alkaline H2O2?


a)
Both I and IV
b)
Only II
c)
Both III and IV
d)
Both II and II
|
|
Shail Chawla answered |
In hydroboration oxidation reaction anti-Markownikoff addition of water takes place. Also both "H" and "OH" groups are attached from same side.
If 3, 3-dimethyl-2, 4-pentanedione is treated with a Grignard reagent consisting of mixture of CH3MgBr and C2H5MgBr and finally hydrolysing product with dilute H2SO4 results in the formation of how many different diols?
Correct answer is '6'. Can you explain this answer?
If 3, 3-dimethyl-2, 4-pentanedione is treated with a Grignard reagent consisting of mixture of CH3MgBr and C2H5MgBr and finally hydrolysing product with dilute H2SO4 results in the formation of how many different diols?
|
|
Om Desai answered |
(II) has two chiral carbon but symmetrical, hence, three stereoisomers.
(III) has only one chiral carbon hence, two stereoisomers (pair of enantiomers).
Phenol is less acidic than ______________.
- a)o – nitrophenol
- b)o – methoxyphenol
- c)ethanol
- d)o – methylphenol
Correct answer is option 'A'. Can you explain this answer?
Phenol is less acidic than ______________.
a)
o – nitrophenol
b)
o – methoxyphenol
c)
ethanol
d)
o – methylphenol

|
Samridhi Bajaj answered |
- Phenol is not as acidic as O-nitrophenol.
- The nitro group is a type of group that pulls electrons away.
- This pulling of electrons reduces the electron density on the benzene ring and spreads out the negative charge of the phenoxide ion.
- Because of this, the acidity increases, making O-nitrophenol more acidic than phenol.
- In contrast, the methoxy group is a group that releases electrons.
- This release of electrons raises the electron density on the benzene ring and does not spread out the negative charge of the phenoxide ion.
- As a result, p-methoxyphenol is less acidic than phenol.
- Additionally, alcohols, such as 2-methylpropanol and 2,2-dimethylpentanol, are considered neutral.
Ketones are reduced to the corresponding alcohols by catalytic hydrogenation to form- a)secondary alcohols
- b)primary alcohols
- c)tertiary alcohols
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Ketones are reduced to the corresponding alcohols by catalytic hydrogenation to form
a)
secondary alcohols
b)
primary alcohols
c)
tertiary alcohols
d)
None of these
|
|
Pooja Mehta answered |
Aldehydes and ketones can be reduced catalytically to form alcohols. When aldehydes and ketones are heated with hydrogen gas in presence of raney Nickel as a catalyst, then primary and secondary alcohols are obtained respectively .Reaction is carried out at high temperature of about 413 Kelvin.
The reaction C2H5ONa + C2H5I → C2H5OCH5 + NaI is known as
- a)Williamson's synthesis
- b)Grignard's synthesis
- c)Wurtz's synthesis
- d)Kolbe's synthesis
Correct answer is option 'A'. Can you explain this answer?
The reaction C2H5ONa + C2H5I → C2H5OCH5 + NaI is known as
a)
Williamson's synthesis
b)
Grignard's synthesis
c)
Wurtz's synthesis
d)
Kolbe's synthesis
|
|
Nabanita Pillai answered |
The reaction between C2H5ONa (sodium ethoxide) and C2H5I (ethyl iodide) is a nucleophilic substitution reaction.
In this reaction, the sodium ethoxide (C2H5ONa) acts as a strong nucleophile, while the ethyl iodide (C2H5I) acts as an alkyl halide.
The reaction can be represented as follows:
C2H5ONa + C2H5I → C2H5OC2H5 + NaI
Here, the sodium ethoxide attacks the carbon of the ethyl iodide, displacing the iodide ion. This results in the formation of diethyl ether (C2H5OC2H5) and sodium iodide (NaI) as a byproduct.
This reaction is an example of an SN2 (substitution nucleophilic bimolecular) reaction, where the nucleophile (sodium ethoxide) and the alkyl halide (ethyl iodide) are involved in the rate-determining step. The reaction proceeds through a one-step mechanism, where the nucleophile directly displaces the leaving group (iodide ion) from the alkyl halide, resulting in the formation of the desired product.
In this reaction, the sodium ethoxide (C2H5ONa) acts as a strong nucleophile, while the ethyl iodide (C2H5I) acts as an alkyl halide.
The reaction can be represented as follows:
C2H5ONa + C2H5I → C2H5OC2H5 + NaI
Here, the sodium ethoxide attacks the carbon of the ethyl iodide, displacing the iodide ion. This results in the formation of diethyl ether (C2H5OC2H5) and sodium iodide (NaI) as a byproduct.
This reaction is an example of an SN2 (substitution nucleophilic bimolecular) reaction, where the nucleophile (sodium ethoxide) and the alkyl halide (ethyl iodide) are involved in the rate-determining step. The reaction proceeds through a one-step mechanism, where the nucleophile directly displaces the leaving group (iodide ion) from the alkyl halide, resulting in the formation of the desired product.
Primary alcohols are prepared by reduction of carboxylic acids. Though lithium aluminium hydride is a strong reducing agent, it is not used in the reaction. Because
- a)it is an expensive reagent
- b)only used for secondary and tertiary alcohols
- c)yield is low
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Primary alcohols are prepared by reduction of carboxylic acids. Though lithium aluminium hydride is a strong reducing agent, it is not used in the reaction. Because
a)
it is an expensive reagent
b)
only used for secondary and tertiary alcohols
c)
yield is low
d)
None of these

|
Mehul Choudhary answered |
- Lithium aluminium hydride (LiAlH4) is indeed a strong reducing agent capable of reducing carboxylic acids to primary alcohols.
- Despite its effectiveness, it is not frequently used for this purpose because:
- Cost: LiAlH4 is relatively expensive, making it less economical for large-scale or routine synthesis.
- Handling: It requires careful handling as it reacts violently with water and moisture.
- Therefore, the correct answer is option A: it is an expensive reagent.
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
- a)CrO3 in anhydrous medium..
- b)Heat in the presence of Cu at 573K.
- c)Pyridinium chlorochromate.
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
a)
CrO3 in anhydrous medium..
b)
Heat in the presence of Cu at 573K.
c)
Pyridinium chlorochromate.
d)
All of these

|
Arnab Chavan answered |
- CrO3 in anhydrous medium: This reagent can oxidize primary alcohols to aldehydes in controlled conditions without over-oxidizing to carboxylic acids.
- Heat in the presence of Cu at 573K: Copper can dehydrogenate primary alcohols, forming aldehydes at this temperature.
- Pyridinium chlorochromate (PCC): PCC is well-known for oxidizing primary alcohols to aldehydes without over-oxidizing them to carboxylic acids.
Thus, all these reagents can effectively oxidize primary alcohols to aldehydes, making the correct answer D: All of these.
Direction (Q. Nos. 9-15) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
In the following reaction,
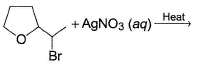 The possible substitution product(s) is/are
The possible substitution product(s) is/are
- a)
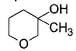
- b)
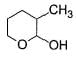
- c)
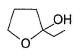
- d)
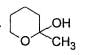
Correct answer is option 'B,C'. Can you explain this answer?
Direction (Q. Nos. 9-15) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
In the following reaction,
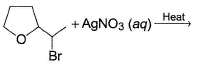
The possible substitution product(s) is/are
a)

b)

c)

d)


|
Yash Kumar answered |
It is a SN 1 reaction.
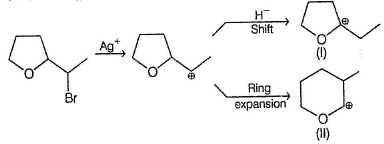
(I) and (II) undergo nucleophilic attack by H2O giving the desired products.
(I) and (II) undergo nucleophilic attack by H2O giving the desired products.
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q, Which of the following pairs of compounds can be used as starting material in the synthesis of 2-phenyl-2-pentanol?- a)CH3— (CH2)2— CH2Br and C6H5COOH
- b)(CH3)2CHCH2Br and PhCOCH3
- c)PhBr and CH3CH2CH2COCH3
- d)PhBr and (CH3)2CHCH2COCH3
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q,
Which of the following pairs of compounds can be used as starting material in the synthesis of 2-phenyl-2-pentanol?
a)
CH3— (CH2)2— CH2Br and C6H5COOH
b)
(CH3)2CHCH2Br and PhCOCH3
c)
PhBr and CH3CH2CH2COCH3
d)
PhBr and (CH3)2CHCH2COCH3

|
Srishti Kaur answered |
Answer:
Introduction:
The synthesis of 2-phenyl-2-pentanol requires starting materials that can provide the necessary functional groups and carbon skeleton for the desired product. In this case, we need a compound that can provide a phenyl group (C6H5) and a pentyl group (CH3CH2CH2CH2) connected by an alcohol functional group (-OH). Let's analyze each option to determine which one can be used as a starting material in the synthesis of 2-phenyl-2-pentanol.
Options Analysis:
a) CH3(CH2)2CH2Br and C6H5COOH:
- CH3(CH2)2CH2Br: This compound does not contain a phenyl group or a pentyl group, so it cannot be used as a starting material for the synthesis of 2-phenyl-2-pentanol.
- C6H5COOH: This compound contains a phenyl group, but it does not contain a pentyl group. It is an acid (carboxylic acid), which is not suitable for the synthesis of 2-phenyl-2-pentanol.
b) (CH3)2CHCH2Br and PhCOCH3:
- (CH3)2CHCH2Br: This compound does not contain a phenyl group but contains a pentyl group. However, it lacks the hydroxyl (-OH) functional group required for the synthesis of 2-phenyl-2-pentanol.
- PhCOCH3: This compound contains a phenyl group but does not contain a pentyl group. It also lacks the hydroxyl (-OH) functional group required for the synthesis of 2-phenyl-2-pentanol.
c) PhBr and CH3CH2CH2COCH3:
- PhBr: This compound contains a phenyl group, which is one of the required functional groups for the synthesis of 2-phenyl-2-pentanol.
- CH3CH2CH2COCH3: This compound contains a pentyl group and a carbonyl functional group (C=O). The carbonyl group can be converted to an alcohol (-OH) group through reduction, which is suitable for the synthesis of 2-phenyl-2-pentanol.
d) PhBr and (CH3)2CHCH2COCH3:
- PhBr: This compound contains a phenyl group, which is one of the required functional groups for the synthesis of 2-phenyl-2-pentanol.
- (CH3)2CHCH2COCH3: This compound does not contain a pentyl group but contains a carbonyl functional group (C=O). Similar to option (c), the carbonyl group can be converted to an alcohol (-OH) group through reduction, which is suitable for the synthesis of 2-phenyl-2-pentanol.
Conclusion:
Among the given options, only option (c) - PhBr and CH3CH2CH2COCH3 - provides both the phenyl group and the pentyl group required for the synthesis of 2-phenyl-2-pentanol. Therefore, option (c) is the correct choice for the
Introduction:
The synthesis of 2-phenyl-2-pentanol requires starting materials that can provide the necessary functional groups and carbon skeleton for the desired product. In this case, we need a compound that can provide a phenyl group (C6H5) and a pentyl group (CH3CH2CH2CH2) connected by an alcohol functional group (-OH). Let's analyze each option to determine which one can be used as a starting material in the synthesis of 2-phenyl-2-pentanol.
Options Analysis:
a) CH3(CH2)2CH2Br and C6H5COOH:
- CH3(CH2)2CH2Br: This compound does not contain a phenyl group or a pentyl group, so it cannot be used as a starting material for the synthesis of 2-phenyl-2-pentanol.
- C6H5COOH: This compound contains a phenyl group, but it does not contain a pentyl group. It is an acid (carboxylic acid), which is not suitable for the synthesis of 2-phenyl-2-pentanol.
b) (CH3)2CHCH2Br and PhCOCH3:
- (CH3)2CHCH2Br: This compound does not contain a phenyl group but contains a pentyl group. However, it lacks the hydroxyl (-OH) functional group required for the synthesis of 2-phenyl-2-pentanol.
- PhCOCH3: This compound contains a phenyl group but does not contain a pentyl group. It also lacks the hydroxyl (-OH) functional group required for the synthesis of 2-phenyl-2-pentanol.
c) PhBr and CH3CH2CH2COCH3:
- PhBr: This compound contains a phenyl group, which is one of the required functional groups for the synthesis of 2-phenyl-2-pentanol.
- CH3CH2CH2COCH3: This compound contains a pentyl group and a carbonyl functional group (C=O). The carbonyl group can be converted to an alcohol (-OH) group through reduction, which is suitable for the synthesis of 2-phenyl-2-pentanol.
d) PhBr and (CH3)2CHCH2COCH3:
- PhBr: This compound contains a phenyl group, which is one of the required functional groups for the synthesis of 2-phenyl-2-pentanol.
- (CH3)2CHCH2COCH3: This compound does not contain a pentyl group but contains a carbonyl functional group (C=O). Similar to option (c), the carbonyl group can be converted to an alcohol (-OH) group through reduction, which is suitable for the synthesis of 2-phenyl-2-pentanol.
Conclusion:
Among the given options, only option (c) - PhBr and CH3CH2CH2COCH3 - provides both the phenyl group and the pentyl group required for the synthesis of 2-phenyl-2-pentanol. Therefore, option (c) is the correct choice for the
Ketones react with Grignard reagent to produce- a)secondary alcohols
- b)tertiary alcohols
- c)primary alcohols
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Ketones react with Grignard reagent to produce
a)
secondary alcohols
b)
tertiary alcohols
c)
primary alcohols
d)
None of the above
|
|
Anjana Sharma answered |
The Grignard reaction with ketones is very similar to the reaction with aldehydes, except we end up with a tertiary alcohol instead of a secondary alcohol. A tertiary alcohol is when the carbon that it is attached to is not attached to any hydrogens (the rest of the bonds are filled with carbons). A secondary alcohol is when the carbon that the alcohol is attached to is attached to only one hydrogen. Then there are also primary alcohols where the carbon is attached to two hydrogens.
Comprehension TypeDirection (Q. Nos. 16-18) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageConsider the following sequence of reaction,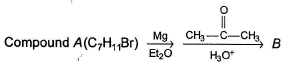
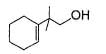
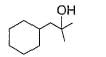
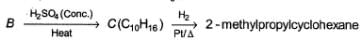 Q. The structure of compound B is
Q. The structure of compound B is- a)
- b)
- c)
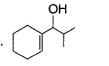
- d)
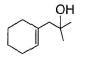
Correct answer is option 'D'. Can you explain this answer?
Comprehension Type
Direction (Q. Nos. 16-18) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Consider the following sequence of reaction,
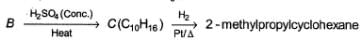
Q.
The structure of compound B is
a)

b)

c)

d)


|
Amar Jain answered |
B is an alcohol formed by the attack of Grignard's reagent on acetone. Hence, alcohol must have the skeleton
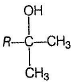
Also, R is C7H11 with two degree of unsaturation it must be cyclohexenyl methyl not cyclohexyl methyl.
Also, R is C7H11 with two degree of unsaturation it must be cyclohexenyl methyl not cyclohexyl methyl.
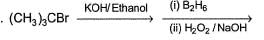
Primary alcohol can easily be prepared from primary alkyl halide via SN2 reaction with aqueous NaOH. However, similar method does not work for the preparation of tertiary alcohol. Which reaction can be used for the efficient preparation of tertiary alcohol {tertiary butanol) from tertiary butyl bromide?- a)
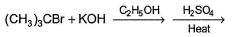
- b)
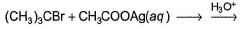
- c)
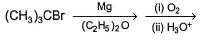
- d)
Correct answer is option 'B'. Can you explain this answer?
Primary alcohol can easily be prepared from primary alkyl halide via SN2 reaction with aqueous NaOH. However, similar method does not work for the preparation of tertiary alcohol. Which reaction can be used for the efficient preparation of tertiary alcohol {tertiary butanol) from tertiary butyl bromide?
a)
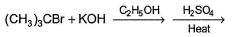
b)
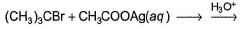
c)
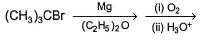
d)


|
Chirag Banerjee answered |
Chapter doubts & questions for MCQ Corner - NCERT on your Fingertips 2025-2026 Edition 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of MCQ Corner - NCERT on your Fingertips 2025-2026 Edition in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup