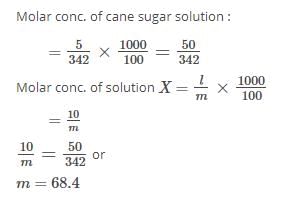All Exams >
NEET >
Chemistry 31 Years NEET Chapterwise Solved Papers >
All Questions
All questions of Solutions for NEET Exam
1 M, 2.5 litre NaOH solution is mixed with another 0.5 M, 3 litre NaOH solution. Then find out the molarity of resultant solution [2002]- a)0.80 M
- b)1.0 M
- c)0.73 M
- d)0.50 M
Correct answer is option 'C'. Can you explain this answer?
1 M, 2.5 litre NaOH solution is mixed with another 0.5 M, 3 litre NaOH solution. Then find out the molarity of resultant solution [2002]
a)
0.80 M
b)
1.0 M
c)
0.73 M
d)
0.50 M
|
|
User5881746 answered |
M = M1V1+M2V2 / V1+V2. M = 1*2.5+0.5*3 / 2.5+3.
M = 4 / 5.5. M = 0.73
M = 4 / 5.5. M = 0.73
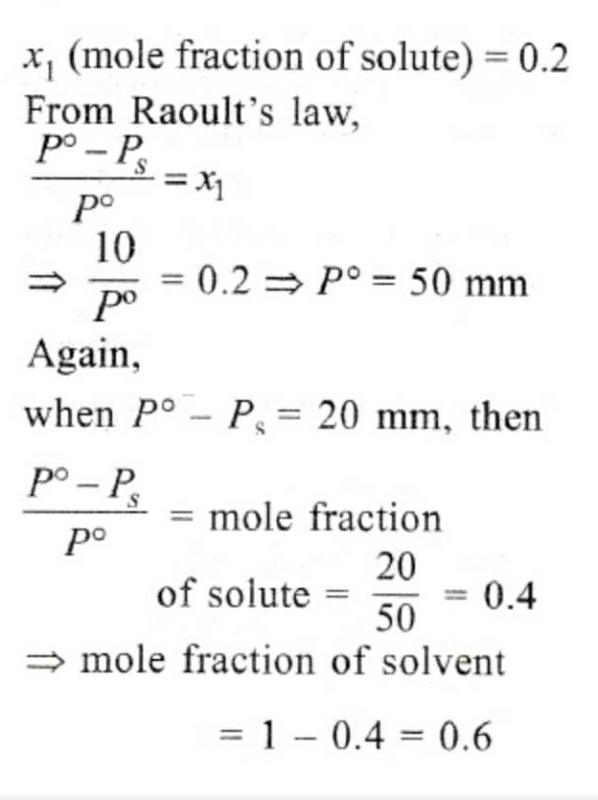
The vapour pressure of a solvent decreased by 10mm of mercury when a non-volatile solute was added to the solvent. The mole fraction of the solute in the solution is 0.2. What should be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20mm of mercury?- a)0.8
- b)0.6 [1998]
- c)0.4
- d)0.2
Correct answer is option 'B'. Can you explain this answer?
The vapour pressure of a solvent decreased by 10mm of mercury when a non-volatile solute was added to the solvent. The mole fraction of the solute in the solution is 0.2. What should be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20mm of mercury?
a)
0.8
b)
0.6 [1998]
c)
0.4
d)
0.2
|
|
Rocky Handsome answered |
All form ideal solution except [1988]- a)C6H6 and C6H5 CH3
- b)C2H6 and C2H5I
- c)C6H5Cl and C6H5 Br
- d)C2H5 I and C2H5 OH.
Correct answer is option 'D'. Can you explain this answer?
All form ideal solution except [1988]
a)
C6H6 and C6H5 CH3
b)
C2H6 and C2H5I
c)
C6H5Cl and C6H5 Br
d)
C2H5 I and C2H5 OH.
|
|
Ananya Das answered |
C2H5I and C2H5OH form non-ideal solution.
Which of the following aqueous solution has minimum freezing point ? [1991]- a)0.01 m NaCl
- b)0.005 m C2H5OH
- c)0.005 m MgI2
- d)0.005 m MgSO4.
Correct answer is option 'A'. Can you explain this answer?
Which of the following aqueous solution has minimum freezing point ? [1991]
a)
0.01 m NaCl
b)
0.005 m C2H5OH
c)
0.005 m MgI2
d)
0.005 m MgSO4.

|
Swara Desai answered |
ΔTf = i × Kf × m Van't Hoff factor, i = 2 for NaCl, hence ΔTf = 0.02 Kf which is maximum in the present case.
Hence ΔTf is maximum or freezing point is minimum.
Hence ΔTf is maximum or freezing point is minimum.
Vapour pressure of benzene at 30°C is 121.8 mm.When 15 g of a non volatile solute is dissolved in 250 g of benzene its vapour pressure decreased to 120.2 mm. The molecular weight of the solute (Mo. wt. of solvent = 78) [1995]- a)356.2
- b)456.8
- c)530.1
- d)656.7
Correct answer is option 'A'. Can you explain this answer?
Vapour pressure of benzene at 30°C is 121.8 mm.When 15 g of a non volatile solute is dissolved in 250 g of benzene its vapour pressure decreased to 120.2 mm. The molecular weight of the solute (Mo. wt. of solvent = 78) [1995]
a)
356.2
b)
456.8
c)
530.1
d)
656.7

|
Ruchi Chakraborty answered |
Give vapour pressure of pur e solute (P0) = 121.8 mm;
Weight of solute (w) = 15 g
Weight of solvent (W) = 250 g;
Vapour pressure of pure solvent (P) = 120.2 mm and Molecular weight of solvent (M) = 78 From Raoult’s law
Weight of solute (w) = 15 g
Weight of solvent (W) = 250 g;
Vapour pressure of pure solvent (P) = 120.2 mm and Molecular weight of solvent (M) = 78 From Raoult’s law
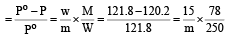
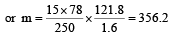
During osmosis, flow of water through a semi-permeable membrane [2006]
- a)from both sides of semipermeable membrane with equal flow rates
- b)from solution having lower concentration only
- c)from both sides of semipermeable membrane with unequal flow rates
- d)from solution having higher concentration only
Correct answer is option 'C'. Can you explain this answer?
During osmosis, flow of water through a semi-permeable membrane [2006]
a)
from both sides of semipermeable membrane with equal flow rates
b)
from solution having lower concentration only
c)
from both sides of semipermeable membrane with unequal flow rates
d)
from solution having higher concentration only

|
Anjana Dasgupta answered |
During osmosis water flows through semipermeable membrane from lower concnetration to higher concentration.
According to Raoult's law, relative lowering of vapour pressure for a solution is equal to [1995- a)moles of solute
- b)moles of solvent
- c)mole fraction of solute
- d)mole fraction of solvent
Correct answer is option 'C'. Can you explain this answer?
According to Raoult's law, relative lowering of vapour pressure for a solution is equal to [1995
a)
moles of solute
b)
moles of solvent
c)
mole fraction of solute
d)
mole fraction of solvent
|
|
Rocky Handsome answered |
Since relative lowering of vapour pressure is a colligative property, therefore it depends upon the number of solute particles or mole fraction of solute.
How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0M HNO3 ? The concentrated acid is 70% HNO3 [NEET 2013]- a)90.0 g conc. HNO3
- b)70.0 g conc. HNO3
- c)54.0 g conc. HNO3
- d)45.0 g conc. HNO3
Correct answer is option 'D'. Can you explain this answer?
How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0M HNO3 ? The concentrated acid is 70% HNO3 [NEET 2013]
a)
90.0 g conc. HNO3
b)
70.0 g conc. HNO3
c)
54.0 g conc. HNO3
d)
45.0 g conc. HNO3

|
Mamali . answered |
The required number of Moles =(250/1000)2=0•5 moles
(no of Moles = molarity×vol.)
so reqd.mass of HNO3 =0•5×63
=31•5
given mass = no of moles × molar mass
Given,
70gms of HNO3 are present in 100 gms of the Sol.
so,1gm will be present in 100/70 gms of sol.
hense, 31•5 gms will be present in
100/70 × 31•5 gms of sol.
so amount of concentrated nitric acid solution used is 45 gms.
(no of Moles = molarity×vol.)
so reqd.mass of HNO3 =0•5×63
=31•5
given mass = no of moles × molar mass
Given,
70gms of HNO3 are present in 100 gms of the Sol.
so,1gm will be present in 100/70 gms of sol.
hense, 31•5 gms will be present in
100/70 × 31•5 gms of sol.
so amount of concentrated nitric acid solution used is 45 gms.
The vapour pressure at a given temperature of an ideal solution containing 0.2 mol of a nonvolatile solute and 0.8 mol of solvent is 60 mm of Hg. The vapour pressure of the pure solvent at the same temperature is [1996]- a)150 mm of Hg
- b)60 mm of Hg
- c)75 mm of Hg
- d)120 mm of Hg
Correct answer is option 'C'. Can you explain this answer?
The vapour pressure at a given temperature of an ideal solution containing 0.2 mol of a nonvolatile solute and 0.8 mol of solvent is 60 mm of Hg. The vapour pressure of the pure solvent at the same temperature is [1996]
a)
150 mm of Hg
b)
60 mm of Hg
c)
75 mm of Hg
d)
120 mm of Hg
|
|
Hansa Sharma answered |
According to Raoult's law
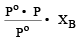
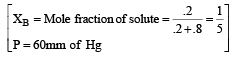
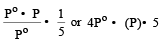
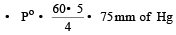
The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]- a)72 torr
- b)140 torr
- c)68 torr
- d)20 torr
Correct answer is option 'A'. Can you explain this answer?
The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]
a)
72 torr
b)
140 torr
c)
68 torr
d)
20 torr

|
Shivani Rane answered |
Given V.PP = 80 torr V.PQ = 60 torr
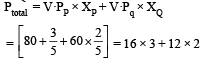
Ptotal = 48 + 24 = 72 torr
A solution of acetone in ethanol [2006]- a)shows a positive deviation from Raoult’s law
- b)behaves like a non ideal solution
- c)obeys Raoult’s law
- d)shows a negative deviation from Raoult’s law
Correct answer is option 'A'. Can you explain this answer?
A solution of acetone in ethanol [2006]
a)
shows a positive deviation from Raoult’s law
b)
behaves like a non ideal solution
c)
obeys Raoult’s law
d)
shows a negative deviation from Raoult’s law

|
Shanaya Rane answered |
A solution of acetone in ethanol shows positive deviation from Raoult's law. It is because ethanol molecules are strongly hydrogen bonded. When acetone is added, these molecules break the hydrogen bonds and ethanol becomes more volatile. Therefore its vapour pressure is increased.
The number of moles of oxygen in one litre of air containing 21% oxygen by volume, in standard conditions, is [1995]- a)0.186
- b)0.21
- c)0.0093
- d)2.10
Correct answer is option 'C'. Can you explain this answer?
The number of moles of oxygen in one litre of air containing 21% oxygen by volume, in standard conditions, is [1995]
a)
0.186
b)
0.21
c)
0.0093
d)
2.10

|
Isha Kar answered |
1 litre =0.21 L Oxygen
We know that, 22.4 L=1 mol of Oxygen
So in 0.21L there will be 0.21/22.4 mols.
=0.0093 mol
The van’t Hoff factor i for a compound which undergoes dissociation in one solvent and association in other solvent is respectively : [2011]- a)less than one and greater than one.
- b)less than one and less than one.
- c)greater than one and less than one.
- d)greater than one and greater than one.
Correct answer is option 'C'. Can you explain this answer?
The van’t Hoff factor i for a compound which undergoes dissociation in one solvent and association in other solvent is respectively : [2011]
a)
less than one and greater than one.
b)
less than one and less than one.
c)
greater than one and less than one.
d)
greater than one and greater than one.

|
Moumita Khanna answered |
If compound dissociates in solvent i > 1 and on association i < 1.
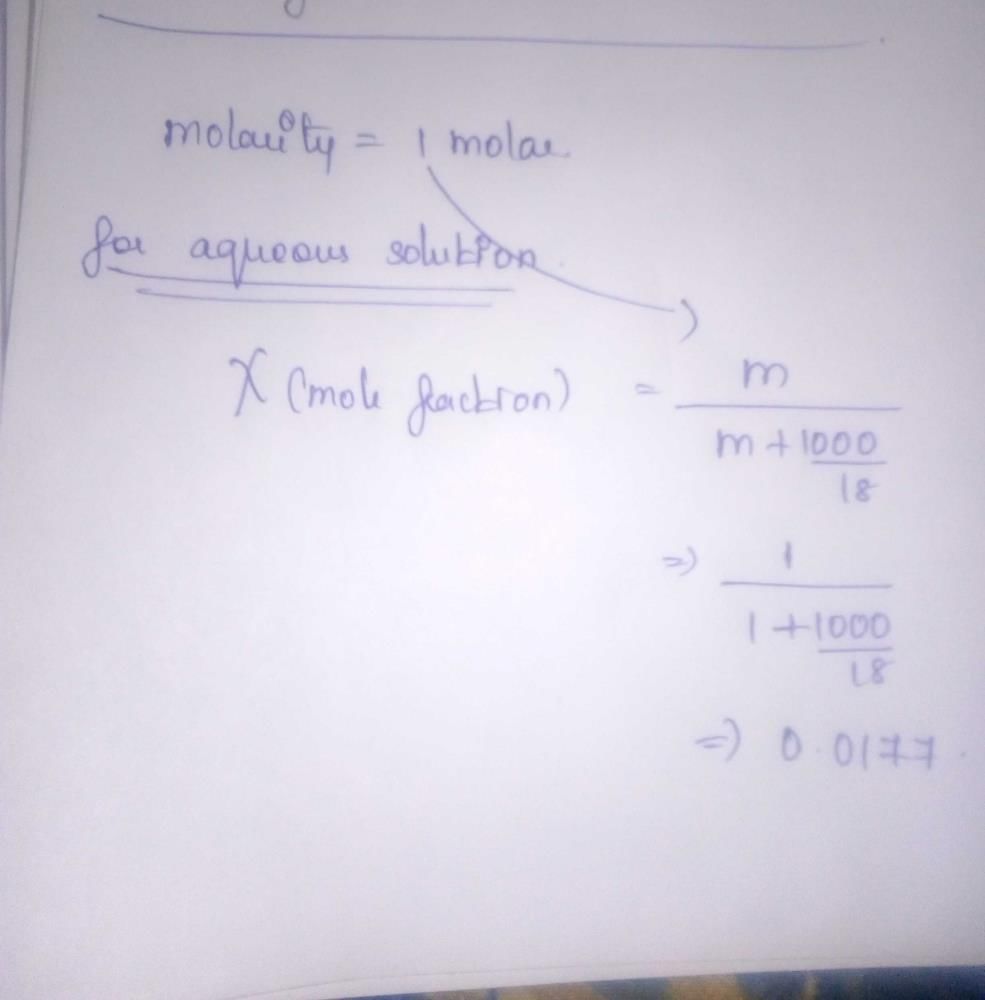
The mole fraction of the solute in one molal aqueous solution is: [2 00 5]- a)0.009
- b)0.018
- c)0.027
- d)0.036
Correct answer is option 'B'. Can you explain this answer?
The mole fraction of the solute in one molal aqueous solution is: [2 00 5]
a)
0.009
b)
0.018
c)
0.027
d)
0.036

|
Aashna Mukherjee answered |
One molal solution means one mole solute present in 1 kg (1000 g) solvent i.e., mole of solute = 1
Mole of solvent 

Mole fraction of solute = 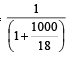
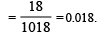
The beans are cooked earlier in pressure cooker, because [2001]- a)Boiling point in creases with in creasing pressure
- b)Boiling point decreases with in creasing pressure
- c)Internal energy is not lost while cooking in pressure cooker
- d)Extra pressure of pressure cooker, softens the beans
Correct answer is option 'A'. Can you explain this answer?
The beans are cooked earlier in pressure cooker, because [2001]
a)
Boiling point in creases with in creasing pressure
b)
Boiling point decreases with in creasing pressure
c)
Internal energy is not lost while cooking in pressure cooker
d)
Extra pressure of pressure cooker, softens the beans

|
Rajeev Sharma answered |
The beans are cooked earlier in pressure cooker because boiling point increases with increasing pressure.
Which of the following modes of expressing concentration is independent of temperature ? [1992,1995]- a)Molarity
- b)Molality
- c)Formality
- d)Normality
Correct answer is option 'B'. Can you explain this answer?
Which of the following modes of expressing concentration is independent of temperature ? [1992,1995]
a)
Molarity
b)
Molality
c)
Formality
d)
Normality
|
|
Rocky Handsome answered |
Molality involved a fixed mass of solvent.Hence,this is independent of temperature.
Camphoris often used in molecular mass determination because [2004]- a)it is readily available
- b)it has a very high cryoscopic constant
- c)it is volatile
- d)it is solvent for organic substances
Correct answer is option 'B'. Can you explain this answer?
Camphoris often used in molecular mass determination because [2004]
a)
it is readily available
b)
it has a very high cryoscopic constant
c)
it is volatile
d)
it is solvent for organic substances

|
Dipika Das answered |
Solvent having high cryoscopic constant can be used in determination of molecular mass by cryoscopic method.
Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25ºC are 200 mm Hg and 415 mm Hg respectively. Vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40 g of CH2Cl2 at the same temperature will be : (Molecular mass of CHCl3 = 119.5 u and molecular mass of CH2Cl2 = 85 u). [2012 M]
- a)173.9 mm Hg
- b)615.0 mm Hg
- c)347.9 mm Hg
- d)285.5 mm Hg
Correct answer is option 'C'. Can you explain this answer?
Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25ºC are 200 mm Hg and 415 mm Hg respectively. Vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40 g of CH2Cl2 at the same temperature will be : (Molecular mass of CHCl3 = 119.5 u and molecular mass of CH2Cl2 = 85 u). [2012 M]
a)
173.9 mm Hg
b)
615.0 mm Hg
c)
347.9 mm Hg
d)
285.5 mm Hg
|
|
Naina Datta answered |
The correct answer is option C
Molar mass of CH2Cl2 =12×1+1×2+35.5×2=85 g mol–1
Molar mass of CHCl3 =12×1+1×1+35.5×3=119.5 g mol−1
Moles of CH2Cl2= 40 g /85 g mol−1 = 0.47 mol
Moles of CHCl3 = 25.5 g /119.5 g mol−1 = 0.213 mol
Total number of moles = 0.47+0.213=0.683 mol
Mole fraction of component 2
=0.47mol/0.683mol= 0.688
Mole fraction of component 1
=1.00–0.688=0.312
We know that:
PT=p10+(p20−p10)x2
=200+(415–200)×0.688
=200+147.9
=347.9 mm Hg
Molar mass of CH2Cl2 =12×1+1×2+35.5×2=85 g mol–1
Molar mass of CHCl3 =12×1+1×1+35.5×3=119.5 g mol−1
Moles of CH2Cl2= 40 g /85 g mol−1 = 0.47 mol
Moles of CHCl3 = 25.5 g /119.5 g mol−1 = 0.213 mol
Total number of moles = 0.47+0.213=0.683 mol
Mole fraction of component 2
=0.47mol/0.683mol= 0.688
Mole fraction of component 1
=1.00–0.688=0.312
We know that:
PT=p10+(p20−p10)x2
=200+(415–200)×0.688
=200+147.9
=347.9 mm Hg
A solution containing 10 g per dm 3 of urea (molecular mass = 60 g mol–1) is isotonic with a 5% solution of a non-volatile solute. The molecular mass of this nonvolatile solute is [2006]- a)300 g mol–1
- b)350 g mol–1
- c)200 g mol–1
- d)250 g mol–1
Correct answer is option 'A'. Can you explain this answer?
A solution containing 10 g per dm 3 of urea (molecular mass = 60 g mol–1) is isotonic with a 5% solution of a non-volatile solute. The molecular mass of this nonvolatile solute is [2006]
a)
300 g mol–1
b)
350 g mol–1
c)
200 g mol–1
d)
250 g mol–1

|
Rajat Roy answered |
Osmotic pressure of urea from the formula
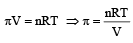
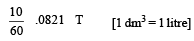
5% solution means
100 ml 5gm
1000 ml 50 g/L
Osmotic pressure of solution having nonvolatile solute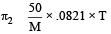
100 ml 5gm
1000 ml 50 g/L
Osmotic pressure of solution having nonvolatile solute
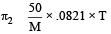
For isotonic solution, π1=π2
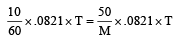
M = 300 gm mol–1
0.5 molal aqueous solution of a weak acid (HX) is 20% ionised. If Kf for water is 1.86 K kg mol– 1,the lowering in freezing point of the solution is- a)0.56 K
- b)1.12 K [2007]
- c)– 0.56 K
- d)– 1.12 K
Correct answer is option 'B'. Can you explain this answer?
0.5 molal aqueous solution of a weak acid (HX) is 20% ionised. If Kf for water is 1.86 K kg mol– 1,the lowering in freezing point of the solution is
a)
0.56 K
b)
1.12 K [2007]
c)
– 0.56 K
d)
– 1.12 K

|
Kunal Rane answered |
As ΔTf = iKfm
For
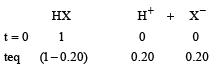
Total no. of moles = 1 – 0.20 + 0.20 + 0.20 = 1 + 0.20 = 1.2
∴ ΔTf = 1.2 × 1.86 × 0.5 = 1.1160 ≈ 1.12 K
∴ ΔTf = 1.2 × 1.86 × 0.5 = 1.1160 ≈ 1.12 K
Blood cells retain their normal shape in solution which are [1991]- a)hypotonic to blood
- b)isotonic to blood
- c)hypertonic to blood
- d)equinormal to blood.
Correct answer is option 'B'. Can you explain this answer?
Blood cells retain their normal shape in solution which are [1991]
a)
hypotonic to blood
b)
isotonic to blood
c)
hypertonic to blood
d)
equinormal to blood.
|
|
Rocky Handsome answered |
In isotonic solutions blood cells retain their normal size and shape.
If 0.1 M solution of glucose and 0.1 M solution of urea are placed on two sides of the semipermeable membrane to equal heights, then it will be correct to say that [1992]- a)There will be no net movement across the membrane
- b)Glucose will flow towards urea solution
- c)urea will flow towards glucose solution
- d)water will flow from urea solution to glucose
Correct answer is option 'A'. Can you explain this answer?
If 0.1 M solution of glucose and 0.1 M solution of urea are placed on two sides of the semipermeable membrane to equal heights, then it will be correct to say that [1992]
a)
There will be no net movement across the membrane
b)
Glucose will flow towards urea solution
c)
urea will flow towards glucose solution
d)
water will flow from urea solution to glucose
|
|
Yash Modi answered |
The van't Hoff factor for both glucose and urea is 1 and the given concentrations are also same. since π=CRT , The osmotic pressure of both the solutions is same. Hence none of the solutions will move across the membrane.
Which one of the following salts will have the same value of van’t Hoff factor (i) as that of K4[Fe (CN)6]. [1994]- a)Al2(SO4)3
- b)NaCl
- c)Al (NO3)3
- d)Na2SO4.
Correct answer is option 'A'. Can you explain this answer?
Which one of the following salts will have the same value of van’t Hoff factor (i) as that of K4[Fe (CN)6]. [1994]
a)
Al2(SO4)3
b)
NaCl
c)
Al (NO3)3
d)
Na2SO4.

|
Arya Nair answered |
K4 [Fe(CN)6] and Al2(SO4)3 both dissociates to give 5 ions or i = 5

and
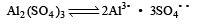
A 0. 00 20 m aqueous solution of an ionic compound Co(NH3)5(NO2)Cl freezes at – 0.00732 °C. Number of moles of ions which 1 mol of ionic compound produces on being dissolved in water will be (Kf = – 1.86°C/m)- a)3
- b)4 [2009]
- c)1
- d)2
Correct answer is option 'D'. Can you explain this answer?
A 0. 00 20 m aqueous solution of an ionic compound Co(NH3)5(NO2)Cl freezes at – 0.00732 °C. Number of moles of ions which 1 mol of ionic compound produces on being dissolved in water will be (Kf = – 1.86°C/m)
a)
3
b)
4 [2009]
c)
1
d)
2

|
Arya Nair answered |
ΔTf = 0 – (0.00732°) = 0.00732
ΔTf = i × Kf × m
ΔTf = i × Kf × m
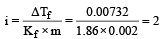
A solution of urea (m ol. mass 56 g mol-1) boils at 100.18°C at the atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol-1 respectively, the above solution will freeze at [2005]- a)0.654°C
- b)-0.654°C
- c)6.54°C
- d)-6.54°C
Correct answer is option 'B'. Can you explain this answer?
A solution of urea (m ol. mass 56 g mol-1) boils at 100.18°C at the atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol-1 respectively, the above solution will freeze at [2005]
a)
0.654°C
b)
-0.654°C
c)
6.54°C
d)
-6.54°C

|
Shruti Chauhan answered |
As ΔTf = Kf. m
ΔTb = Kb. m
ΔTb = Kb. m
Hence, we have 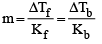
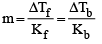
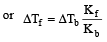
= [ΔTb = 100.18 - 100 = 0.18°C]
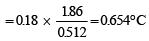
As the Freezing Point of pure water is 0°C,
ΔTf = 0 –Tf
0.654 = 0 – Tf
∴ Tf = – 0.654 thus the freezing point of solution will be – 0.654°C.
ΔTf = 0 –Tf
0.654 = 0 – Tf
∴ Tf = – 0.654 thus the freezing point of solution will be – 0.654°C.
At 25°C, the highest osmotic pressure is exhibited by 0.1 M solution of [1994]- a)CaCl2
- b)KCl
- c)Glucose
- d)Urea.
Correct answer is option 'A'. Can you explain this answer?
At 25°C, the highest osmotic pressure is exhibited by 0.1 M solution of [1994]
a)
CaCl2
b)
KCl
c)
Glucose
d)
Urea.

|
Smruti Sucharita answered |
Highest osmotic pressure depends on only i value... Bcoz temp and concentration r same....
For cacl2 i=3
Kcl=2
Glucose =1
Urea =1
So as i is more for cacl2.. It has more osmotic pressure
(i is the vant Hoff s factor..)
For cacl2 i=3
Kcl=2
Glucose =1
Urea =1
So as i is more for cacl2.. It has more osmotic pressure
(i is the vant Hoff s factor..)
A solution of sucrose (molar mass = 342 g mol–1) has been prepared by dissolving 68.5 g of sucrose in 1000 g of water. The freezing point of the solution obtained will be ( f for water = 1.86 K kg mol–1). [2010]- a)– 0.372°C
- b)– 0.520°C
- c)+ 0.372°C
- d)– 0.570°C
Correct answer is option 'A'. Can you explain this answer?
A solution of sucrose (molar mass = 342 g mol–1) has been prepared by dissolving 68.5 g of sucrose in 1000 g of water. The freezing point of the solution obtained will be ( f for water = 1.86 K kg mol–1). [2010]
a)
– 0.372°C
b)
– 0.520°C
c)
+ 0.372°C
d)
– 0.570°C
|
|
Sanchita Nair answered |
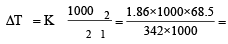 0.372
0.372T = – 0.372°C
2 5. 3 g of sodium carbonate, Na2 CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ions, Na+ and carbonate ions, CO32– are respectively (Molar mass of Na2CO3 = 106 g mol–1) [2010]- a)0.955 M and 1.910 M
- b)1.910 M and 0.955 M
- c)1.90 M and 1.910 M
- d)0.477 M and 0.477 M
Correct answer is option 'B'. Can you explain this answer?
2 5. 3 g of sodium carbonate, Na2 CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ions, Na+ and carbonate ions, CO32– are respectively (Molar mass of Na2CO3 = 106 g mol–1) [2010]
a)
0.955 M and 1.910 M
b)
1.910 M and 0.955 M
c)
1.90 M and 1.910 M
d)
0.477 M and 0.477 M

|
Nilotpal Gupta answered |
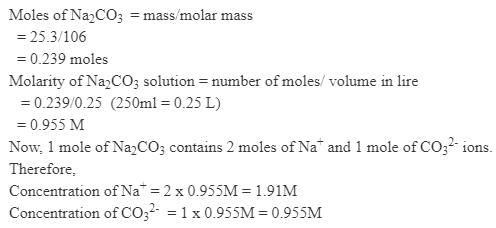
Which of the following statements, regarding the mole fraction (x) of a component in solution, is incorrect? [1999]- a)–2 ≤ x ≤ 2
- b)x ≤ 1
- c)x is always non-negative
- d)0 ≤ x ≤ 1
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements, regarding the mole fraction (x) of a component in solution, is incorrect? [1999]
a)
–2 ≤ x ≤ 2
b)
x ≤ 1
c)
x is always non-negative
d)
0 ≤ x ≤ 1
|
|
Mansi Nair answered |
Mole fraction of any component A

As total no. of moles > no. of moles of A thus x can never be equal to one on zero.
The relative lowering of the vapour pressure is equal to the ratio between the number of [1991]- a)solute molecules to the solvent molecules
- b)solute molecules to the total molecules in the solution
- c)solvent molecules to the total molecules in the solution
- d)solvent molecules to the total number of ions of the solute.
Correct answer is option 'B'. Can you explain this answer?
The relative lowering of the vapour pressure is equal to the ratio between the number of [1991]
a)
solute molecules to the solvent molecules
b)
solute molecules to the total molecules in the solution
c)
solvent molecules to the total molecules in the solution
d)
solvent molecules to the total number of ions of the solute.
|
|
Sankar Banerjee answered |
Relative lowering of vapor pressure is a colligative property, which means it depends on the number of particles in a solution rather than their nature. It is defined as the ratio of the decrease in vapor pressure of a solvent to the vapor pressure of the pure solvent. This property is directly related to the concentration of solute particles in a solution.
Explanation:
Colligative Properties:
- Colligative properties are properties of a solution that depend on the number of solute particles present, rather than the specific type of solute.
- These properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Vapor Pressure:
- Vapor pressure is the pressure exerted by the vapor molecules above the surface of a liquid in a closed container at equilibrium.
- It is a measure of the tendency of molecules to escape from the liquid phase and enter the vapor phase.
Relative Lowering of Vapor Pressure:
- The relative lowering of vapor pressure is defined as the ratio of the decrease in vapor pressure of the solvent in the presence of a non-volatile solute to the vapor pressure of the pure solvent.
- It can be calculated using the formula: Relative Lowering of Vapor Pressure = (P° - P) / P°, where P° is the vapor pressure of the pure solvent and P is the vapor pressure of the solvent in the presence of the solute.
Number of Particles:
- According to Raoult's Law, the vapor pressure of a solvent is directly proportional to the mole fraction of the solvent in the solution.
- When a non-volatile solute is added to the solvent, it lowers the vapor pressure of the solvent.
- The extent of vapor pressure lowering depends on the number of solute particles present in the solution.
Explanation of the Correct Answer:
- The relative lowering of vapor pressure is equal to the ratio between the number of solute molecules to the total molecules in the solution.
- This is because the vapor pressure of the solvent is affected by the presence of solute particles, and the relative lowering of vapor pressure is a measure of this effect.
- The more solute particles there are in the solution, the greater the decrease in vapor pressure of the solvent.
- Therefore, option B is the correct answer as it correctly represents the relationship between the number of solute molecules and the relative lowering of vapor pressure.
In conclusion, the relative lowering of vapor pressure is directly related to the ratio between the number of solute molecules to the total molecules in the solution. This colligative property depends on the concentration of solute particles and is independent of the nature of the solute.
Explanation:
Colligative Properties:
- Colligative properties are properties of a solution that depend on the number of solute particles present, rather than the specific type of solute.
- These properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Vapor Pressure:
- Vapor pressure is the pressure exerted by the vapor molecules above the surface of a liquid in a closed container at equilibrium.
- It is a measure of the tendency of molecules to escape from the liquid phase and enter the vapor phase.
Relative Lowering of Vapor Pressure:
- The relative lowering of vapor pressure is defined as the ratio of the decrease in vapor pressure of the solvent in the presence of a non-volatile solute to the vapor pressure of the pure solvent.
- It can be calculated using the formula: Relative Lowering of Vapor Pressure = (P° - P) / P°, where P° is the vapor pressure of the pure solvent and P is the vapor pressure of the solvent in the presence of the solute.
Number of Particles:
- According to Raoult's Law, the vapor pressure of a solvent is directly proportional to the mole fraction of the solvent in the solution.
- When a non-volatile solute is added to the solvent, it lowers the vapor pressure of the solvent.
- The extent of vapor pressure lowering depends on the number of solute particles present in the solution.
Explanation of the Correct Answer:
- The relative lowering of vapor pressure is equal to the ratio between the number of solute molecules to the total molecules in the solution.
- This is because the vapor pressure of the solvent is affected by the presence of solute particles, and the relative lowering of vapor pressure is a measure of this effect.
- The more solute particles there are in the solution, the greater the decrease in vapor pressure of the solvent.
- Therefore, option B is the correct answer as it correctly represents the relationship between the number of solute molecules and the relative lowering of vapor pressure.
In conclusion, the relative lowering of vapor pressure is directly related to the ratio between the number of solute molecules to the total molecules in the solution. This colligative property depends on the concentration of solute particles and is independent of the nature of the solute.
Which one is a colligative property ? [1992]- a)boiling point
- b)vapour pressure
- c)osmotic pressure
- d)freezing point
Correct answer is option 'C'. Can you explain this answer?
Which one is a colligative property ? [1992]
a)
boiling point
b)
vapour pressure
c)
osmotic pressure
d)
freezing point

|
Anu Bajaj answered |
Osmotic pressure is a colligative property.
A solution containing components A and B follows Raoult's law when [2002]- a)A – B attraction force is greater than A – A and B – B
- b)A – B attraction force is less than A – A and B – B
- c)A – B attraction force remains same as A–A and B –B
- d)Volume of solution is different from sum of volume of solute and solvent
Correct answer is option 'C'. Can you explain this answer?
A solution containing components A and B follows Raoult's law when [2002]
a)
A – B attraction force is greater than A – A and B – B
b)
A – B attraction force is less than A – A and B – B
c)
A – B attraction force remains same as A–A and B –B
d)
Volume of solution is different from sum of volume of solute and solvent

|
Raghav Khanna answered |
Raoult's law is valid for ideal solution only. The element of non - ideality enters into the picture when the molecules of the solute and solvent affect each others intermolecular forces. A solution containing components of A and B behaves as ideal solution when A - B attraction force remains same as A - A and B - B.
Which condition is not satisfied by an ideal solution? [NEET Kar. 2013] - a)Δmix H = 0
- b)Δmix V = 0
- c)Δmix S = 0
- d)Obeyance to Raoult’s Law
Correct answer is option 'C'. Can you explain this answer?
Which condition is not satisfied by an ideal solution? [NEET Kar. 2013]
a)
Δmix H = 0
b)
Δmix V = 0
c)
Δmix S = 0
d)
Obeyance to Raoult’s Law

|
Deepak Joshi answered |
An ideal solution is that solution in which each component obeys Raoult’s law under all conditions of temperatures and concentrations. For an ideal solution.
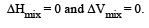
Which of the following colligative property can provide molar mass of proteins (or polymers or colloids) with greatest precision ? [2000]- a)Osmotic pressure
- b)Elevation of boiling point
- c)Depression of freezing point
- d)Relative lowering of vapour pressure
Correct answer is option 'A'. Can you explain this answer?
Which of the following colligative property can provide molar mass of proteins (or polymers or colloids) with greatest precision ? [2000]
a)
Osmotic pressure
b)
Elevation of boiling point
c)
Depression of freezing point
d)
Relative lowering of vapour pressure

|
Krish Khanna answered |
Osmotic pressure is a colligative property which is used to find the molecular weight of polymer.
A 0.1 molal aqueous solution of a weak acid is 30% ionized. If Kf for water is 1.86°C/m, the freezing point of the solution will be : [2011 M]- a)– 0.18°C
- b)– 0.54°C
- c)– 0.36°C
- d)– 0.24°C
Correct answer is option 'D'. Can you explain this answer?
A 0.1 molal aqueous solution of a weak acid is 30% ionized. If Kf for water is 1.86°C/m, the freezing point of the solution will be : [2011 M]
a)
– 0.18°C
b)
– 0.54°C
c)
– 0.36°C
d)
– 0.24°C
|
|
Sahil Menon answered |
To find the percent ionization of a weak acid, we need to use the equation:
% ionization = (concentration of ionized acid)/(initial concentration of weak acid) * 100
Given that the solution is 0.1 molal, we can assume that the initial concentration of the weak acid is also 0.1 molal.
Let x represent the concentration of ionized acid. Therefore, the concentration of the weak acid that remains unionized is (0.1 - x) molal.
The equation for the percent ionization can be written as:
30 = (x)/(0.1) * 100
Simplifying the equation, we have:
0.3 = x/0.1
x = 0.03 molal
Therefore, the concentration of the ionized acid is 0.03 molal.
Now, let's calculate the molality of water in the solution using the equation:
Kf = molality of water * boiling point elevation constant
Given that Kf for water is 1.86, we can rearrange the equation to solve for the molality of water:
molality of water = Kf / boiling point elevation constant
molality of water = 1.86 / 0.512 (boiling point elevation constant for water)
molality of water = 3.63 molal
Therefore, the molality of water in the solution is 3.63 molal.
% ionization = (concentration of ionized acid)/(initial concentration of weak acid) * 100
Given that the solution is 0.1 molal, we can assume that the initial concentration of the weak acid is also 0.1 molal.
Let x represent the concentration of ionized acid. Therefore, the concentration of the weak acid that remains unionized is (0.1 - x) molal.
The equation for the percent ionization can be written as:
30 = (x)/(0.1) * 100
Simplifying the equation, we have:
0.3 = x/0.1
x = 0.03 molal
Therefore, the concentration of the ionized acid is 0.03 molal.
Now, let's calculate the molality of water in the solution using the equation:
Kf = molality of water * boiling point elevation constant
Given that Kf for water is 1.86, we can rearrange the equation to solve for the molality of water:
molality of water = Kf / boiling point elevation constant
molality of water = 1.86 / 0.512 (boiling point elevation constant for water)
molality of water = 3.63 molal
Therefore, the molality of water in the solution is 3.63 molal.
Molarity of liquid HCl will be, if density of solution is 1.17 gm/cc [2001]- a)36.5
- b)32.05
- c)18.25
- d)42.10
Correct answer is option 'B'. Can you explain this answer?
Molarity of liquid HCl will be, if density of solution is 1.17 gm/cc [2001]
a)
36.5
b)
32.05
c)
18.25
d)
42.10

|
Shivani Tiwari answered |
Density = 1.17 gm/cc (Given)
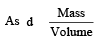
volume = 1cc ∴ mass = d = 1.17g
now molarity 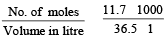
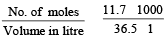
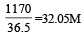
Which of the following 0.10 m aqueous solutions will have the lowest freezing point ? [1997]- a)Al2(SO4)3
- b)C6H12O6
- c)KCl
- d)C12H22O11
Correct answer is option 'A'. Can you explain this answer?
Which of the following 0.10 m aqueous solutions will have the lowest freezing point ? [1997]
a)
Al2(SO4)3
b)
C6H12O6
c)
KCl
d)
C12H22O11

|
Gowri Nair answered |
Depression in F.P. ∝ No. of particles.
Al2(SO4)3 provides five ions on ionisation
Al2(SO4)3 provides five ions on ionisation
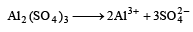
while KCl provides two ions
KCl K Cl
C6H12O6 and C12H22O11 are not ionised so they have single particle.
Hence, Al2(SO4)3 have maximum value of depression in F.P or lowest F.P
KCl K Cl
C6H12O6 and C12H22O11 are not ionised so they have single particle.
Hence, Al2(SO4)3 have maximum value of depression in F.P or lowest F.P
Formation of a solution from two components can be considered as [2003]
(i) Pure solvent → separated solvent molecules, ΔH1
(ii) Pure solute → separated solute molecules, ΔH2
(iii) Separted solvent & solute molecules → Solution, ΔH3
Solution so formed will be ideal if(a) ΔHsoln = ΔH1 + ΔH2 - ΔH3(b) ΔHsoln = ΔH1 + ΔH2 + ΔH3(c) ΔHsoln = ΔH1 - ΔH2 - ΔH3(d) ΔHsoln = ΔH3 - ΔH1 - ΔH2- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
Formation of a solution from two components can be considered as [2003]
(i) Pure solvent → separated solvent molecules, ΔH1
(ii) Pure solute → separated solute molecules, ΔH2
(iii) Separted solvent & solute molecules → Solution, ΔH3
Solution so formed will be ideal if
(i) Pure solvent → separated solvent molecules, ΔH1
(ii) Pure solute → separated solute molecules, ΔH2
(iii) Separted solvent & solute molecules → Solution, ΔH3
Solution so formed will be ideal if
(a) ΔHsoln = ΔH1 + ΔH2 - ΔH3
(b) ΔHsoln = ΔH1 + ΔH2 + ΔH3
(c) ΔHsoln = ΔH1 - ΔH2 - ΔH3
(d) ΔHsoln = ΔH3 - ΔH1 - ΔH2
a)
a
b)
b
c)
c
d)
d
|
|
Nisha Kulkarni answered |
For an ideal solution, ΔHmixing = 0
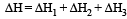 (Accroding to Hess's law) i.e., for ideal solutions there is no change in magnitude of the attractive forces in the two components present.
(Accroding to Hess's law) i.e., for ideal solutions there is no change in magnitude of the attractive forces in the two components present.
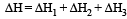 (Accroding to Hess's law) i.e., for ideal solutions there is no change in magnitude of the attractive forces in the two components present.
(Accroding to Hess's law) i.e., for ideal solutions there is no change in magnitude of the attractive forces in the two components present.An aqueous solution is 1.00 molal in KI. Which change will cause the vapour pressure of the solution to increase? [2010]- a)Addition of NaCI
- b)Addition of Na 2SO4
- c)Addition of 1.00 molal KI
- d)Addition of water
Correct answer is option 'D'. Can you explain this answer?
An aqueous solution is 1.00 molal in KI. Which change will cause the vapour pressure of the solution to increase? [2010]
a)
Addition of NaCI
b)
Addition of Na 2SO4
c)
Addition of 1.00 molal KI
d)
Addition of water

|
Pallabi Reddy answered |
When the aqueous solution of one molal KI is diluted with water, concentration decreases, therefore the vapour pressure of the resulting solution increases.
200 mL of an aqueous solution of a protein contains its 1.26 g. The osmotic pressure of this solution at 300 K is found to be 2.57 × 10–3 bar.The molar mass of protein will be (R = 0.083 L bar mol–1 K–1) [2011 M]- a)51022 g mol–1
- b)122044 g mol–1
- c)31011 g mol–1
- d)61038 g mol–1
Correct answer is option 'D'. Can you explain this answer?
200 mL of an aqueous solution of a protein contains its 1.26 g. The osmotic pressure of this solution at 300 K is found to be 2.57 × 10–3 bar.The molar mass of protein will be (R = 0.083 L bar mol–1 K–1) [2011 M]
a)
51022 g mol–1
b)
122044 g mol–1
c)
31011 g mol–1
d)
61038 g mol–1

|
Lekshmi Banerjee answered |
(π) = CRT Osmotic pressure
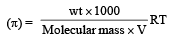
= 2.57 10 3
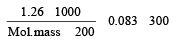
Molecular mass = 61038 g
The freezing point depression constant for water is – 1.86ºC m–1. If 5.00 g Na2SO4 is dissolved in 45.0 g H2O, the freezing point is changed by – 3.82ºC. Calculate the van’t Hoff factor for Na2SO4. [2011]- a)2.05
- b)2.63
- c)3.11
- d)0.381
Correct answer is option 'B'. Can you explain this answer?
The freezing point depression constant for water is – 1.86ºC m–1. If 5.00 g Na2SO4 is dissolved in 45.0 g H2O, the freezing point is changed by – 3.82ºC. Calculate the van’t Hoff factor for Na2SO4. [2011]
a)
2.05
b)
2.63
c)
3.11
d)
0.381

|
Lekshmi Banerjee answered |
Given Kf = – 1.86º cm–1, mass of solute = 5.00 g, mass of solvent = 45.0 g
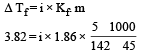
∴ i = 2.63 (Molecular mass of Na2SO4 = 142)
PA and PB are the vapour pressure of pure liquid components, A and B, respectively of an ideal binary solution. If XA represents the mole fraction of component A, the total pressure of the solution will be. [2012]- a)PA + XA (PB – PA)
- b)PA + XA (PA – PB)
- c)PB + XA (PB – PA)
- d)PB + XA (PA – PB)
Correct answer is option 'D'. Can you explain this answer?
PA and PB are the vapour pressure of pure liquid components, A and B, respectively of an ideal binary solution. If XA represents the mole fraction of component A, the total pressure of the solution will be. [2012]
a)
PA + XA (PB – PA)
b)
PA + XA (PA – PB)
c)
PB + XA (PB – PA)
d)
PB + XA (PA – PB)

|
Krish Patel answered |
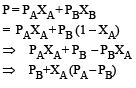
1.00 g of a non-electrolyte solute (molar mass 250 g mol–1) was dissolved in 51.2 g of benzene. If the freezing point depression constant, Kf of benzene is 5.12 K kg mol–1, the freezing point of benzene will be lowered by [2006]- a)0.3 K
- b)0.5 K
- c)0.4 K
- d)0.2
Correct answer is option 'C'. Can you explain this answer?
1.00 g of a non-electrolyte solute (molar mass 250 g mol–1) was dissolved in 51.2 g of benzene. If the freezing point depression constant, Kf of benzene is 5.12 K kg mol–1, the freezing point of benzene will be lowered by [2006]
a)
0.3 K
b)
0.5 K
c)
0.4 K
d)
0.2

|
Rohan Unni answered |
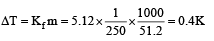
Chapter doubts & questions for Solutions - Chemistry 31 Years NEET Chapterwise Solved Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Solutions - Chemistry 31 Years NEET Chapterwise Solved Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup