All Exams >
Mechanical Engineering >
Topicwise Question Bank for Mechanical Engineering >
All Questions
All questions of Engineering Materials for Mechanical Engineering Exam
The percentage of pro-eutectoid ferrite and pearlite in a slowly cooled 0.5% carbon steel is- a)25% proeutectoid ferrite and 75% pearlite
- b)37.5% proeutectoid ferrite and 62.5% pearlite
- c)62.5% proeutectoid ferrite and 37.5% pearlite
- d)40% proeutectoid ferrite and 60% pearlite
Correct answer is option 'B'. Can you explain this answer?
The percentage of pro-eutectoid ferrite and pearlite in a slowly cooled 0.5% carbon steel is
a)
25% proeutectoid ferrite and 75% pearlite
b)
37.5% proeutectoid ferrite and 62.5% pearlite
c)
62.5% proeutectoid ferrite and 37.5% pearlite
d)
40% proeutectoid ferrite and 60% pearlite
|
|
Avinash Sharma answered |
Let fulcrum is at 0.5% carbon to apply lever rule. % Proeutectoid ferrite
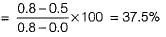
% Pearlite = 100 - 37.5 = 62.5%
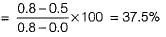
% Pearlite = 100 - 37.5 = 62.5%
Following is WRONG about a phase diagram:- a)It gives information on transformation rates.
- b)Relative amount of different phases can be found under given equilibrium conditions.
- c)It indicates the temperature at which different phases start to melt.
- d)Solid solubility limits are depicted by it.
Correct answer is option 'A'. Can you explain this answer?
Following is WRONG about a phase diagram:
a)
It gives information on transformation rates.
b)
Relative amount of different phases can be found under given equilibrium conditions.
c)
It indicates the temperature at which different phases start to melt.
d)
Solid solubility limits are depicted by it.

|
Ashwin Gupta answered |
Time dependence phase changes is studied by kinetics of the phase transformations.
Brittle fracture is more dangerous than ductile fracture because- a)no warning sign
- b)crack propagates at very high speed
- c)no need for extra stress during crack propagation
- d)all of these
Correct answer is option 'D'. Can you explain this answer?
Brittle fracture is more dangerous than ductile fracture because
a)
no warning sign
b)
crack propagates at very high speed
c)
no need for extra stress during crack propagation
d)
all of these
|
|
Athul Kumar answered |
Brittle fracture is more dangerous than ductile fracture because of several reasons. Let's discuss each reason in detail.
No warning sign:
- Brittle materials do not show any warning signs before fracturing, unlike ductile materials.
- Ductile materials undergo plastic deformation before fracturing, which gives a warning sign to prevent catastrophic failure.
- Brittle fracture can occur suddenly without any warning sign, making it more dangerous.
Crack propagates at a very high speed:
- In brittle materials, the crack propagates at a very high speed once it starts.
- Due to the absence of warning signs, there is no time to detect and control the crack propagation.
- The high-speed crack propagation can cause catastrophic failure in a very short time.
No need for extra stress during crack propagation:
- Brittle fracture can occur even at a low level of stress, which makes it more dangerous.
- In ductile fracture, the crack propagation requires extra stress to continue, which can be controlled to prevent catastrophic failure.
All of these:
- All the above reasons make brittle fracture more dangerous than ductile fracture.
- The absence of warning signs, high-speed crack propagation, and no need for extra stress during crack propagation can cause catastrophic failure in a very short time.
Therefore, it is important to understand the differences between brittle and ductile fracture and take appropriate precautions to prevent catastrophic failure in brittle materials.
No warning sign:
- Brittle materials do not show any warning signs before fracturing, unlike ductile materials.
- Ductile materials undergo plastic deformation before fracturing, which gives a warning sign to prevent catastrophic failure.
- Brittle fracture can occur suddenly without any warning sign, making it more dangerous.
Crack propagates at a very high speed:
- In brittle materials, the crack propagates at a very high speed once it starts.
- Due to the absence of warning signs, there is no time to detect and control the crack propagation.
- The high-speed crack propagation can cause catastrophic failure in a very short time.
No need for extra stress during crack propagation:
- Brittle fracture can occur even at a low level of stress, which makes it more dangerous.
- In ductile fracture, the crack propagation requires extra stress to continue, which can be controlled to prevent catastrophic failure.
All of these:
- All the above reasons make brittle fracture more dangerous than ductile fracture.
- The absence of warning signs, high-speed crack propagation, and no need for extra stress during crack propagation can cause catastrophic failure in a very short time.
Therefore, it is important to understand the differences between brittle and ductile fracture and take appropriate precautions to prevent catastrophic failure in brittle materials.
In structure, all metals are- a)granular
- b)crystalline
- c)wrought
- d)amorphous
Correct answer is option 'B'. Can you explain this answer?
In structure, all metals are
a)
granular
b)
crystalline
c)
wrought
d)
amorphous
|
|
Isha Nambiar answered |
Metallic Structure
Metallic structure refers to the arrangement of metal atoms in a solid. The arrangement of metal atoms can be granular, crystalline, or amorphous.
Granular Structure
A granular structure refers to a metal structure that is composed of granules or grains. The grains are usually visible to the naked eye and are formed when molten metal solidifies.
Wrought Structure
Wrought structure refers to the arrangement of metal atoms in a metal that has been worked or shaped by mechanical or thermal means. This type of structure is often found in metals that have been forged, rolled, or drawn.
Amorphous Structure
An amorphous structure refers to a metal structure that lacks long-range order or crystalline structure. This type of structure is usually found in metals that have been rapidly cooled, such as in the case of metallic glasses.
Crystalline Structure
A crystalline structure refers to a metal structure that has a repeating pattern of atoms in three dimensions. This type of structure is often found in metals that have been slowly cooled, allowing the atoms to arrange themselves in an ordered pattern.
Conclusion
In structure, all metals are crystalline, meaning they have a repeating pattern of atoms in three dimensions. This is due to the way in which metals solidify, allowing the atoms to arrange themselves in an ordered pattern as the metal cools.
Metallic structure refers to the arrangement of metal atoms in a solid. The arrangement of metal atoms can be granular, crystalline, or amorphous.
Granular Structure
A granular structure refers to a metal structure that is composed of granules or grains. The grains are usually visible to the naked eye and are formed when molten metal solidifies.
Wrought Structure
Wrought structure refers to the arrangement of metal atoms in a metal that has been worked or shaped by mechanical or thermal means. This type of structure is often found in metals that have been forged, rolled, or drawn.
Amorphous Structure
An amorphous structure refers to a metal structure that lacks long-range order or crystalline structure. This type of structure is usually found in metals that have been rapidly cooled, such as in the case of metallic glasses.
Crystalline Structure
A crystalline structure refers to a metal structure that has a repeating pattern of atoms in three dimensions. This type of structure is often found in metals that have been slowly cooled, allowing the atoms to arrange themselves in an ordered pattern.
Conclusion
In structure, all metals are crystalline, meaning they have a repeating pattern of atoms in three dimensions. This is due to the way in which metals solidify, allowing the atoms to arrange themselves in an ordered pattern as the metal cools.
Alloy steel which is work hard enable and which is used to make the blade of bull dozers, bucket wheel excavators and other earth moving equipment contain Iron, Carbon and- a)Chromium
- b)Silicon
- c)Maganese
- d)Magnesium
Correct answer is option 'C'. Can you explain this answer?
Alloy steel which is work hard enable and which is used to make the blade of bull dozers, bucket wheel excavators and other earth moving equipment contain Iron, Carbon and
a)
Chromium
b)
Silicon
c)
Maganese
d)
Magnesium
|
|
Anshul Sharma answered |
When maganese in steel is more than 12%. It is called hadfield steel and used to make the blade of bull dozers and other earth moving equipment.
There occurs an increase in softness, flexibility and ease in processing of plastics with the addition of- a)fillers
- b)catalysts
- c)plasticizers
- d)asbestos
Correct answer is option 'C'. Can you explain this answer?
There occurs an increase in softness, flexibility and ease in processing of plastics with the addition of
a)
fillers
b)
catalysts
c)
plasticizers
d)
asbestos
|
|
Shruti Bose answered |
There occurs an increase in softness, flexibility and ease in processing of plastics with the addition of (a) fillers (b) catalysts (c) plasticizers (d) asbestos
If a particular Fe-C alloy contains less than 0.83% carbon. It is called
- a)hypoeutectoid steel
- b)hypereutectoid steel
- c)lhigh carbon steel
- d)cast iron
Correct answer is option 'A'. Can you explain this answer?
If a particular Fe-C alloy contains less than 0.83% carbon. It is called
a)
hypoeutectoid steel
b)
hypereutectoid steel
c)
lhigh carbon steel
d)
cast iron
|
|
Shruti Bose answered |
Pearlite is the eutectoid composition of ferrite and cementite in pearlite ferrite and cementite are found in alternate structure.
Ternary stage creep is associated with- a)strain hardening
- b)recovery
- c)necking
- d)none of these
Correct answer is option 'C'. Can you explain this answer?
Ternary stage creep is associated with
a)
strain hardening
b)
recovery
c)
necking
d)
none of these

|
Swati Patel answered |
Ternary stage creep is associated with necking.
Explanation:
Creep is the time-dependent deformation that occurs in a material under a constant load or stress. It is a common phenomenon in materials exposed to high temperatures and constant loading conditions. Creep can be divided into three stages: primary (or transient), secondary (or steady-state), and tertiary (or accelerated) creep.
Ternary stage creep, also known as tertiary creep, is the final stage of creep where the deformation rate increases rapidly. During this stage, the material experiences significant necking.
Necking is the localized reduction in cross-sectional area of a material under tension. It typically occurs in ductile materials and is characterized by the formation of a narrow region of reduced diameter or thickness. Necking is a result of strain localization and can lead to fracture.
During the primary and secondary creep stages, the material undergoes uniform deformation without significant necking. The deformation is distributed evenly across the material. However, as the material enters the tertiary creep stage, the deformation becomes highly localized, and necking occurs. This is often accompanied by a rapid increase in the deformation rate.
Necking in the context of ternary stage creep is an important phenomenon to consider because it indicates that the material is approaching its ultimate failure. It signifies that the material has undergone significant plastic deformation and is getting closer to fracture. Therefore, the occurrence of necking during ternary stage creep is a critical factor in assessing the remaining life and structural integrity of the material.
In conclusion, ternary stage creep is associated with necking, which is the localized reduction in cross-sectional area of a material under tension. Necking occurs during the final stage of creep when the material undergoes rapid deformation and is an important indicator of impending failure.
Explanation:
Creep is the time-dependent deformation that occurs in a material under a constant load or stress. It is a common phenomenon in materials exposed to high temperatures and constant loading conditions. Creep can be divided into three stages: primary (or transient), secondary (or steady-state), and tertiary (or accelerated) creep.
Ternary stage creep, also known as tertiary creep, is the final stage of creep where the deformation rate increases rapidly. During this stage, the material experiences significant necking.
Necking is the localized reduction in cross-sectional area of a material under tension. It typically occurs in ductile materials and is characterized by the formation of a narrow region of reduced diameter or thickness. Necking is a result of strain localization and can lead to fracture.
During the primary and secondary creep stages, the material undergoes uniform deformation without significant necking. The deformation is distributed evenly across the material. However, as the material enters the tertiary creep stage, the deformation becomes highly localized, and necking occurs. This is often accompanied by a rapid increase in the deformation rate.
Necking in the context of ternary stage creep is an important phenomenon to consider because it indicates that the material is approaching its ultimate failure. It signifies that the material has undergone significant plastic deformation and is getting closer to fracture. Therefore, the occurrence of necking during ternary stage creep is a critical factor in assessing the remaining life and structural integrity of the material.
In conclusion, ternary stage creep is associated with necking, which is the localized reduction in cross-sectional area of a material under tension. Necking occurs during the final stage of creep when the material undergoes rapid deformation and is an important indicator of impending failure.
In which of the following phases of steel, cementite is in lamellar form?- a)Ferrite
- b)Bainite
- c)Martensite
- d)Pearlite
Correct answer is option 'D'. Can you explain this answer?
In which of the following phases of steel, cementite is in lamellar form?
a)
Ferrite
b)
Bainite
c)
Martensite
d)
Pearlite
|
|
Vaibhav Khanna answered |
Pearlite phase in steel is made up of alternate layers of ferrite and cementite.
Hardness of steel greatly improves with- a)Annealing
- b)Cyaniding
- c)Normalizing
- d)Tempering
Correct answer is option 'B'. Can you explain this answer?
Hardness of steel greatly improves with
a)
Annealing
b)
Cyaniding
c)
Normalizing
d)
Tempering

|
Jyoti Choudhury answered |
Understanding Cyaniding
Cyaniding is a surface hardening process that enhances the hardness of steel, making it suitable for applications requiring high wear resistance. Here’s how it works:
Process Overview
- Cyaniding involves the introduction of carbon and nitrogen into the surface of low-carbon steel.
- Temperature Range: The process typically occurs at temperatures between 850°C and 950°C, where the steel is immersed in a cyanide salt bath.
Mechanism of Hardening
- Diffusion: The cyanide compounds diffuse into the steel's surface, enriching it with carbon and nitrogen.
- Formation of Hard Phases: This results in the formation of hard phases such as cyanide compounds (e.g., iron nitrides), which significantly increase surface hardness.
Advantages of Cyaniding
- High Hardness: The surface can achieve hardness levels between 60-70 HRC (Rockwell Hardness Scale).
- Improved Wear Resistance: The enhanced hardness contributes to superior wear resistance, making it ideal for components like gears and shafts.
- Tough Core: While the surface becomes hard, the core remains tough, preventing brittleness.
Comparison with Other Processes
- Annealing: This process softens steel, making it more ductile.
- Normalizing: Focuses on refining grain structure without significantly increasing hardness.
- Tempering: Reduces brittleness of hardened steel, but does not enhance its hardness.
In conclusion, cyaniding is the most effective method among the options for significantly increasing the hardness of steel, making it valuable for engineering applications that require durability and wear resistance.
Cyaniding is a surface hardening process that enhances the hardness of steel, making it suitable for applications requiring high wear resistance. Here’s how it works:
Process Overview
- Cyaniding involves the introduction of carbon and nitrogen into the surface of low-carbon steel.
- Temperature Range: The process typically occurs at temperatures between 850°C and 950°C, where the steel is immersed in a cyanide salt bath.
Mechanism of Hardening
- Diffusion: The cyanide compounds diffuse into the steel's surface, enriching it with carbon and nitrogen.
- Formation of Hard Phases: This results in the formation of hard phases such as cyanide compounds (e.g., iron nitrides), which significantly increase surface hardness.
Advantages of Cyaniding
- High Hardness: The surface can achieve hardness levels between 60-70 HRC (Rockwell Hardness Scale).
- Improved Wear Resistance: The enhanced hardness contributes to superior wear resistance, making it ideal for components like gears and shafts.
- Tough Core: While the surface becomes hard, the core remains tough, preventing brittleness.
Comparison with Other Processes
- Annealing: This process softens steel, making it more ductile.
- Normalizing: Focuses on refining grain structure without significantly increasing hardness.
- Tempering: Reduces brittleness of hardened steel, but does not enhance its hardness.
In conclusion, cyaniding is the most effective method among the options for significantly increasing the hardness of steel, making it valuable for engineering applications that require durability and wear resistance.
The defect which takes place due to imperfect packing of atoms during crystallization is known as
- a)line defect
- b)Both (a) and (c)
- c)surface defect
- d)point defect
Correct answer is option 'D'. Can you explain this answer?
The defect which takes place due to imperfect packing of atoms during crystallization is known as
a)
line defect
b)
Both (a) and (c)
c)
surface defect
d)
point defect

|
Gate Funda answered |
Point defects are where an atom is missing or is in an irregular place in the lattice structure.
Point defects include self interstitial atoms, interstitial impurity atoms, substitutional atoms and vacancies
Point defects include self interstitial atoms, interstitial impurity atoms, substitutional atoms and vacancies
Heat treatment that requires heating in a part below A, temperature, i.e, between 550°C and 650°C is called as- a)hardening
- b)normalizing
- c)process annealing
- d)fullanealing
Correct answer is option 'C'. Can you explain this answer?
Heat treatment that requires heating in a part below A, temperature, i.e, between 550°C and 650°C is called as
a)
hardening
b)
normalizing
c)
process annealing
d)
fullanealing

|
Shraddha Datta answered |
Process annealing is a sub-critical treatment given to metals to soften them during mechanical processing. It may or may not involve full recrystallization of the cold worked metal.
Which of the following elements is not a metalloid?
- a)Copper
- b)germanium
- c)boron
- d)Silicon
Correct answer is option 'A'. Can you explain this answer?
Which of the following elements is not a metalloid?
a)
Copper
b)
germanium
c)
boron
d)
Silicon
|
|
Vibhor Goyal answered |
Correct option is A: Copper elements is not a metalloid. The known metalloids are boron, silicon, germanium.
The Iron-carbon diagram and the TTT curves are determined under- a)equilibrium and non-equilibrium conditions respectively
- b)non-equilibrium and equilibrium conditions respectively
- c)equilibrium conditions for both
- d)non-equilibrium conditions for both
Correct answer is option 'A'. Can you explain this answer?
The Iron-carbon diagram and the TTT curves are determined under
a)
equilibrium and non-equilibrium conditions respectively
b)
non-equilibrium and equilibrium conditions respectively
c)
equilibrium conditions for both
d)
non-equilibrium conditions for both
|
|
Kiran Basu answered |
Iron-carbon diagram are draw in equilibrium condition that is why it is also called iron-carbon equilibrium diagram while T-T-T diagram is drawn for non-equilibrium conditions.
Hardness of martensite is about- a)RC 65
- b)RC48
- c)RC 57
- d)RC 80
Correct answer is option 'A'. Can you explain this answer?
Hardness of martensite is about
a)
RC 65
b)
RC48
c)
RC 57
d)
RC 80
|
|
Akshara Rane answered |
0.2% C Martensite →50 RC
0.4% C Martensite →58 RC
0.8% C Martensite→ 65 RC
0.4% C Martensite →58 RC
0.8% C Martensite→ 65 RC
Which among the following has poorest weldability- a)Low carbon steel
- b)Medium carbon steel
- c)High carbon steel
- d)Wrought iron
Correct answer is option 'C'. Can you explain this answer?
Which among the following has poorest weldability
a)
Low carbon steel
b)
Medium carbon steel
c)
High carbon steel
d)
Wrought iron
|
|
Gopal Choudhury answered |
As the amount of carbon increases, its weldability increases since from the material given, high carbon steel has maximum amount of carbon, it will posses poorest weldability.
Free carbon in iron makes the iron
- a)soft and gives a fine crystalline structure
- b)soft and gives a coarse grained crystalline structure
- c)hard and gives a coarse grained crystalline structure
- d)hard and gives a fine grained crystalline structure
Correct answer is option 'B'. Can you explain this answer?
Free carbon in iron makes the iron
a)
soft and gives a fine crystalline structure
b)
soft and gives a coarse grained crystalline structure
c)
hard and gives a coarse grained crystalline structure
d)
hard and gives a fine grained crystalline structure
|
|
Sinjini Nambiar answered |
Hyper eutectoid steel ⇒ 0.76 to 2.1 percent carbon.
The grain structure obtained by isothermal hardening operation is- a)martensite
- b)aciculartroostite
- c)sorbite
- d)bainite
Correct answer is option 'D'. Can you explain this answer?
The grain structure obtained by isothermal hardening operation is
a)
martensite
b)
aciculartroostite
c)
sorbite
d)
bainite
|
|
Kajal Tiwari answered |
Understanding Isothermal Hardening
Isothermal hardening is a heat treatment process that enhances the mechanical properties of steel, particularly its hardness and strength. The process involves holding the steel at a specific temperature for a designated time, allowing for transformations in the microstructure.
Microstructures Resulting from Isothermal Hardening
- Martensite:
- Formed by rapid cooling, martensite is a very hard and brittle phase, typically produced by quenching from a high temperature.
- Acicular Troostite:
- This microstructure appears as needle-like formations and is not typically associated with isothermal treatments.
- Sorbite:
- A mixture of ferrite and cementite, sorbite results from tempering martensite but is not a direct product of isothermal hardening.
- Bainite:
- This is the correct answer. Bainite is produced through isothermal transformation at intermediate temperatures. It displays a fine, elongated structure and offers a balance of strength and ductility.
Why Bainite is the Correct Answer
- Temperature Range:
- Isothermal hardening occurs at specific temperatures (around 250-550°C), which favor the formation of bainite rather than martensite.
- Mechanical Properties:
- Bainite provides superior toughness compared to martensite, making it ideal for applications requiring both strength and ductility.
- Transformation Mechanism:
- The isothermal process allows for a slower transformation, leading to a more refined microstructure, characteristic of bainite.
In summary, the grain structure obtained from isothermal hardening is bainite, which is preferred for its improved balance of mechanical properties.
Isothermal hardening is a heat treatment process that enhances the mechanical properties of steel, particularly its hardness and strength. The process involves holding the steel at a specific temperature for a designated time, allowing for transformations in the microstructure.
Microstructures Resulting from Isothermal Hardening
- Martensite:
- Formed by rapid cooling, martensite is a very hard and brittle phase, typically produced by quenching from a high temperature.
- Acicular Troostite:
- This microstructure appears as needle-like formations and is not typically associated with isothermal treatments.
- Sorbite:
- A mixture of ferrite and cementite, sorbite results from tempering martensite but is not a direct product of isothermal hardening.
- Bainite:
- This is the correct answer. Bainite is produced through isothermal transformation at intermediate temperatures. It displays a fine, elongated structure and offers a balance of strength and ductility.
Why Bainite is the Correct Answer
- Temperature Range:
- Isothermal hardening occurs at specific temperatures (around 250-550°C), which favor the formation of bainite rather than martensite.
- Mechanical Properties:
- Bainite provides superior toughness compared to martensite, making it ideal for applications requiring both strength and ductility.
- Transformation Mechanism:
- The isothermal process allows for a slower transformation, leading to a more refined microstructure, characteristic of bainite.
In summary, the grain structure obtained from isothermal hardening is bainite, which is preferred for its improved balance of mechanical properties.
Which one of the following elements is a ferritic stabilizer?- a)Nickel
- b)Manganese
- c)Copper
- d)Chromium
Correct answer is option 'D'. Can you explain this answer?
Which one of the following elements is a ferritic stabilizer?
a)
Nickel
b)
Manganese
c)
Copper
d)
Chromium
|
|
Avantika Sen answered |
Chromium, Tungsten and Molybdenum are ferritic stabilizer.
Maximum surface hardness is attained by- a)cyaniding
- b)carburising
- c)flame hardening
- d)nitriding
Correct answer is option 'D'. Can you explain this answer?
Maximum surface hardness is attained by
a)
cyaniding
b)
carburising
c)
flame hardening
d)
nitriding
|
|
Sai Reddy answered |
Maximum surface hardness is produced by nitriding; next come cyaniding and carburising.
Tempering is a process of annealing- a)Martensite at low temperatures
- b)Martensite at higher temperatures
- c)Bainite at low temperatures
- d)Bainite at higher temperatures
Correct answer is option 'A'. Can you explain this answer?
Tempering is a process of annealing
a)
Martensite at low temperatures
b)
Martensite at higher temperatures
c)
Bainite at low temperatures
d)
Bainite at higher temperatures
|
|
Sharmila Chauhan answered |
Tempering is the process of annealing martensite at low temperature. Although martensite is strong and wear resistant, it is brittle. Its toughness is low, primarily because of residual stresses introduced by the transformation. When tempering is carried out at a occurs. The martensite grains retain their strength, but improve their toughness and donot change shape on storage.
Which one of the following sets of constituents is expected in equilibrium cooling of a hypereutectoid steel from austenitic state?- a)Ferrite and pearlite
- b)Cementite and pearlite
- c)Ferrite and bainite
- d)Cementite and martensite
Correct answer is option 'B'. Can you explain this answer?
Which one of the following sets of constituents is expected in equilibrium cooling of a hypereutectoid steel from austenitic state?
a)
Ferrite and pearlite
b)
Cementite and pearlite
c)
Ferrite and bainite
d)
Cementite and martensite
|
|
Shreya Choudhury answered |
Hypereutectoid steel when cooled in equilibrium will result in proeutectoid cementite and pearlite whereas hypoeutectoid steel when cooled in equilibrium will result in proeutectoid ferrite and pearlite.
Sam _______ by next week. (to leave)Correct answer is 'will have left'. Can you explain this answer?
Sam _______ by next week. (to leave)

|
Arshiya Mehta answered |
This answer implies that the action of leaving will have been completed by the specified time. The verb "leave" is in the future perfect tense, which is used to indicate an action that will have been completed by a certain point in the future. In this case, the action of leaving will have been completed by next week.
Tempering temperature of most of the material is of the order of- a)100-150°C
- b)200-300°C
- c)350-400°C
- d)400-500°C
Correct answer is option 'B'. Can you explain this answer?
Tempering temperature of most of the material is of the order of
a)
100-150°C
b)
200-300°C
c)
350-400°C
d)
400-500°C
|
|
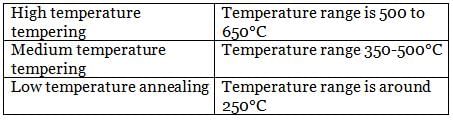
Anjali Shah answered |
Tempering process is categorised in three category

Addition of vanadium to steel results in improvement of- a)heat-treatabiiity by quenching '
- b)hardenabiiity
- c)fatigue strength
- d)resistance to oxidation at elevated temperature
Correct answer is option 'B'. Can you explain this answer?
Addition of vanadium to steel results in improvement of
a)
heat-treatabiiity by quenching '
b)
hardenabiiity
c)
fatigue strength
d)
resistance to oxidation at elevated temperature
|
|
Anuj Chauhan answered |
Vanadium is added in Sow and medium carbon steels in order to increase their yield and tensile strength properties. In combination with chromium. It produces a marked effect on the properties of steel and makes the steel extremely tough and strong, these steels are largely used for making spring steel,.high speed tool steels, crankshaft etc.
Which of the following regions of the electromagnetic spectrum would be used to determine the structure of crystalline solids?- a)Microwave
- b)Infrared
- c)X-ray
- d)Visible
Correct answer is option 'C'. Can you explain this answer?
Which of the following regions of the electromagnetic spectrum would be used to determine the structure of crystalline solids?
a)
Microwave
b)
Infrared
c)
X-ray
d)
Visible

|
Nilesh Kapoor answered |
X-ray crystallography: A technique in which the patterns formed by the diffraction of X-rays on passing through a crystalline substance yield information on the lattice structure of the crystal, and the molecular structure of the substance.
Which of the following polymers produces HCI as condensate?- a)Phenon formaldehyde
- b)Polycarbonate
- c)Urea formaldehyde
- d)Nylon-6, 6
Correct answer is option 'B'. Can you explain this answer?
Which of the following polymers produces HCI as condensate?
a)
Phenon formaldehyde
b)
Polycarbonate
c)
Urea formaldehyde
d)
Nylon-6, 6
|
|
Ameya Kaur answered |
The main polycarbonate material is produced by the reaction of bisphenol A(BPA) and phosgene CO CI2 HCI is final by product.
Cast steel crankshaft surface is hardened by- a)Nitriding
- b)Normalizing
- c)Induction heating
- d)Carburizing
Correct answer is option 'C'. Can you explain this answer?
Cast steel crankshaft surface is hardened by
a)
Nitriding
b)
Normalizing
c)
Induction heating
d)
Carburizing
|
|
Priyanka Tiwari answered |
Nitriding:
Nitriding is a surface hardening process in which nitrogen is diffused into the surface of the steel to create a hardened layer. While nitriding can be a suitable method for hardening certain types of steel components, it is not typically used for surface hardening cast steel crankshafts.
Normalizing:
Normalizing is a heat treatment process that involves heating the steel to a specific temperature, holding it at that temperature for a period of time, and then cooling it in air. Normalizing is primarily used to refine the grain structure of the steel and improve its mechanical properties, but it does not provide surface hardening.
Induction Heating:
Induction heating is a process that uses electromagnetic induction to heat a metal object. In the case of a cast steel crankshaft, induction heating can be used to selectively heat the surface of the crankshaft to a high temperature, allowing for rapid quenching and subsequent hardening of the surface.
Carburizing:
Carburizing is a surface hardening process in which carbon is diffused into the surface of the steel to create a hardened layer. This process is commonly used for hardening steel components such as crankshafts, gears, and bearings. In the case of a cast steel crankshaft, carburizing can be an effective method for achieving the desired surface hardness.
Nitriding is a surface hardening process in which nitrogen is diffused into the surface of the steel to create a hardened layer. While nitriding can be a suitable method for hardening certain types of steel components, it is not typically used for surface hardening cast steel crankshafts.
Normalizing:
Normalizing is a heat treatment process that involves heating the steel to a specific temperature, holding it at that temperature for a period of time, and then cooling it in air. Normalizing is primarily used to refine the grain structure of the steel and improve its mechanical properties, but it does not provide surface hardening.
Induction Heating:
Induction heating is a process that uses electromagnetic induction to heat a metal object. In the case of a cast steel crankshaft, induction heating can be used to selectively heat the surface of the crankshaft to a high temperature, allowing for rapid quenching and subsequent hardening of the surface.
Carburizing:
Carburizing is a surface hardening process in which carbon is diffused into the surface of the steel to create a hardened layer. This process is commonly used for hardening steel components such as crankshafts, gears, and bearings. In the case of a cast steel crankshaft, carburizing can be an effective method for achieving the desired surface hardness.
The co-ordination number of BCC crystal structure is- a)8
- b)16
- c)12
- d)10
Correct answer is option 'A'. Can you explain this answer?
The co-ordination number of BCC crystal structure is
a)
8
b)
16
c)
12
d)
10
|
|
Anjali Sengupta answered |
The co-ordination number of a crystal structure refers to the number of nearest neighboring atoms surrounding a central atom. In the case of a body-centered cubic (BCC) crystal structure, the co-ordination number is 8.
Explanation:
1. Body-Centered Cubic (BCC) Structure:
- The BCC crystal structure is one of the common arrangements of atoms in a solid material.
- In this structure, the atoms are arranged in a cubic lattice, with an additional atom located in the center of the cube.
- Each corner of the cube is shared by 8 adjacent unit cells, while the center atom is not shared with any other unit cells.
2. Identifying Nearest Neighbors:
- To determine the co-ordination number of a BCC structure, we need to identify the nearest neighboring atoms surrounding a central atom.
- In this case, the central atom is located at the center of the cube.
- The nearest neighboring atoms are the 8 atoms situated at the corners of the cube.
- Each corner atom is shared by 8 unit cells, with one unit cell belonging to the central atom.
3. Co-ordination Number:
- The co-ordination number is the total number of nearest neighboring atoms surrounding a central atom.
- In the BCC crystal structure, there are 8 corner atoms surrounding the central atom.
- Therefore, the co-ordination number of a BCC crystal structure is 8.
In summary, the co-ordination number of a BCC crystal structure is 8 because there are 8 corner atoms surrounding the central atom.
Explanation:
1. Body-Centered Cubic (BCC) Structure:
- The BCC crystal structure is one of the common arrangements of atoms in a solid material.
- In this structure, the atoms are arranged in a cubic lattice, with an additional atom located in the center of the cube.
- Each corner of the cube is shared by 8 adjacent unit cells, while the center atom is not shared with any other unit cells.
2. Identifying Nearest Neighbors:
- To determine the co-ordination number of a BCC structure, we need to identify the nearest neighboring atoms surrounding a central atom.
- In this case, the central atom is located at the center of the cube.
- The nearest neighboring atoms are the 8 atoms situated at the corners of the cube.
- Each corner atom is shared by 8 unit cells, with one unit cell belonging to the central atom.
3. Co-ordination Number:
- The co-ordination number is the total number of nearest neighboring atoms surrounding a central atom.
- In the BCC crystal structure, there are 8 corner atoms surrounding the central atom.
- Therefore, the co-ordination number of a BCC crystal structure is 8.
In summary, the co-ordination number of a BCC crystal structure is 8 because there are 8 corner atoms surrounding the central atom.
The eutectoid of carbon in iron, above lower critical temperature, when cooled, result in- a)ferrite and austenite
- b)ferrite and cementite
- c)cementite and austenite
- d)ferrite, cementite and austenite
Correct answer is option 'B'. Can you explain this answer?
The eutectoid of carbon in iron, above lower critical temperature, when cooled, result in
a)
ferrite and austenite
b)
ferrite and cementite
c)
cementite and austenite
d)
ferrite, cementite and austenite
|
|
Neha Joshi answered |
The correct answer should be the second option - Ferrite and Cementite.
A eutectoid reaction is referred to the phase transformation or change of one solid into two other different solids.
Eutectoid reaction occurs at the eutectoid point of 727°C and 0.77% Carbon, which when cooled gives α-Ferrite and Cementite, also known as Pearlite. Below the critical temperature of 723°C, austenite is no more stable and it gets converted into pearlite.
A eutectoid reaction is referred to the phase transformation or change of one solid into two other different solids.
Eutectoid reaction occurs at the eutectoid point of 727°C and 0.77% Carbon, which when cooled gives α-Ferrite and Cementite, also known as Pearlite. Below the critical temperature of 723°C, austenite is no more stable and it gets converted into pearlite.
Alloying element which can replace tungsten in high speed steels is- a)Nickel
- b)Vanadium
- c)Cobait
- d)Molybdenum
Correct answer is option 'D'. Can you explain this answer?
Alloying element which can replace tungsten in high speed steels is
a)
Nickel
b)
Vanadium
c)
Cobait
d)
Molybdenum

|
Jyoti Choudhury answered |
Introduction:
High-speed steels are a class of tool steels that are widely used in cutting tools, such as drills, taps, and milling cutters, due to their excellent combination of hardness, toughness, and wear resistance at high temperatures. Tungsten is one of the key alloying elements in high-speed steels, but there are other elements that can be used as a replacement for tungsten.
Molybdenum as a replacement for tungsten:
Molybdenum (Mo) is an effective alloying element that can replace tungsten in high-speed steels. It has similar properties to tungsten and can provide several benefits.
1. Increased hardenability:
Molybdenum improves the hardenability of high-speed steels, allowing them to be hardened throughout their cross-section. This results in uniform hardness and improved strength and wear resistance.
2. Improved high-temperature strength:
Molybdenum enhances the high-temperature strength of high-speed steels. It forms a stable carbide phase, which helps to retain the hardness and strength of the steel even at elevated temperatures.
3. Increased red hardness:
Red hardness refers to the ability of a material to maintain its hardness at high temperatures. Molybdenum improves the red hardness of high-speed steels, allowing them to retain their cutting ability even at high operating temperatures.
4. Enhanced toughness:
Molybdenum contributes to the toughness of high-speed steels, preventing the formation of cracks and improving their resistance to chipping and breakage during use.
5. Improved machinability:
Molybdenum can also improve the machinability of high-speed steels, making them easier to machine into complex shapes and reducing tool wear during the machining process.
Conclusion:
In summary, molybdenum is an excellent alloying element that can replace tungsten in high-speed steels. It provides increased hardenability, improved high-temperature strength, increased red hardness, enhanced toughness, and improved machinability. These properties make molybdenum a valuable addition to high-speed steels, allowing them to perform effectively in demanding cutting tool applications.
High-speed steels are a class of tool steels that are widely used in cutting tools, such as drills, taps, and milling cutters, due to their excellent combination of hardness, toughness, and wear resistance at high temperatures. Tungsten is one of the key alloying elements in high-speed steels, but there are other elements that can be used as a replacement for tungsten.
Molybdenum as a replacement for tungsten:
Molybdenum (Mo) is an effective alloying element that can replace tungsten in high-speed steels. It has similar properties to tungsten and can provide several benefits.
1. Increased hardenability:
Molybdenum improves the hardenability of high-speed steels, allowing them to be hardened throughout their cross-section. This results in uniform hardness and improved strength and wear resistance.
2. Improved high-temperature strength:
Molybdenum enhances the high-temperature strength of high-speed steels. It forms a stable carbide phase, which helps to retain the hardness and strength of the steel even at elevated temperatures.
3. Increased red hardness:
Red hardness refers to the ability of a material to maintain its hardness at high temperatures. Molybdenum improves the red hardness of high-speed steels, allowing them to retain their cutting ability even at high operating temperatures.
4. Enhanced toughness:
Molybdenum contributes to the toughness of high-speed steels, preventing the formation of cracks and improving their resistance to chipping and breakage during use.
5. Improved machinability:
Molybdenum can also improve the machinability of high-speed steels, making them easier to machine into complex shapes and reducing tool wear during the machining process.
Conclusion:
In summary, molybdenum is an excellent alloying element that can replace tungsten in high-speed steels. It provides increased hardenability, improved high-temperature strength, increased red hardness, enhanced toughness, and improved machinability. These properties make molybdenum a valuable addition to high-speed steels, allowing them to perform effectively in demanding cutting tool applications.
Eutectic product in Fe-C system is called- a)pearlite
- b)bainite
- c)ledeburite
- d)spheroidite
Correct answer is option 'C'. Can you explain this answer?
Eutectic product in Fe-C system is called
a)
pearlite
b)
bainite
c)
ledeburite
d)
spheroidite

|
Gowri Sharma answered |
A eutectic reaction occurs at 1146°C
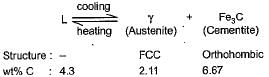
On cooling through the eutectic temperature, the lowest melting liquid of the system decomposes to solid phases, austenite and cementite. This eutectic mixture is knonw as ledeburite.

On cooling through the eutectic temperature, the lowest melting liquid of the system decomposes to solid phases, austenite and cementite. This eutectic mixture is knonw as ledeburite.
What is the planer density of (100) plane in FCC (face-centred cubic) crystal with unit cell side a equa! to?- a)1.484/a2
- b)2/a2
- c)1/a2
- d)√2/a2
Correct answer is option 'B'. Can you explain this answer?
What is the planer density of (100) plane in FCC (face-centred cubic) crystal with unit cell side a equa! to?
a)
1.484/a2
b)
2/a2
c)
1/a2
d)
√2/a2
|
|
Stuti Mishra answered |
FCC on (100) plane

No. of atoms = 1/4 x 4 +1 = 2
Area = a x a = a2
Planar density = 2/a2

No. of atoms = 1/4 x 4 +1 = 2
Area = a x a = a2
Planar density = 2/a2
A continuous and aligned glass fibre-reinforced composite consists of 40 vol% of glass fibres modulus of elasticity 69 GPa and 60 vol% of a polyester resin that, when-hardened, displays a modulus of 3.4 GPa. What is the modulus of elasticity of this composite in the longitudinal direction?- a)15 GPa
- b)30 GPa
- c)45 GPa
- d)60 GPa
Correct answer is option 'B'. Can you explain this answer?
A continuous and aligned glass fibre-reinforced composite consists of 40 vol% of glass fibres modulus of elasticity 69 GPa and 60 vol% of a polyester resin that, when-hardened, displays a modulus of 3.4 GPa. What is the modulus of elasticity of this composite in the longitudinal direction?
a)
15 GPa
b)
30 GPa
c)
45 GPa
d)
60 GPa
|
|
Hrishikesh Chakraborty answered |
ECL = Em Vm + Ef Vf = 3.4 x 0.6 + 69 x 0.4
= 2.04 + 27.6 = 30 GPa
= 2.04 + 27.6 = 30 GPa
The effective number of lattice points in the unit celi of simple cubic, body centered cubic and face centered cubic space lattices respectively are- a)1, 2, 2
- b)1, 2, 4
- c)2, 3, 4
- d)2, 4, 4
Correct answer is option 'B'. Can you explain this answer?
The effective number of lattice points in the unit celi of simple cubic, body centered cubic and face centered cubic space lattices respectively are
a)
1, 2, 2
b)
1, 2, 4
c)
2, 3, 4
d)
2, 4, 4

|
Anuj Chakraborty answered |
For simple cubic effective number of lattice point = 1
For B.C.C. effective number of lattice point = 2
For F.C.C. effective number of lattice point = 4
For B.C.C. effective number of lattice point = 2
For F.C.C. effective number of lattice point = 4
For good weldability, the carbon equivalent (%) of steel should be in the range of- a)0.2 - 0.4
- b)0.5 - 0.8
- c)0.7 - 0.8
- d)0.9 - 1.0
Correct answer is option 'A'. Can you explain this answer?
For good weldability, the carbon equivalent (%) of steel should be in the range of
a)
0.2 - 0.4
b)
0.5 - 0.8
c)
0.7 - 0.8
d)
0.9 - 1.0
|
|
Meera Bose answered |
Carbon Equivalent and Weldability of Steel
The carbon equivalent (CE) is a parameter used to measure the weldability of steel. Weldability refers to the ease with which a material can be welded without developing defects or experiencing problems during the welding process. The carbon equivalent value helps to predict the risk of cracking and other issues that may arise during welding.
Definition of Carbon Equivalent (CE)
The carbon equivalent is calculated based on the chemical composition of the steel, particularly the carbon content and the presence of other alloying elements. It is a numerical value that indicates the relative contribution of carbon and other elements to the weldability of the steel.
Range of Carbon Equivalent for Good Weldability
For good weldability, the carbon equivalent (%) of steel should be in the range of 0.2 - 0.4. This means that if the carbon equivalent value falls within this range, the steel is considered to have good weldability.
Explanation of the Correct Answer
The correct answer, option 'A' (0.2 - 0.4), is the range of carbon equivalent values that indicate good weldability. This range is widely accepted and used in various welding codes and standards.
Reason for the Range of 0.2 - 0.4
- Low Carbon Equivalent: A low carbon equivalent value indicates a low risk of cracking and other welding-related issues. This is because a lower carbon equivalent means a lower carbon content in the steel, which reduces the likelihood of hardening and cracking during welding.
- Optimum Carbon Equivalent: The range of 0.2 - 0.4 is considered the optimum range for good weldability. It strikes a balance between reducing the risk of welding defects and maintaining desirable mechanical properties in the welded joint.
- Effect of Alloying Elements: The presence of alloying elements such as manganese, silicon, and other elements affects the carbon equivalent value. These elements can help reduce the carbon equivalent and improve the weldability of the steel.
- Consideration of Welding Process: The carbon equivalent value is also influenced by the specific welding process being used. Some welding processes, such as high heat input processes, may require a lower carbon equivalent to ensure good weldability.
Conclusion
In summary, the carbon equivalent is an important parameter for assessing the weldability of steel. A carbon equivalent value in the range of 0.2 - 0.4 indicates good weldability, as it reduces the risk of welding defects while maintaining desirable mechanical properties in the welded joint. It is crucial to consider the carbon equivalent when selecting steel for welding applications to ensure successful and reliable welds.
The carbon equivalent (CE) is a parameter used to measure the weldability of steel. Weldability refers to the ease with which a material can be welded without developing defects or experiencing problems during the welding process. The carbon equivalent value helps to predict the risk of cracking and other issues that may arise during welding.
Definition of Carbon Equivalent (CE)
The carbon equivalent is calculated based on the chemical composition of the steel, particularly the carbon content and the presence of other alloying elements. It is a numerical value that indicates the relative contribution of carbon and other elements to the weldability of the steel.
Range of Carbon Equivalent for Good Weldability
For good weldability, the carbon equivalent (%) of steel should be in the range of 0.2 - 0.4. This means that if the carbon equivalent value falls within this range, the steel is considered to have good weldability.
Explanation of the Correct Answer
The correct answer, option 'A' (0.2 - 0.4), is the range of carbon equivalent values that indicate good weldability. This range is widely accepted and used in various welding codes and standards.
Reason for the Range of 0.2 - 0.4
- Low Carbon Equivalent: A low carbon equivalent value indicates a low risk of cracking and other welding-related issues. This is because a lower carbon equivalent means a lower carbon content in the steel, which reduces the likelihood of hardening and cracking during welding.
- Optimum Carbon Equivalent: The range of 0.2 - 0.4 is considered the optimum range for good weldability. It strikes a balance between reducing the risk of welding defects and maintaining desirable mechanical properties in the welded joint.
- Effect of Alloying Elements: The presence of alloying elements such as manganese, silicon, and other elements affects the carbon equivalent value. These elements can help reduce the carbon equivalent and improve the weldability of the steel.
- Consideration of Welding Process: The carbon equivalent value is also influenced by the specific welding process being used. Some welding processes, such as high heat input processes, may require a lower carbon equivalent to ensure good weldability.
Conclusion
In summary, the carbon equivalent is an important parameter for assessing the weldability of steel. A carbon equivalent value in the range of 0.2 - 0.4 indicates good weldability, as it reduces the risk of welding defects while maintaining desirable mechanical properties in the welded joint. It is crucial to consider the carbon equivalent when selecting steel for welding applications to ensure successful and reliable welds.
Steel widely used for making precision measuring instruments is- a)Nickel steel
- b)Nickel-Chrome steel
- c)High speed steel
- d)Chrome-vanadium steel
Correct answer is option 'C'. Can you explain this answer?
Steel widely used for making precision measuring instruments is
a)
Nickel steel
b)
Nickel-Chrome steel
c)
High speed steel
d)
Chrome-vanadium steel
|
|
Divyansh Goyal answered |
Free cutting steels also called free machining steel. The free cutting steel have higher amount of Sulphur as compared to other Steel and used where rapid machining and high quality surface finish is primary requirement.
Cold working of steel is defined as working- a)at its recrystallization temperature
- b)above its recrystallization temperature
- c)below its recrystallization temperature
- d)at two third of melting temperature of the metal
Correct answer is option 'C'. Can you explain this answer?
Cold working of steel is defined as working
a)
at its recrystallization temperature
b)
above its recrystallization temperature
c)
below its recrystallization temperature
d)
at two third of melting temperature of the metal

|
Sameer Verma answered |
Understanding Cold Working of Steel
Cold working refers to the process of deforming metal at temperatures below its recrystallization point. For steel, this generally occurs at room temperature or slightly above.
Definition and Characteristics
- Temperature Range: Cold working is performed below the recrystallization temperature, typically less than 0.6 times the melting temperature of the steel.
- Deformation Mechanism: The process involves plastic deformation, where the steel is shaped or stretched, leading to an increase in dislocation density within the metal’s structure.
Benefits of Cold Working
- Increased Strength: The dislocations created during cold working hinder the movement of other dislocations, resulting in work hardening, which significantly increases the strength and hardness of steel.
- Improved Surface Finish: Cold working processes like rolling and drawing can enhance the surface finish of the steel, making it smoother.
Applications
- Manufacturing: Cold working is widely used in manufacturing components such as wires, sheets, and rods, where precise dimensions and properties are crucial.
- Structural Integrity: The enhanced mechanical properties obtained through cold working make it suitable for applications requiring high strength, such as in aerospace and automotive industries.
Conclusion
Cold working is a vital process in metalworking that allows for the manipulation of steel without elevated temperatures, leading to improved mechanical properties and surface characteristics. Understanding the significance of this process is essential for materials engineers and manufacturers alike.
Cold working refers to the process of deforming metal at temperatures below its recrystallization point. For steel, this generally occurs at room temperature or slightly above.
Definition and Characteristics
- Temperature Range: Cold working is performed below the recrystallization temperature, typically less than 0.6 times the melting temperature of the steel.
- Deformation Mechanism: The process involves plastic deformation, where the steel is shaped or stretched, leading to an increase in dislocation density within the metal’s structure.
Benefits of Cold Working
- Increased Strength: The dislocations created during cold working hinder the movement of other dislocations, resulting in work hardening, which significantly increases the strength and hardness of steel.
- Improved Surface Finish: Cold working processes like rolling and drawing can enhance the surface finish of the steel, making it smoother.
Applications
- Manufacturing: Cold working is widely used in manufacturing components such as wires, sheets, and rods, where precise dimensions and properties are crucial.
- Structural Integrity: The enhanced mechanical properties obtained through cold working make it suitable for applications requiring high strength, such as in aerospace and automotive industries.
Conclusion
Cold working is a vital process in metalworking that allows for the manipulation of steel without elevated temperatures, leading to improved mechanical properties and surface characteristics. Understanding the significance of this process is essential for materials engineers and manufacturers alike.
Which of the following is defined as the ability of the structure to transform into martensite?- a)Strength
- b)Hardenability
- c)Toughness
- d)Hardness
Correct answer is option 'B'. Can you explain this answer?
Which of the following is defined as the ability of the structure to transform into martensite?
a)
Strength
b)
Hardenability
c)
Toughness
d)
Hardness
|
|
Sharmila Chauhan answered |
Hardenability
Hardenability is defined as the ability of a material to be hardened through heat treatment. It specifically refers to the ability of a structure to transform into martensite, a hard and brittle phase, when cooled rapidly from a high temperature.
Explanation
When a metal is heated to a high temperature and then rapidly cooled, it undergoes a phase transformation from austenite to martensite. Austenite is a high-temperature phase with a face-centered cubic (FCC) crystal structure, while martensite is a low-temperature phase with a body-centered tetragonal (BCT) crystal structure.
The formation of martensite is accompanied by a significant increase in hardness and strength. This is because the BCT crystal structure of martensite is highly distorted, leading to a high dislocation density and a high resistance to deformation. Therefore, the ability of a structure to transform into martensite directly affects its hardness and strength.
Factors Affecting Hardenability
The hardenability of a material depends on several factors, including:
1. Alloying elements: Alloying elements such as carbon, chromium, and nickel can significantly affect the hardenability of a material. For example, increasing the carbon content in steel increases its hardenability.
2. Cooling rate: The rate at which a material is cooled from the austenitizing temperature influences its hardenability. Rapid cooling, such as quenching in water or oil, promotes the formation of martensite.
3. Grain size: Fine-grained materials have higher hardenability compared to coarse-grained materials. This is because smaller grains provide more nucleation sites for the formation of martensite.
4. Heat treatment: Heat treatment processes such as quenching and tempering can be used to manipulate the hardenability of a material. Quenching involves rapid cooling to maximize the formation of martensite, while tempering is a subsequent heat treatment to improve toughness and reduce brittleness.
Importance of Hardenability
Hardenability is an important property to consider in the design and selection of materials. It determines the depth and distribution of the hardened layer, which affects the overall mechanical properties of the material. Materials with high hardenability are suitable for applications requiring high strength and hardness, such as cutting tools and gears. On the other hand, materials with low hardenability are preferred for applications that require increased toughness and resistance to fracture, such as structural components.
In conclusion, hardenability is the ability of a structure to transform into martensite, a hard and brittle phase, upon rapid cooling. It is influenced by factors such as alloying elements, cooling rate, grain size, and heat treatment. Hardenability directly affects the hardness and strength of a material, making it an important consideration in material selection and design.
Hardenability is defined as the ability of a material to be hardened through heat treatment. It specifically refers to the ability of a structure to transform into martensite, a hard and brittle phase, when cooled rapidly from a high temperature.
Explanation
When a metal is heated to a high temperature and then rapidly cooled, it undergoes a phase transformation from austenite to martensite. Austenite is a high-temperature phase with a face-centered cubic (FCC) crystal structure, while martensite is a low-temperature phase with a body-centered tetragonal (BCT) crystal structure.
The formation of martensite is accompanied by a significant increase in hardness and strength. This is because the BCT crystal structure of martensite is highly distorted, leading to a high dislocation density and a high resistance to deformation. Therefore, the ability of a structure to transform into martensite directly affects its hardness and strength.
Factors Affecting Hardenability
The hardenability of a material depends on several factors, including:
1. Alloying elements: Alloying elements such as carbon, chromium, and nickel can significantly affect the hardenability of a material. For example, increasing the carbon content in steel increases its hardenability.
2. Cooling rate: The rate at which a material is cooled from the austenitizing temperature influences its hardenability. Rapid cooling, such as quenching in water or oil, promotes the formation of martensite.
3. Grain size: Fine-grained materials have higher hardenability compared to coarse-grained materials. This is because smaller grains provide more nucleation sites for the formation of martensite.
4. Heat treatment: Heat treatment processes such as quenching and tempering can be used to manipulate the hardenability of a material. Quenching involves rapid cooling to maximize the formation of martensite, while tempering is a subsequent heat treatment to improve toughness and reduce brittleness.
Importance of Hardenability
Hardenability is an important property to consider in the design and selection of materials. It determines the depth and distribution of the hardened layer, which affects the overall mechanical properties of the material. Materials with high hardenability are suitable for applications requiring high strength and hardness, such as cutting tools and gears. On the other hand, materials with low hardenability are preferred for applications that require increased toughness and resistance to fracture, such as structural components.
In conclusion, hardenability is the ability of a structure to transform into martensite, a hard and brittle phase, upon rapid cooling. It is influenced by factors such as alloying elements, cooling rate, grain size, and heat treatment. Hardenability directly affects the hardness and strength of a material, making it an important consideration in material selection and design.
Whiskers are- a)very thin wires
- b)very thin copper wires
- c)very thin single crystals
- d)none of these
Correct answer is option 'C'. Can you explain this answer?
Whiskers are
a)
very thin wires
b)
very thin copper wires
c)
very thin single crystals
d)
none of these
|
|
Shreya Kulkarni answered |
Whiskers are very thin filaments, hair-like single crystals of about 13 mm length and 10-4 cm diameter (approx). These are produced as dislocations of free - crystals and are without any structural defect. Whiskers are far stronger than polycrystals of same materials.
Which of the following is not an example of laminar composite?- a)Wood
- b)Bimetallic
- c)Coatings/Paints
- d)Claddings
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not an example of laminar composite?
a)
Wood
b)
Bimetallic
c)
Coatings/Paints
d)
Claddings
|
|
Amrita Chauhan answered |
When multidirectional stresses are imposed within a single plane, a ligned layers that are fastened together one on top of another at different orientations are frequently utilized. These are called laminar composites. These are generally designed to provide high strength and low cost at a lighter weight.
Which is closest to the purest form of iron- a)Cast iron
- b)Wrought iron
- c)Grey iron
- d)Mild Steel
Correct answer is option 'B'. Can you explain this answer?
Which is closest to the purest form of iron
a)
Cast iron
b)
Wrought iron
c)
Grey iron
d)
Mild Steel
|
|
Rajat Khanna answered |
Wrought iron contains as much as 99.9% of iron. Since it contains very little amount of Carbon, it is very pure iron.
Consider the following effects of yield point:
1. Luders bands
2. Strain ageing
3. Blue brittleness
4. Orange peel effect
Q. Which of the above are true?- a)1,2 and 4
- b)2 and 4
- c)1,3 and 4
- d)all of these
Correct answer is option 'B'. Can you explain this answer?
Consider the following effects of yield point:
1. Luders bands
2. Strain ageing
3. Blue brittleness
4. Orange peel effect
Q. Which of the above are true?
1. Luders bands
2. Strain ageing
3. Blue brittleness
4. Orange peel effect
Q. Which of the above are true?
a)
1,2 and 4
b)
2 and 4
c)
1,3 and 4
d)
all of these
|
|
Suyash Patel answered |
Luders Bands: These represents the marking on the surface of a tensile test sample, formed at the points of stress concentration like fillets.
Strain Ageing: It refers to changes in the properties of an over strained alloy with time. Strain ageing or strain-age-hardening is accompanied by hardening due to increase in stress value.
Blue Brittleness: When the temperature range of the test is raised, the yield point becomes less pronounced and blue brittleness effect is produced.
Orange Peel effects: During stretching, these markings appear on metals.
Strain Ageing: It refers to changes in the properties of an over strained alloy with time. Strain ageing or strain-age-hardening is accompanied by hardening due to increase in stress value.
Blue Brittleness: When the temperature range of the test is raised, the yield point becomes less pronounced and blue brittleness effect is produced.
Orange Peel effects: During stretching, these markings appear on metals.
Which is false statement about properties of aluminium?- a)modulus of elasticity is fairly low
- b)wear resistance is very good
- c)fatigue strength is not high
- d)corrosion resistance is good
Correct answer is option 'B'. Can you explain this answer?
Which is false statement about properties of aluminium?
a)
modulus of elasticity is fairly low
b)
wear resistance is very good
c)
fatigue strength is not high
d)
corrosion resistance is good
|
|
Ruchi Ahuja answered |
Properties of Aluminium
Aluminium is a lightweight, strong, and durable metal that is widely used in various industries. Some of the properties of aluminium are:
1. Modulus of Elasticity is Fairly Low:
Aluminium has a relatively low modulus of elasticity compared to other metals. This means that it is more flexible and less stiff than materials like steel. However, this property also makes it more susceptible to deformation and bending under stress.
2. Wear Resistance is not Very Good:
Contrary to the statement given in the question, the wear resistance of aluminium is not very good. It has a relatively low hardness and can easily scratch, scuff, or dent under abrasive or impact forces. Therefore, it is often coated or treated with other materials to enhance its wear resistance.
3. Fatigue Strength is not High:
Aluminium has a low fatigue strength, which means that it can fail or crack under cyclic loading or repeated stresses. This property makes it less suitable for applications that require high durability and long-term reliability.
4. Corrosion Resistance is Good:
Aluminium has a natural oxide layer that forms on its surface when exposed to air or water. This layer provides a protective barrier against corrosion, rust, or tarnishing. Therefore, aluminium is often used in applications that require resistance to environmental or chemical exposure.
Conclusion:
In conclusion, the false statement about properties of aluminium is option 'B' that the wear resistance of aluminium is very good. Aluminium has a relatively low wear resistance and is susceptible to scratches, scuffs, or dents under abrasive or impact forces.
Aluminium is a lightweight, strong, and durable metal that is widely used in various industries. Some of the properties of aluminium are:
1. Modulus of Elasticity is Fairly Low:
Aluminium has a relatively low modulus of elasticity compared to other metals. This means that it is more flexible and less stiff than materials like steel. However, this property also makes it more susceptible to deformation and bending under stress.
2. Wear Resistance is not Very Good:
Contrary to the statement given in the question, the wear resistance of aluminium is not very good. It has a relatively low hardness and can easily scratch, scuff, or dent under abrasive or impact forces. Therefore, it is often coated or treated with other materials to enhance its wear resistance.
3. Fatigue Strength is not High:
Aluminium has a low fatigue strength, which means that it can fail or crack under cyclic loading or repeated stresses. This property makes it less suitable for applications that require high durability and long-term reliability.
4. Corrosion Resistance is Good:
Aluminium has a natural oxide layer that forms on its surface when exposed to air or water. This layer provides a protective barrier against corrosion, rust, or tarnishing. Therefore, aluminium is often used in applications that require resistance to environmental or chemical exposure.
Conclusion:
In conclusion, the false statement about properties of aluminium is option 'B' that the wear resistance of aluminium is very good. Aluminium has a relatively low wear resistance and is susceptible to scratches, scuffs, or dents under abrasive or impact forces.
Select the wrong statement:- a)In flame hardening, oxy-acetylene flame is used.
- b)In induction hardening, frequency is kept high.
- c)In the nitriding process, both carbon and nitrogen are absorbed by the metal surface to get it hardened.
- d)Carburising, cyaniding and nitriding are case hardening processes.
Correct answer is option 'C'. Can you explain this answer?
Select the wrong statement:
a)
In flame hardening, oxy-acetylene flame is used.
b)
In induction hardening, frequency is kept high.
c)
In the nitriding process, both carbon and nitrogen are absorbed by the metal surface to get it hardened.
d)
Carburising, cyaniding and nitriding are case hardening processes.

|
Moumita Rane answered |
In nitriding, only nitrogen is used.
Puddling is the process employed for converting- a)Iron ore into Pig Iron
- b)Pig Iron into Cast Iron
- c)Pig Iron into Wrought Iron
- d)Cast Iron into Mild Steel
Correct answer is option 'C'. Can you explain this answer?
Puddling is the process employed for converting
a)
Iron ore into Pig Iron
b)
Pig Iron into Cast Iron
c)
Pig Iron into Wrought Iron
d)
Cast Iron into Mild Steel
|
|
Yash Das answered |
Puddling Process Overview
The puddling process is a crucial step in the metallurgy of iron, specifically for converting pig iron into wrought iron. This method involves the removal of excess carbon and impurities present in pig iron, resulting in a more malleable and ductile form of iron.
Key Steps in the Puddling Process
- Furnace Setup: The process takes place in a reverberatory furnace, where pig iron is heated to high temperatures.
- Oxidation: The pig iron is stirred to facilitate the oxidation of carbon and other impurities. This is essential for transforming the brittle pig iron into a workable form.
- Formation of Wrought Iron: As carbon is oxidized, it escapes as gas, and the remaining iron becomes purer. The final product is wrought iron, which is characterized by its low carbon content and improved mechanical properties.
Characteristics of Wrought Iron
- High Ductility: Wrought iron is known for its excellent ductility, allowing it to be easily shaped and worked.
- Corrosion Resistance: It exhibits better resistance to corrosion compared to cast iron due to its lower carbon content.
- Applications: Wrought iron is used in various applications, including construction, automotive components, and decorative items, owing to its strength and flexibility.
Conclusion
In summary, the puddling process is significant for converting pig iron into wrought iron, enhancing the material's properties and making it suitable for various applications in engineering and manufacturing industries.
The puddling process is a crucial step in the metallurgy of iron, specifically for converting pig iron into wrought iron. This method involves the removal of excess carbon and impurities present in pig iron, resulting in a more malleable and ductile form of iron.
Key Steps in the Puddling Process
- Furnace Setup: The process takes place in a reverberatory furnace, where pig iron is heated to high temperatures.
- Oxidation: The pig iron is stirred to facilitate the oxidation of carbon and other impurities. This is essential for transforming the brittle pig iron into a workable form.
- Formation of Wrought Iron: As carbon is oxidized, it escapes as gas, and the remaining iron becomes purer. The final product is wrought iron, which is characterized by its low carbon content and improved mechanical properties.
Characteristics of Wrought Iron
- High Ductility: Wrought iron is known for its excellent ductility, allowing it to be easily shaped and worked.
- Corrosion Resistance: It exhibits better resistance to corrosion compared to cast iron due to its lower carbon content.
- Applications: Wrought iron is used in various applications, including construction, automotive components, and decorative items, owing to its strength and flexibility.
Conclusion
In summary, the puddling process is significant for converting pig iron into wrought iron, enhancing the material's properties and making it suitable for various applications in engineering and manufacturing industries.
Consider the following polymers:
1. Nylon-6
2. Nylon-6,6
3. Polyvinyl chloride
4, Poly styrene
Polyamide includes- a)1 only
- b)1 and 2
- c)1, 3 and 4
- d)all of the above
Correct answer is option 'B'. Can you explain this answer?
Consider the following polymers:
1. Nylon-6
2. Nylon-6,6
3. Polyvinyl chloride
4, Poly styrene
Polyamide includes
1. Nylon-6
2. Nylon-6,6
3. Polyvinyl chloride
4, Poly styrene
Polyamide includes
a)
1 only
b)
1 and 2
c)
1, 3 and 4
d)
all of the above
|
|
Manoj Pillai answered |
Understanding Polyamides
Polyamides are a class of polymers characterized by the presence of amide linkages (-CONH-). They are known for their strength, durability, and resistance to wear and tear.
Polymers in Question
1. Nylon-6:
- A synthetic polymer made from caprolactam.
- Contains amide linkages, classifying it as a polyamide.
2. Nylon-6,6:
- Formed from hexamethylenediamine and adipic acid.
- Also contains amide linkages, making it another example of a polyamide.
3. Polyvinyl Chloride (PVC):
- A widely used plastic made from vinyl chloride monomer.
- It does not contain amide linkages and is not classified as a polyamide.
4. Polystyrene:
- A polymer made from the monomer styrene.
- Lacks amide linkages and is not classified as a polyamide.
Correct Answer Explanation
- The correct answer is option 'B' (Nylon-6 and Nylon-6,6).
- Both Nylon-6 and Nylon-6,6 are polyamides due to their amide linkages.
- Polyvinyl chloride and polystyrene do not fit into the polyamide category because they lack these linkages.
Summary
In conclusion, the only polymers that are classified as polyamides from the given options are Nylon-6 and Nylon-6,6. PVC and polystyrene do not meet the criteria for polyamides, thus reinforcing that option 'B' is the correct choice.
Polyamides are a class of polymers characterized by the presence of amide linkages (-CONH-). They are known for their strength, durability, and resistance to wear and tear.
Polymers in Question
1. Nylon-6:
- A synthetic polymer made from caprolactam.
- Contains amide linkages, classifying it as a polyamide.
2. Nylon-6,6:
- Formed from hexamethylenediamine and adipic acid.
- Also contains amide linkages, making it another example of a polyamide.
3. Polyvinyl Chloride (PVC):
- A widely used plastic made from vinyl chloride monomer.
- It does not contain amide linkages and is not classified as a polyamide.
4. Polystyrene:
- A polymer made from the monomer styrene.
- Lacks amide linkages and is not classified as a polyamide.
Correct Answer Explanation
- The correct answer is option 'B' (Nylon-6 and Nylon-6,6).
- Both Nylon-6 and Nylon-6,6 are polyamides due to their amide linkages.
- Polyvinyl chloride and polystyrene do not fit into the polyamide category because they lack these linkages.
Summary
In conclusion, the only polymers that are classified as polyamides from the given options are Nylon-6 and Nylon-6,6. PVC and polystyrene do not meet the criteria for polyamides, thus reinforcing that option 'B' is the correct choice.
Chapter doubts & questions for Engineering Materials - Topicwise Question Bank for Mechanical Engineering 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Engineering Materials - Topicwise Question Bank for Mechanical Engineering in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Topicwise Question Bank for Mechanical Engineering
45 videos|314 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup