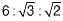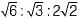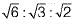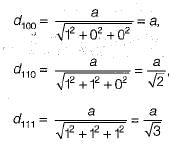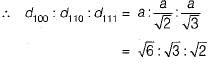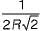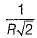All Exams >
Mechanical Engineering >
Engineering Materials >
All Questions
All questions of Structure of Engineering Materials for Mechanical Engineering Exam
In structure, all metals are- a)granular
- b)crystalline
- c)wrought
- d)amorphous
Correct answer is option 'B'. Can you explain this answer?
In structure, all metals are
a)
granular
b)
crystalline
c)
wrought
d)
amorphous
|
|
Isha Nambiar answered |
Metallic Structure
Metallic structure refers to the arrangement of metal atoms in a solid. The arrangement of metal atoms can be granular, crystalline, or amorphous.
Granular Structure
A granular structure refers to a metal structure that is composed of granules or grains. The grains are usually visible to the naked eye and are formed when molten metal solidifies.
Wrought Structure
Wrought structure refers to the arrangement of metal atoms in a metal that has been worked or shaped by mechanical or thermal means. This type of structure is often found in metals that have been forged, rolled, or drawn.
Amorphous Structure
An amorphous structure refers to a metal structure that lacks long-range order or crystalline structure. This type of structure is usually found in metals that have been rapidly cooled, such as in the case of metallic glasses.
Crystalline Structure
A crystalline structure refers to a metal structure that has a repeating pattern of atoms in three dimensions. This type of structure is often found in metals that have been slowly cooled, allowing the atoms to arrange themselves in an ordered pattern.
Conclusion
In structure, all metals are crystalline, meaning they have a repeating pattern of atoms in three dimensions. This is due to the way in which metals solidify, allowing the atoms to arrange themselves in an ordered pattern as the metal cools.
Metallic structure refers to the arrangement of metal atoms in a solid. The arrangement of metal atoms can be granular, crystalline, or amorphous.
Granular Structure
A granular structure refers to a metal structure that is composed of granules or grains. The grains are usually visible to the naked eye and are formed when molten metal solidifies.
Wrought Structure
Wrought structure refers to the arrangement of metal atoms in a metal that has been worked or shaped by mechanical or thermal means. This type of structure is often found in metals that have been forged, rolled, or drawn.
Amorphous Structure
An amorphous structure refers to a metal structure that lacks long-range order or crystalline structure. This type of structure is usually found in metals that have been rapidly cooled, such as in the case of metallic glasses.
Crystalline Structure
A crystalline structure refers to a metal structure that has a repeating pattern of atoms in three dimensions. This type of structure is often found in metals that have been slowly cooled, allowing the atoms to arrange themselves in an ordered pattern.
Conclusion
In structure, all metals are crystalline, meaning they have a repeating pattern of atoms in three dimensions. This is due to the way in which metals solidify, allowing the atoms to arrange themselves in an ordered pattern as the metal cools.
The defect which takes place due to imperfect packing of atoms during crystallization is known as
- a)line defect
- b)Both (a) and (c)
- c)surface defect
- d)point defect
Correct answer is option 'D'. Can you explain this answer?
The defect which takes place due to imperfect packing of atoms during crystallization is known as
a)
line defect
b)
Both (a) and (c)
c)
surface defect
d)
point defect

|
Gate Funda answered |
Point defects are where an atom is missing or is in an irregular place in the lattice structure.
Point defects include self interstitial atoms, interstitial impurity atoms, substitutional atoms and vacancies
Point defects include self interstitial atoms, interstitial impurity atoms, substitutional atoms and vacancies
Which of the following elements is not a metalloid?
- a)Copper
- b)germanium
- c)boron
- d)Silicon
Correct answer is option 'A'. Can you explain this answer?
Which of the following elements is not a metalloid?
a)
Copper
b)
germanium
c)
boron
d)
Silicon

|
Simran Saha answered |
Answer:
To determine which of the given elements is not a metalloid, we need to understand the properties and characteristics of metalloids and identify the one element that does not possess these characteristics.
Metalloids:
Metalloids, also known as semi-metals, are a group of elements that have properties intermediate between those of metals and nonmetals. They exhibit both metallic and nonmetallic properties, making them unique. Metalloids are typically solid at room temperature and have a shiny or metallic appearance. They are also generally brittle and semiconductors of electricity.
Given Elements:
a) Copper: Copper is a metal and not a metalloid. It is a good conductor of electricity and heat, possesses metallic luster, and has high ductility and malleability. Therefore, copper is not a metalloid.
b) Carbon: Carbon is a nonmetal and not a metalloid. It exists in various forms, such as graphite and diamond, which have different properties. Carbon is a poor conductor of electricity and heat, and it does not possess metallic luster. Therefore, carbon is not a metalloid.
c) Sulphur: Sulphur is a nonmetal and not a metalloid. It is a poor conductor of electricity and heat and does not possess metallic luster. Sulphur is brittle and can exist in different allotropes. Therefore, sulphur is not a metalloid.
d) Silicon: Silicon is a metalloid. It is a semiconductor of electricity and possesses some metallic properties like luster. Silicon is brittle and is used extensively in the electronics industry. Therefore, silicon is a metalloid.
Conclusion:
Among the given elements, copper is the only one that is not a metalloid. Copper is a metal, while carbon, sulphur, and silicon are nonmetals or metalloids.
To determine which of the given elements is not a metalloid, we need to understand the properties and characteristics of metalloids and identify the one element that does not possess these characteristics.
Metalloids:
Metalloids, also known as semi-metals, are a group of elements that have properties intermediate between those of metals and nonmetals. They exhibit both metallic and nonmetallic properties, making them unique. Metalloids are typically solid at room temperature and have a shiny or metallic appearance. They are also generally brittle and semiconductors of electricity.
Given Elements:
a) Copper: Copper is a metal and not a metalloid. It is a good conductor of electricity and heat, possesses metallic luster, and has high ductility and malleability. Therefore, copper is not a metalloid.
b) Carbon: Carbon is a nonmetal and not a metalloid. It exists in various forms, such as graphite and diamond, which have different properties. Carbon is a poor conductor of electricity and heat, and it does not possess metallic luster. Therefore, carbon is not a metalloid.
c) Sulphur: Sulphur is a nonmetal and not a metalloid. It is a poor conductor of electricity and heat and does not possess metallic luster. Sulphur is brittle and can exist in different allotropes. Therefore, sulphur is not a metalloid.
d) Silicon: Silicon is a metalloid. It is a semiconductor of electricity and possesses some metallic properties like luster. Silicon is brittle and is used extensively in the electronics industry. Therefore, silicon is a metalloid.
Conclusion:
Among the given elements, copper is the only one that is not a metalloid. Copper is a metal, while carbon, sulphur, and silicon are nonmetals or metalloids.
Which of the following regions of the electromagnetic spectrum would be used to determine the structure of crystalline solids?- a)Microwave
- b)Infrared
- c)X-ray
- d)Visible
Correct answer is option 'C'. Can you explain this answer?
Which of the following regions of the electromagnetic spectrum would be used to determine the structure of crystalline solids?
a)
Microwave
b)
Infrared
c)
X-ray
d)
Visible

|
Nilesh Kapoor answered |
X-ray crystallography: A technique in which the patterns formed by the diffraction of X-rays on passing through a crystalline substance yield information on the lattice structure of the crystal, and the molecular structure of the substance.
The co-ordination number of BCC crystal structure is- a)8
- b)16
- c)12
- d)10
Correct answer is option 'A'. Can you explain this answer?
The co-ordination number of BCC crystal structure is
a)
8
b)
16
c)
12
d)
10
|
|
Anjali Sengupta answered |
The co-ordination number of a crystal structure refers to the number of nearest neighboring atoms surrounding a central atom. In the case of a body-centered cubic (BCC) crystal structure, the co-ordination number is 8.
Explanation:
1. Body-Centered Cubic (BCC) Structure:
- The BCC crystal structure is one of the common arrangements of atoms in a solid material.
- In this structure, the atoms are arranged in a cubic lattice, with an additional atom located in the center of the cube.
- Each corner of the cube is shared by 8 adjacent unit cells, while the center atom is not shared with any other unit cells.
2. Identifying Nearest Neighbors:
- To determine the co-ordination number of a BCC structure, we need to identify the nearest neighboring atoms surrounding a central atom.
- In this case, the central atom is located at the center of the cube.
- The nearest neighboring atoms are the 8 atoms situated at the corners of the cube.
- Each corner atom is shared by 8 unit cells, with one unit cell belonging to the central atom.
3. Co-ordination Number:
- The co-ordination number is the total number of nearest neighboring atoms surrounding a central atom.
- In the BCC crystal structure, there are 8 corner atoms surrounding the central atom.
- Therefore, the co-ordination number of a BCC crystal structure is 8.
In summary, the co-ordination number of a BCC crystal structure is 8 because there are 8 corner atoms surrounding the central atom.
Explanation:
1. Body-Centered Cubic (BCC) Structure:
- The BCC crystal structure is one of the common arrangements of atoms in a solid material.
- In this structure, the atoms are arranged in a cubic lattice, with an additional atom located in the center of the cube.
- Each corner of the cube is shared by 8 adjacent unit cells, while the center atom is not shared with any other unit cells.
2. Identifying Nearest Neighbors:
- To determine the co-ordination number of a BCC structure, we need to identify the nearest neighboring atoms surrounding a central atom.
- In this case, the central atom is located at the center of the cube.
- The nearest neighboring atoms are the 8 atoms situated at the corners of the cube.
- Each corner atom is shared by 8 unit cells, with one unit cell belonging to the central atom.
3. Co-ordination Number:
- The co-ordination number is the total number of nearest neighboring atoms surrounding a central atom.
- In the BCC crystal structure, there are 8 corner atoms surrounding the central atom.
- Therefore, the co-ordination number of a BCC crystal structure is 8.
In summary, the co-ordination number of a BCC crystal structure is 8 because there are 8 corner atoms surrounding the central atom.
What is the planer density of (100) plane in FCC (face-centred cubic) crystal with unit cell side a equa! to?- a)1.484/a2
- b)2/a2
- c)1/a2
- d)√2/a2
Correct answer is option 'B'. Can you explain this answer?
What is the planer density of (100) plane in FCC (face-centred cubic) crystal with unit cell side a equa! to?
a)
1.484/a2
b)
2/a2
c)
1/a2
d)
√2/a2
|
|
Stuti Mishra answered |
FCC on (100) plane

No. of atoms = 1/4 x 4 +1 = 2
Area = a x a = a2
Planar density = 2/a2

No. of atoms = 1/4 x 4 +1 = 2
Area = a x a = a2
Planar density = 2/a2
The effective number of lattice points in the unit celi of simple cubic, body centered cubic and face centered cubic space lattices respectively are- a)1, 2, 2
- b)1, 2, 4
- c)2, 3, 4
- d)2, 4, 4
Correct answer is option 'B'. Can you explain this answer?
The effective number of lattice points in the unit celi of simple cubic, body centered cubic and face centered cubic space lattices respectively are
a)
1, 2, 2
b)
1, 2, 4
c)
2, 3, 4
d)
2, 4, 4

|
Anuj Chakraborty answered |
For simple cubic effective number of lattice point = 1
For B.C.C. effective number of lattice point = 2
For F.C.C. effective number of lattice point = 4
For B.C.C. effective number of lattice point = 2
For F.C.C. effective number of lattice point = 4
Which is false statement about properties of aluminium?- a)modulus of elasticity is fairly low
- b)wear resistance is very good
- c)fatigue strength is not high
- d)corrosion resistance is good
Correct answer is option 'B'. Can you explain this answer?
Which is false statement about properties of aluminium?
a)
modulus of elasticity is fairly low
b)
wear resistance is very good
c)
fatigue strength is not high
d)
corrosion resistance is good
|
|
Ruchi Ahuja answered |
Properties of Aluminium
Aluminium is a lightweight, strong, and durable metal that is widely used in various industries. Some of the properties of aluminium are:
1. Modulus of Elasticity is Fairly Low:
Aluminium has a relatively low modulus of elasticity compared to other metals. This means that it is more flexible and less stiff than materials like steel. However, this property also makes it more susceptible to deformation and bending under stress.
2. Wear Resistance is not Very Good:
Contrary to the statement given in the question, the wear resistance of aluminium is not very good. It has a relatively low hardness and can easily scratch, scuff, or dent under abrasive or impact forces. Therefore, it is often coated or treated with other materials to enhance its wear resistance.
3. Fatigue Strength is not High:
Aluminium has a low fatigue strength, which means that it can fail or crack under cyclic loading or repeated stresses. This property makes it less suitable for applications that require high durability and long-term reliability.
4. Corrosion Resistance is Good:
Aluminium has a natural oxide layer that forms on its surface when exposed to air or water. This layer provides a protective barrier against corrosion, rust, or tarnishing. Therefore, aluminium is often used in applications that require resistance to environmental or chemical exposure.
Conclusion:
In conclusion, the false statement about properties of aluminium is option 'B' that the wear resistance of aluminium is very good. Aluminium has a relatively low wear resistance and is susceptible to scratches, scuffs, or dents under abrasive or impact forces.
Aluminium is a lightweight, strong, and durable metal that is widely used in various industries. Some of the properties of aluminium are:
1. Modulus of Elasticity is Fairly Low:
Aluminium has a relatively low modulus of elasticity compared to other metals. This means that it is more flexible and less stiff than materials like steel. However, this property also makes it more susceptible to deformation and bending under stress.
2. Wear Resistance is not Very Good:
Contrary to the statement given in the question, the wear resistance of aluminium is not very good. It has a relatively low hardness and can easily scratch, scuff, or dent under abrasive or impact forces. Therefore, it is often coated or treated with other materials to enhance its wear resistance.
3. Fatigue Strength is not High:
Aluminium has a low fatigue strength, which means that it can fail or crack under cyclic loading or repeated stresses. This property makes it less suitable for applications that require high durability and long-term reliability.
4. Corrosion Resistance is Good:
Aluminium has a natural oxide layer that forms on its surface when exposed to air or water. This layer provides a protective barrier against corrosion, rust, or tarnishing. Therefore, aluminium is often used in applications that require resistance to environmental or chemical exposure.
Conclusion:
In conclusion, the false statement about properties of aluminium is option 'B' that the wear resistance of aluminium is very good. Aluminium has a relatively low wear resistance and is susceptible to scratches, scuffs, or dents under abrasive or impact forces.
Surface imperfections that separate two orientations that are mirror image of one- another is called- a)stacking fault
- b)grain boundary
- c)tilt boundary
- d)twinned boundary
Correct answer is option 'D'. Can you explain this answer?
Surface imperfections that separate two orientations that are mirror image of one- another is called
a)
stacking fault
b)
grain boundary
c)
tilt boundary
d)
twinned boundary
|
|
Mrinalini Sharma answered |
The grain boundary defects are called twin boundary when the surface imperfect orientations on the side are mirror image of those in the opposite side.
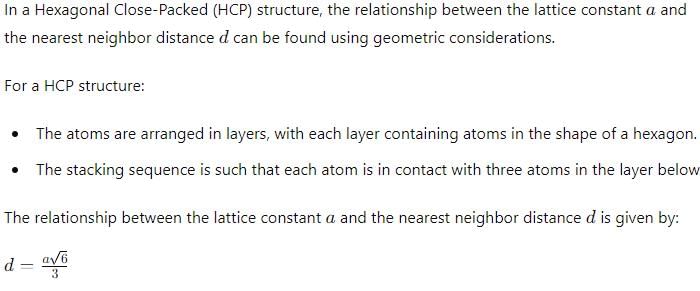
The ratio of long and short unit cell dimensions of ideal HCP crystal structure should be- a)1.56
- b)1.89
- c)1.633
- d)1.59
Correct answer is option 'C'. Can you explain this answer?
The ratio of long and short unit cell dimensions of ideal HCP crystal structure should be
a)
1.56
b)
1.89
c)
1.633
d)
1.59

|
Navya Saha answered |
For the ideal HCP packing, the ratio of c/a is 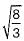 i.e. 1.633. The actual HCP metals deviate from ideal c/a ratio.
i.e. 1.633. The actual HCP metals deviate from ideal c/a ratio.
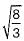 i.e. 1.633. The actual HCP metals deviate from ideal c/a ratio.
i.e. 1.633. The actual HCP metals deviate from ideal c/a ratio.Atomic packing factor in case of Copper Crystal is- a)0.52
- b)0.68
- c)0.74
- d)1.633
Correct answer is option 'C'. Can you explain this answer?
Atomic packing factor in case of Copper Crystal is
a)
0.52
b)
0.68
c)
0.74
d)
1.633
|
|
Akshara Rane answered |
Since Copper .is having FCC structure APF = 0.74
Which of the following is structure sensitive property?- a)Elastic contacts
- b)Density
- c)Creep strength
- d)Specific heat
Correct answer is option 'C'. Can you explain this answer?
Which of the following is structure sensitive property?
a)
Elastic contacts
b)
Density
c)
Creep strength
d)
Specific heat
|
|
Manoj Pillai answered |
Structure sensitive properties: Electrical conductivity, semiconductor properties, yield stress, fracture strength, creep strength.
The linear density along the direction (110) will be equal to, if lattice constant of copper unit cell is 3.61 Å.- a)3.91 x 106 atoms/mm
- b)39.1 x 106 atoms/mm
- c)4.91 x 106 atoms/mm
- d)49.1 x 106 atoms/mm
Correct answer is option 'A'. Can you explain this answer?
The linear density along the direction (110) will be equal to, if lattice constant of copper unit cell is 3.61 Å.
a)
3.91 x 106 atoms/mm
b)
39.1 x 106 atoms/mm
c)
4.91 x 106 atoms/mm
d)
49.1 x 106 atoms/mm
|
|
Isha Nambiar answered |

= 3.91 x 109 atoms/m
ρ = 3.91 x 106 atoms/mm
Which one of the following pairs of axis lengths (a, b, c) and inter axial angles (a, β, γ) represents the tetragonal crystal system?- a)a = b = c α = β = γ = 90°
- b)a = b ≠ c α = β = γ = 90°
- c)a ≠ b ≠ c α = β = γ = 90°
- d)a = b = c α = β = γ ≠ 90°
Correct answer is option 'B'. Can you explain this answer?
Which one of the following pairs of axis lengths (a, b, c) and inter axial angles (a, β, γ) represents the tetragonal crystal system?
a)
a = b = c α = β = γ = 90°
b)
a = b ≠ c α = β = γ = 90°
c)
a ≠ b ≠ c α = β = γ = 90°
d)
a = b = c α = β = γ ≠ 90°
|
|
Neha Joshi answered |

Dislocation in a material is a _____ defect.- a)point
- b)iine
- c)plane
- d)volumetric
Correct answer is option 'B'. Can you explain this answer?
Dislocation in a material is a _____ defect.
a)
point
b)
iine
c)
plane
d)
volumetric
|
|
Mansi Rane answered |
Line imperfections are known as dislocations.
The crystal structure of brass is- a)FCC
- b)BCC
- c)HCP
- d)Orthorhombic
Correct answer is option 'A'. Can you explain this answer?
The crystal structure of brass is
a)
FCC
b)
BCC
c)
HCP
d)
Orthorhombic
|
|
Nitin Joshi answered |
Crystal Structure of Brass
Brass is an alloy of copper and zinc. The crystal structure of brass is determined by the arrangement of atoms in the alloy. The crystal structure of brass is FCC (face-centered cubic).
Explanation
FCC (face-centered cubic) is a crystal structure where atoms are arranged in a cubic lattice with an atom at each corner and one in the center of each face. Brass is a solid solution of copper and zinc, and the FCC structure is formed due to the similar atomic radii of copper and zinc.
The FCC crystal structure of brass makes it a ductile and malleable material. Due to the FCC structure, the atoms in brass are closely packed, which results in attractive forces between the atoms. These forces are responsible for the high ductility and malleability of the brass.
Conclusion
In conclusion, the crystal structure of brass is FCC. This structure is responsible for the properties of brass, including its ductility and malleability. Understanding the crystal structure of brass is important for understanding its properties and applications in engineering and manufacturing.
Brass is an alloy of copper and zinc. The crystal structure of brass is determined by the arrangement of atoms in the alloy. The crystal structure of brass is FCC (face-centered cubic).
Explanation
FCC (face-centered cubic) is a crystal structure where atoms are arranged in a cubic lattice with an atom at each corner and one in the center of each face. Brass is a solid solution of copper and zinc, and the FCC structure is formed due to the similar atomic radii of copper and zinc.
The FCC crystal structure of brass makes it a ductile and malleable material. Due to the FCC structure, the atoms in brass are closely packed, which results in attractive forces between the atoms. These forces are responsible for the high ductility and malleability of the brass.
Conclusion
In conclusion, the crystal structure of brass is FCC. This structure is responsible for the properties of brass, including its ductility and malleability. Understanding the crystal structure of brass is important for understanding its properties and applications in engineering and manufacturing.
What is the movement of block of atoms along certain crystallographic phase and directions termed as- a)Glide
- b)Twinning
- c)Slip
- d)Jog
Correct answer is option 'C'. Can you explain this answer?
What is the movement of block of atoms along certain crystallographic phase and directions termed as
a)
Glide
b)
Twinning
c)
Slip
d)
Jog
|
|
Ameya Kaur answered |
Slip is defined as a irreversible shear displacement of one part of a crystal relative to another in a definite crystallographic direction and an a specific crystallographic plane.
A screw dislocation
1. Lies parallel to its Burger’s vector
2. Lies perpendicular to its Burger’s vector
3. Moves in a perpendicular direction to the Burger’s vector
4. Moves in an inclined direction to the Burger’s vector
Q. Select the correct answer using the codes given below:- a)1 and 4
- b)1 and 3
- c)2 and 3
- d)2 and 4
Correct answer is option 'B'. Can you explain this answer?
A screw dislocation
1. Lies parallel to its Burger’s vector
2. Lies perpendicular to its Burger’s vector
3. Moves in a perpendicular direction to the Burger’s vector
4. Moves in an inclined direction to the Burger’s vector
Q. Select the correct answer using the codes given below:
1. Lies parallel to its Burger’s vector
2. Lies perpendicular to its Burger’s vector
3. Moves in a perpendicular direction to the Burger’s vector
4. Moves in an inclined direction to the Burger’s vector
Q. Select the correct answer using the codes given below:
a)
1 and 4
b)
1 and 3
c)
2 and 3
d)
2 and 4

|
Akshat Datta answered |
Screw dislocation lies parallel to Burger vector and moves in perpendicular direction to Burger vector.
Edge dislocation - Burger vector in normal to dislocation line and movement will be parallel to Burger vector.
Edge dislocation - Burger vector in normal to dislocation line and movement will be parallel to Burger vector.
An example of amorphous material is - a)brass
- b)glass
- c)silver
- d)lead
Correct answer is option 'B'. Can you explain this answer?
An example of amorphous material is
a)
brass
b)
glass
c)
silver
d)
lead

|
Debolina Chavan answered |
Amorphous material is one in which there is no definite atomic structure and atom exist in a random pattern just as in a liquid.
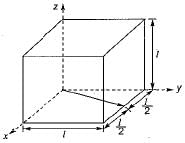
What is the volume of an FCC cell in terms of the atomic radius R?- a)16 R3
- b)
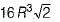
- c)
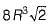
- d)8 R3
Correct answer is option 'B'. Can you explain this answer?
What is the volume of an FCC cell in terms of the atomic radius R?
a)
16 R3
b)
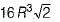
c)
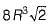
d)
8 R3
|
|
Keerthana Joshi answered |
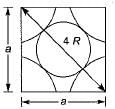
From the right angle on the face,
a2 + a2 = (4 R)2
∴ 2 a2 = 16 R2
∴
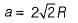
Volume of FCC unit,
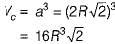
Which of the following material is not a ferromagnetic material?- a)Iron
- b)Nickel
- c)Zinc
- d)Cobalt
Correct answer is option 'C'. Can you explain this answer?
Which of the following material is not a ferromagnetic material?
a)
Iron
b)
Nickel
c)
Zinc
d)
Cobalt

|
Shivani Choudhury answered |
Introduction:
Ferromagnetic materials are substances that exhibit strong magnetic properties and can be permanently magnetized. These materials are composed of atoms or ions that have magnetic moments aligned in the same direction. Iron, nickel, and cobalt are well-known examples of ferromagnetic materials. However, zinc is not a ferromagnetic material.
Explanation:
Zinc is an element that is classified as a diamagnetic material rather than a ferromagnetic material. Diamagnetic materials are substances that have weak magnetic properties and are repelled by a magnetic field. They do not retain any magnetization after the external magnetic field is removed.
Comparison between Ferromagnetic and Diamagnetic Materials:
To understand why zinc is not a ferromagnetic material, let's compare the properties of ferromagnetic and diamagnetic materials:
1. Ferromagnetic Materials:
- Ferromagnetic materials can be permanently magnetized.
- They have a high magnetic susceptibility, meaning they can be strongly magnetized in the presence of a magnetic field.
- Ferromagnetic materials retain their magnetization even after the external magnetic field is removed.
- Iron, nickel, and cobalt are examples of ferromagnetic materials.
2. Diamagnetic Materials:
- Diamagnetic materials have a weak magnetic susceptibility, meaning they are weakly magnetized in the presence of a magnetic field.
- They are repelled by a magnetic field and tend to move away from the region of the strongest magnetic field.
- Diamagnetic materials do not retain any magnetization after the external magnetic field is removed.
- Zinc is an example of a diamagnetic material.
Conclusion:
In conclusion, zinc is not a ferromagnetic material. It is classified as a diamagnetic material, which means it has weak magnetic properties and is repelled by a magnetic field. Iron, nickel, and cobalt are examples of ferromagnetic materials that exhibit strong magnetic properties and can be permanently magnetized.
Ferromagnetic materials are substances that exhibit strong magnetic properties and can be permanently magnetized. These materials are composed of atoms or ions that have magnetic moments aligned in the same direction. Iron, nickel, and cobalt are well-known examples of ferromagnetic materials. However, zinc is not a ferromagnetic material.
Explanation:
Zinc is an element that is classified as a diamagnetic material rather than a ferromagnetic material. Diamagnetic materials are substances that have weak magnetic properties and are repelled by a magnetic field. They do not retain any magnetization after the external magnetic field is removed.
Comparison between Ferromagnetic and Diamagnetic Materials:
To understand why zinc is not a ferromagnetic material, let's compare the properties of ferromagnetic and diamagnetic materials:
1. Ferromagnetic Materials:
- Ferromagnetic materials can be permanently magnetized.
- They have a high magnetic susceptibility, meaning they can be strongly magnetized in the presence of a magnetic field.
- Ferromagnetic materials retain their magnetization even after the external magnetic field is removed.
- Iron, nickel, and cobalt are examples of ferromagnetic materials.
2. Diamagnetic Materials:
- Diamagnetic materials have a weak magnetic susceptibility, meaning they are weakly magnetized in the presence of a magnetic field.
- They are repelled by a magnetic field and tend to move away from the region of the strongest magnetic field.
- Diamagnetic materials do not retain any magnetization after the external magnetic field is removed.
- Zinc is an example of a diamagnetic material.
Conclusion:
In conclusion, zinc is not a ferromagnetic material. It is classified as a diamagnetic material, which means it has weak magnetic properties and is repelled by a magnetic field. Iron, nickel, and cobalt are examples of ferromagnetic materials that exhibit strong magnetic properties and can be permanently magnetized.
Consider the following statements about screw dislocation
1. It forms when crystal displaces angulanly over the remaining parts
2. Burgers vector is parallel to screw dislocation line
3. Screw dislocations are symbolically represented by clockwise & anticiock-wise and reffered to negative & positive screw dislocation respectively
Q. Which of these statement are correct?- a)1 and 2
- b)2 and 3
- c)1 and 3
- d)1, 2 and 3
Correct answer is option 'A'. Can you explain this answer?
Consider the following statements about screw dislocation
1. It forms when crystal displaces angulanly over the remaining parts
2. Burgers vector is parallel to screw dislocation line
3. Screw dislocations are symbolically represented by clockwise & anticiock-wise and reffered to negative & positive screw dislocation respectively
Q. Which of these statement are correct?
1. It forms when crystal displaces angulanly over the remaining parts
2. Burgers vector is parallel to screw dislocation line
3. Screw dislocations are symbolically represented by clockwise & anticiock-wise and reffered to negative & positive screw dislocation respectively
Q. Which of these statement are correct?
a)
1 and 2
b)
2 and 3
c)
1 and 3
d)
1, 2 and 3

|
Shounak Saini answered |
Rotation of the dislocation line4. Screw dislocations have a shear stress component along the dislocation line5. The motion of screw dislocations is responsible for plastic deformation in crystals.
Which of the statements above are true?
A) 1 and 4
B) 2 and 5
C) 3 and 4
D) 2, 3, and 5
The correct answer is D) 2, 3, and 5.
Which of the statements above are true?
A) 1 and 4
B) 2 and 5
C) 3 and 4
D) 2, 3, and 5
The correct answer is D) 2, 3, and 5.
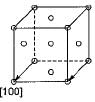
The set of miller indices of the plane shown in the given figure is

- a)(1 0 0)
- b)

- c)(1 0 1)
- d)(1 1 0)
Correct answer is option 'B'. Can you explain this answer?
The set of miller indices of the plane shown in the given figure is


a)
(1 0 0)
b)

c)
(1 0 1)
d)
(1 1 0)
|
|
Nayanika Yadav answered |
Intercept on y and z-axis is ∞. So taking reciprocal it will be 0, 0 on both y and z-axis. Intercept on x-axis is on the negative side of x-axis  .
.
 .
.Match List-I (Miller indices) with List-ll (Denotes) and select the correct answer using the codes given below the lists:
List-I
A. (h, k l)
B. [h k l]
C. {h k l}
D. <h k l>
List-ll
1. direction
2. plane
3. family of direction
4. Family of planes
Codes:
A B C D
(a) 1 2 3 4
(b) 1 2 4 3
(c) 2 1 4 3
(d) 2 1 3 4- a)(a)
- b)(b)
- c)(c)
- d)(d)
Correct answer is option 'C'. Can you explain this answer?
Match List-I (Miller indices) with List-ll (Denotes) and select the correct answer using the codes given below the lists:
List-I
A. (h, k l)
B. [h k l]
C. {h k l}
D. <h k l>
List-ll
1. direction
2. plane
3. family of direction
4. Family of planes
Codes:
A B C D
(a) 1 2 3 4
(b) 1 2 4 3
(c) 2 1 4 3
(d) 2 1 3 4
List-I
A. (h, k l)
B. [h k l]
C. {h k l}
D. <h k l>
List-ll
1. direction
2. plane
3. family of direction
4. Family of planes
Codes:
A B C D
(a) 1 2 3 4
(b) 1 2 4 3
(c) 2 1 4 3
(d) 2 1 3 4
a)
(a)
b)
(b)
c)
(c)
d)
(d)

|
Subham Unni answered |
(hkl)
List-II
1. Crystal plane
2. Crystal direction
3. Family of parallel planes
4. Miller indices
Codes:
A B C D
List-II
1. Crystal plane
2. Crystal direction
3. Family of parallel planes
4. Miller indices
Codes:
A B C D
The correct order of co-ordination number in SC, BCC, FCC and HCP unit cells is- a)12, 12, 8, 6
- b)6, 8, 12, 12
- c)6, 6, 8, 12
- d)12, 8, 6, 12
Correct answer is option 'B'. Can you explain this answer?
The correct order of co-ordination number in SC, BCC, FCC and HCP unit cells is
a)
12, 12, 8, 6
b)
6, 8, 12, 12
c)
6, 6, 8, 12
d)
12, 8, 6, 12
|
|
Disha Nambiar answered |
Co-ordination Number in Different Unit Cells
Co-ordination number is the number of nearest neighbours of an atom in a crystal lattice. The co-ordination number depends on the type of unit cell in the crystal lattice.
The co-ordination number in different unit cells is given below:
- Simple Cubic (SC) Unit Cell: In an SC unit cell, each atom is in contact with 6 nearest neighbours arranged at the corners of a cube. Therefore, the co-ordination number of an SC unit cell is 6.
- Body-Centred Cubic (BCC) Unit Cell: In a BCC unit cell, each atom is in contact with 8 nearest neighbours. Six of these neighbours are arranged at the corners of the cubic unit cell, and the other two are located at the body-centre of the unit cell. Therefore, the co-ordination number of a BCC unit cell is 8.
- Face-Centred Cubic (FCC) Unit Cell: In an FCC unit cell, each atom is in contact with 12 nearest neighbours. Eight of these neighbours are located at the corners of the cubic unit cell, and the other four are located at the centre of the faces of the unit cell. Therefore, the co-ordination number of an FCC unit cell is 12.
- Hexagonal Close Packed (HCP) Unit Cell: In an HCP unit cell, each atom is in contact with 12 nearest neighbours. Six of these neighbours are located in the same plane as the atom, and the other six are located in the adjacent plane. Therefore, the co-ordination number of an HCP unit cell is 12.
Correct Order of Co-ordination Number
The correct order of co-ordination number in SC, BCC, FCC, and HCP unit cells is:
- SC unit cell: 6
- BCC unit cell: 8
- FCC unit cell: 12
- HCP unit cell: 12
Therefore, the correct answer is option B (6, 8, 12, 12).
Co-ordination number is the number of nearest neighbours of an atom in a crystal lattice. The co-ordination number depends on the type of unit cell in the crystal lattice.
The co-ordination number in different unit cells is given below:
- Simple Cubic (SC) Unit Cell: In an SC unit cell, each atom is in contact with 6 nearest neighbours arranged at the corners of a cube. Therefore, the co-ordination number of an SC unit cell is 6.
- Body-Centred Cubic (BCC) Unit Cell: In a BCC unit cell, each atom is in contact with 8 nearest neighbours. Six of these neighbours are arranged at the corners of the cubic unit cell, and the other two are located at the body-centre of the unit cell. Therefore, the co-ordination number of a BCC unit cell is 8.
- Face-Centred Cubic (FCC) Unit Cell: In an FCC unit cell, each atom is in contact with 12 nearest neighbours. Eight of these neighbours are located at the corners of the cubic unit cell, and the other four are located at the centre of the faces of the unit cell. Therefore, the co-ordination number of an FCC unit cell is 12.
- Hexagonal Close Packed (HCP) Unit Cell: In an HCP unit cell, each atom is in contact with 12 nearest neighbours. Six of these neighbours are located in the same plane as the atom, and the other six are located in the adjacent plane. Therefore, the co-ordination number of an HCP unit cell is 12.
Correct Order of Co-ordination Number
The correct order of co-ordination number in SC, BCC, FCC, and HCP unit cells is:
- SC unit cell: 6
- BCC unit cell: 8
- FCC unit cell: 12
- HCP unit cell: 12
Therefore, the correct answer is option B (6, 8, 12, 12).
When atoms are displaced in two separate planes perpendicular to each other, the defect so produced is known as- a)Edge dislocation
- b)Screw dislocation
- c)Stacking fault
- d)Schottky defect
Correct answer is option 'A'. Can you explain this answer?
When atoms are displaced in two separate planes perpendicular to each other, the defect so produced is known as
a)
Edge dislocation
b)
Screw dislocation
c)
Stacking fault
d)
Schottky defect

|
Kirti Sharma answered |
Edge Dislocation:
An edge dislocation is a type of crystallographic defect in which a plane of atoms is displaced in two separate planes perpendicular to each other. This results in a region of excess atoms on one side and a region of missing atoms on the other side of the plane. Edge dislocations are common in materials with a close-packed structure, such as metals and ceramics.
Causes:
Edge dislocations can be caused by a number of factors, including plastic deformation, thermal cycling, and radiation damage. They can also be intentionally introduced into a material through the process of doping, which involves adding impurities to the crystal lattice.
Effects:
Edge dislocations can have a significant impact on the mechanical properties of a material. They can increase the strength and hardness of a material, but they can also decrease its ductility and toughness. In addition, edge dislocations can act as sites for crack initiation and propagation, which can lead to failure of the material under stress.
Conclusion:
In conclusion, edge dislocations are a common crystallographic defect in materials with a close-packed structure. They can be caused by a variety of factors and can have a significant impact on the mechanical properties of a material. Understanding the nature and effects of edge dislocations is important for the design and development of new materials with improved properties.
An edge dislocation is a type of crystallographic defect in which a plane of atoms is displaced in two separate planes perpendicular to each other. This results in a region of excess atoms on one side and a region of missing atoms on the other side of the plane. Edge dislocations are common in materials with a close-packed structure, such as metals and ceramics.
Causes:
Edge dislocations can be caused by a number of factors, including plastic deformation, thermal cycling, and radiation damage. They can also be intentionally introduced into a material through the process of doping, which involves adding impurities to the crystal lattice.
Effects:
Edge dislocations can have a significant impact on the mechanical properties of a material. They can increase the strength and hardness of a material, but they can also decrease its ductility and toughness. In addition, edge dislocations can act as sites for crack initiation and propagation, which can lead to failure of the material under stress.
Conclusion:
In conclusion, edge dislocations are a common crystallographic defect in materials with a close-packed structure. They can be caused by a variety of factors and can have a significant impact on the mechanical properties of a material. Understanding the nature and effects of edge dislocations is important for the design and development of new materials with improved properties.
Crystal structure of metals is studied by- a)metallographic techniques
- b)X-ray techniques
- c)electron microscopy
- d)high powered microscope
Correct answer is option 'B'. Can you explain this answer?
Crystal structure of metals is studied by
a)
metallographic techniques
b)
X-ray techniques
c)
electron microscopy
d)
high powered microscope
|
|
Pankaj Joshi answered |
X-ray Techniques to Study Crystal Structure of Metals
Crystal structure refers to the arrangement of atoms in a crystal. It influences the mechanical, physical, and chemical properties of metals. X-ray diffraction is a powerful tool that is used to study the crystal structure of metals. X-ray techniques are based on the interaction of X-rays with the atoms of a crystal. The X-rays are scattered by the atoms of the crystal, and the resulting pattern of scattered X-rays is used to determine the crystal structure.
There are several X-ray techniques that are used to study the crystal structure of metals, including:
1. Powder diffraction
Powder diffraction is a common X-ray technique used to study the crystal structure of metals. In this technique, a powdered sample is exposed to a beam of X-rays, and the resulting diffraction pattern is measured. The pattern is then analyzed to determine the crystal structure of the sample.
2. Single-crystal diffraction
Single-crystal diffraction is another X-ray technique used to study the crystal structure of metals. In this technique, a single crystal is exposed to a beam of X-rays, and the resulting diffraction pattern is measured. The pattern is then analyzed to determine the crystal structure of the sample.
3. Small-angle X-ray scattering
Small-angle X-ray scattering is a technique used to study the structure of materials on a length scale of nanometers to micrometers. In this technique, X-rays are scattered by the sample, and the pattern of scattered X-rays is analyzed to determine the structure of the sample.
4. X-ray absorption spectroscopy
X-ray absorption spectroscopy is a technique used to study the local structure of a material. In this technique, X-rays are absorbed by the sample, and the resulting absorption spectrum is analyzed to determine the local structure of the material.
In conclusion, X-ray techniques are an important tool used to study the crystal structure of metals. They provide valuable information about the arrangement of atoms in a crystal and help to understand the mechanical, physical, and chemical properties of metals.
Crystal structure refers to the arrangement of atoms in a crystal. It influences the mechanical, physical, and chemical properties of metals. X-ray diffraction is a powerful tool that is used to study the crystal structure of metals. X-ray techniques are based on the interaction of X-rays with the atoms of a crystal. The X-rays are scattered by the atoms of the crystal, and the resulting pattern of scattered X-rays is used to determine the crystal structure.
There are several X-ray techniques that are used to study the crystal structure of metals, including:
1. Powder diffraction
Powder diffraction is a common X-ray technique used to study the crystal structure of metals. In this technique, a powdered sample is exposed to a beam of X-rays, and the resulting diffraction pattern is measured. The pattern is then analyzed to determine the crystal structure of the sample.
2. Single-crystal diffraction
Single-crystal diffraction is another X-ray technique used to study the crystal structure of metals. In this technique, a single crystal is exposed to a beam of X-rays, and the resulting diffraction pattern is measured. The pattern is then analyzed to determine the crystal structure of the sample.
3. Small-angle X-ray scattering
Small-angle X-ray scattering is a technique used to study the structure of materials on a length scale of nanometers to micrometers. In this technique, X-rays are scattered by the sample, and the pattern of scattered X-rays is analyzed to determine the structure of the sample.
4. X-ray absorption spectroscopy
X-ray absorption spectroscopy is a technique used to study the local structure of a material. In this technique, X-rays are absorbed by the sample, and the resulting absorption spectrum is analyzed to determine the local structure of the material.
In conclusion, X-ray techniques are an important tool used to study the crystal structure of metals. They provide valuable information about the arrangement of atoms in a crystal and help to understand the mechanical, physical, and chemical properties of metals.
The packing factor of diamond cubic crystal structure is- a)90%
- b)45%
- c)72%
- d)34%
Correct answer is option 'D'. Can you explain this answer?
The packing factor of diamond cubic crystal structure is
a)
90%
b)
45%
c)
72%
d)
34%
|
|
Divyansh Goyal answered |
Packing factor of Diamond Cubic Crystal Structure
Diamond cubic crystal structure is a type of crystal structure that is commonly found in many elements and compounds such as diamond, silicon, germanium, etc. In this crystal structure, each atom is covalently bonded to four nearest neighbors, forming a tetrahedral structure.
Definition of packing factor
Packing factor is a measure of how efficiently the atoms are arranged in a crystal structure. It is defined as the ratio of the volume occupied by the atoms to the total volume of the unit cell.
Calculation of packing factor for diamond cubic crystal structure
The diamond cubic crystal structure can be thought of as two interpenetrating face-centered cubic (FCC) lattices. Each FCC lattice contains 4 atoms at the corners of the unit cell and 1 atom at the center of the unit cell. Therefore, the total number of atoms in the unit cell is 8.
The volume of the unit cell can be calculated as follows:
a^3 = 4r^3
where a is the edge length of the unit cell and r is the radius of the atom. For the diamond crystal structure, the radius of the atom is half the distance between two nearest neighbor atoms, which is equal to a/2√2.
Substituting the value of r in the above equation, we get:
a = 2r√3
Volume of the unit cell = a^3 = 16r^3√3
The volume occupied by each atom is equal to (4/3)πr^3. Therefore, the total volume occupied by the 8 atoms in the unit cell is:
8 × (4/3)πr^3 = (32/3)πr^3
The packing factor can be calculated as follows:
Packing factor = (Total volume occupied by atoms) / (Volume of the unit cell)
= [(32/3)πr^3] / [16r^3√3]
= 0.34
Therefore, the packing factor of diamond cubic crystal structure is 0.34 or 34%.
Diamond cubic crystal structure is a type of crystal structure that is commonly found in many elements and compounds such as diamond, silicon, germanium, etc. In this crystal structure, each atom is covalently bonded to four nearest neighbors, forming a tetrahedral structure.
Definition of packing factor
Packing factor is a measure of how efficiently the atoms are arranged in a crystal structure. It is defined as the ratio of the volume occupied by the atoms to the total volume of the unit cell.
Calculation of packing factor for diamond cubic crystal structure
The diamond cubic crystal structure can be thought of as two interpenetrating face-centered cubic (FCC) lattices. Each FCC lattice contains 4 atoms at the corners of the unit cell and 1 atom at the center of the unit cell. Therefore, the total number of atoms in the unit cell is 8.
The volume of the unit cell can be calculated as follows:
a^3 = 4r^3
where a is the edge length of the unit cell and r is the radius of the atom. For the diamond crystal structure, the radius of the atom is half the distance between two nearest neighbor atoms, which is equal to a/2√2.
Substituting the value of r in the above equation, we get:
a = 2r√3
Volume of the unit cell = a^3 = 16r^3√3
The volume occupied by each atom is equal to (4/3)πr^3. Therefore, the total volume occupied by the 8 atoms in the unit cell is:
8 × (4/3)πr^3 = (32/3)πr^3
The packing factor can be calculated as follows:
Packing factor = (Total volume occupied by atoms) / (Volume of the unit cell)
= [(32/3)πr^3] / [16r^3√3]
= 0.34
Therefore, the packing factor of diamond cubic crystal structure is 0.34 or 34%.
If (3 2 6) are the Miller indices of a plane, the intercepts made by the plane on the three crystallographic axes are- a)(a, b, c)
- b)(2a, 3b, c)
- c)(a, 2b, 3c)
- d)(2a, b, 3c)
Correct answer is option 'B'. Can you explain this answer?
If (3 2 6) are the Miller indices of a plane, the intercepts made by the plane on the three crystallographic axes are
a)
(a, b, c)
b)
(2a, 3b, c)
c)
(a, 2b, 3c)
d)
(2a, b, 3c)

|
Swati Gupta answered |
Intercepts made by a plane with Miller indices (h k l) on the crystallographic axes can be calculated as follows:
1. Take the reciprocal of each index: (1/h, 1/k, 1/l)
2. Simplify the ratios to the smallest whole numbers: (m, n, p)
3. The intercepts made by the plane on the three axes are (a/m, b/n, c/p)
Using this method, we can calculate the intercepts made by the plane with Miller indices (3 2 6) as follows:
1. Reciprocal of indices: (1/3, 1/2, 1/6)
2. Simplify ratios: (2, 3, 1)
3. Intercepts on axes: (a/2, b/3, c)
However, these intercepts are not in the same form as the options given in the question. We need to manipulate the ratios to match one of the options.
Option B has ratios of (2a, 3b, c), which can be obtained by multiplying the simplified ratios (2, 3, 1) by (a, b, 6c) respectively. This gives us:
(2, 3, 1) x (a, b, 6c) = (2a, 3b, 6c)
Dividing by 2 gives us the ratios in option B: (2a/2, 3b/2, 6c/2) = (a, 3b/2, 3c)
Therefore, option B is the correct answer.
1. Take the reciprocal of each index: (1/h, 1/k, 1/l)
2. Simplify the ratios to the smallest whole numbers: (m, n, p)
3. The intercepts made by the plane on the three axes are (a/m, b/n, c/p)
Using this method, we can calculate the intercepts made by the plane with Miller indices (3 2 6) as follows:
1. Reciprocal of indices: (1/3, 1/2, 1/6)
2. Simplify ratios: (2, 3, 1)
3. Intercepts on axes: (a/2, b/3, c)
However, these intercepts are not in the same form as the options given in the question. We need to manipulate the ratios to match one of the options.
Option B has ratios of (2a, 3b, c), which can be obtained by multiplying the simplified ratios (2, 3, 1) by (a, b, 6c) respectively. This gives us:
(2, 3, 1) x (a, b, 6c) = (2a, 3b, 6c)
Dividing by 2 gives us the ratios in option B: (2a/2, 3b/2, 6c/2) = (a, 3b/2, 3c)
Therefore, option B is the correct answer.
The maximum radius of the interstitial sphere that can fit into the void between the body centred atom of BCC structure is- a)
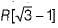
- b)
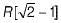
- c)
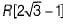
- d)0.707 R
Correct answer is option 'C'. Can you explain this answer?
The maximum radius of the interstitial sphere that can fit into the void between the body centred atom of BCC structure is
a)
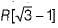
b)
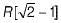
c)
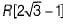
d)
0.707 R
|
|
Anshika Anshika answered |
The maximum radius of the interstitial sphere that can fit into the void between the body centred atom of BCC structure is
The defect responsible for the phenomena of slip, by which most metals deform plastically, is known as- a)fracture
- b)twinning
- c)dislocation
- d)strain hardening
Correct answer is option 'C'. Can you explain this answer?
The defect responsible for the phenomena of slip, by which most metals deform plastically, is known as
a)
fracture
b)
twinning
c)
dislocation
d)
strain hardening
|
|
Kalyan Chakraborty answered |
Defect responsible for slip in metals
Introduction:
When a metal is subjected to external forces, it undergoes deformation. The deformation can be elastic or plastic. In elastic deformation, the metal returns to its original shape when the external force is removed. In plastic deformation, the metal undergoes permanent deformation and does not return to its original shape. The defect responsible for the phenomena of slip, by which most metals deform plastically, is known as dislocation.
Dislocation:
A dislocation is an interruption or disruption in the regular arrangement of atoms in a crystal lattice. It is a line defect that occurs when a plane of atoms is shifted by one or more atomic spacings. Dislocations can move through a crystal lattice when an external force is applied, causing plastic deformation.
Slip:
Slip is the movement of dislocations through a crystal lattice. It occurs when an external force is applied to a metal, causing the dislocations to move. The movement of dislocations allows the metal to deform plastically without fracturing.
Significance:
Dislocations play a significant role in the mechanical properties of metals. The number and arrangement of dislocations in a metal determine its strength, ductility, and toughness. The movement of dislocations through a crystal lattice is also responsible for work hardening, where the metal becomes stronger and harder as it is deformed.
Conclusion:
In conclusion, the defect responsible for slip in metals is dislocation. Dislocations play a significant role in the mechanical properties of metals and allow them to deform plastically without fracturing. Understanding the behavior of dislocations is essential for designing and engineering materials with desired mechanical properties.
Introduction:
When a metal is subjected to external forces, it undergoes deformation. The deformation can be elastic or plastic. In elastic deformation, the metal returns to its original shape when the external force is removed. In plastic deformation, the metal undergoes permanent deformation and does not return to its original shape. The defect responsible for the phenomena of slip, by which most metals deform plastically, is known as dislocation.
Dislocation:
A dislocation is an interruption or disruption in the regular arrangement of atoms in a crystal lattice. It is a line defect that occurs when a plane of atoms is shifted by one or more atomic spacings. Dislocations can move through a crystal lattice when an external force is applied, causing plastic deformation.
Slip:
Slip is the movement of dislocations through a crystal lattice. It occurs when an external force is applied to a metal, causing the dislocations to move. The movement of dislocations allows the metal to deform plastically without fracturing.
Significance:
Dislocations play a significant role in the mechanical properties of metals. The number and arrangement of dislocations in a metal determine its strength, ductility, and toughness. The movement of dislocations through a crystal lattice is also responsible for work hardening, where the metal becomes stronger and harder as it is deformed.
Conclusion:
In conclusion, the defect responsible for slip in metals is dislocation. Dislocations play a significant role in the mechanical properties of metals and allow them to deform plastically without fracturing. Understanding the behavior of dislocations is essential for designing and engineering materials with desired mechanical properties.
Miller indices of a plane will be whose intercepts are a, b/2 and 3c on x, y and z axes respectively in a simple cubic unit cell?- a)123
- b)321
- c)361
- d)631
Correct answer is option 'C'. Can you explain this answer?
Miller indices of a plane will be whose intercepts are a, b/2 and 3c on x, y and z axes respectively in a simple cubic unit cell?
a)
123
b)
321
c)
361
d)
631

|
Anand Mehta answered |
Miller indices of a plane in a simple cubic unit cell can be determined using the following steps:
1. Find the intercepts of the plane on the x, y, and z axes.
In this case, the intercepts are a, b/2, and 3c.
2. Take the reciprocals of the intercepts.
The reciprocals are 1/a, 2/b, and 1/3c.
3. Multiply the reciprocals by a common factor to make them integers.
In this case, we can multiply by 6 to get 6/a, 12/b, and 2/c.
4. Enclose the integers in parentheses and write them as the Miller indices of the plane.
The Miller indices are (6,12,2), which matches option C.
Therefore, the correct answer is option C (361).
1. Find the intercepts of the plane on the x, y, and z axes.
In this case, the intercepts are a, b/2, and 3c.
2. Take the reciprocals of the intercepts.
The reciprocals are 1/a, 2/b, and 1/3c.
3. Multiply the reciprocals by a common factor to make them integers.
In this case, we can multiply by 6 to get 6/a, 12/b, and 2/c.
4. Enclose the integers in parentheses and write them as the Miller indices of the plane.
The Miller indices are (6,12,2), which matches option C.
Therefore, the correct answer is option C (361).
Chapter doubts & questions for Structure of Engineering Materials - Engineering Materials 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Structure of Engineering Materials - Engineering Materials in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Engineering Materials
15 videos|35 docs|13 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup