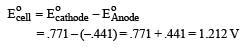All Exams >
NEET >
NEET Past Year Papers >
All Questions
All questions of Electrochemistry for NEET Exam
The equilibrium constant of the reaction: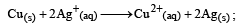 E° = 0.46 V at 298 K is [2007]
E° = 0.46 V at 298 K is [2007]- a)2.0 × 1010
- b)4.0 × 1010
- c)4.0 × 1015
- d)2.4 × 1010
Correct answer is option 'C'. Can you explain this answer?
The equilibrium constant of the reaction:
E° = 0.46 V at 298 K is [2007]
a)
2.0 × 1010
b)
4.0 × 1010
c)
4.0 × 1015
d)
2.4 × 1010

|
Harshitha Chavan answered |
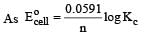
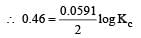
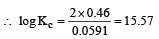
or Kc = Antilog 15.57 = 3.7 × 1015 ≈ 4 × 1015
Consider the following relations for emf of a electrochemical cell: [2010]
(i) emf of cell = (Oxidation potential of anode) – (Reduction potential of cathode)
(ii) emf of cell = (Oxidation potential of anode) + (Reduction potential of cathode)
(iii) emf of cell = (Reduction potential of anode) + (Reduction potential of cathode)
(iv) emf of cell = (Oxidation potential of anode) – (Oxidation potential of cathode)
Which of the above relations are correct?- a)(ii) and (iv)
- b)(iii) and (i)
- c)(i) and (ii)
- d)(iii) and (iv)
Correct answer is option 'A'. Can you explain this answer?
Consider the following relations for emf of a electrochemical cell: [2010]
(i) emf of cell = (Oxidation potential of anode) – (Reduction potential of cathode)
(ii) emf of cell = (Oxidation potential of anode) + (Reduction potential of cathode)
(iii) emf of cell = (Reduction potential of anode) + (Reduction potential of cathode)
(iv) emf of cell = (Oxidation potential of anode) – (Oxidation potential of cathode)
Which of the above relations are correct?
(i) emf of cell = (Oxidation potential of anode) – (Reduction potential of cathode)
(ii) emf of cell = (Oxidation potential of anode) + (Reduction potential of cathode)
(iii) emf of cell = (Reduction potential of anode) + (Reduction potential of cathode)
(iv) emf of cell = (Oxidation potential of anode) – (Oxidation potential of cathode)
Which of the above relations are correct?
a)
(ii) and (iv)
b)
(iii) and (i)
c)
(i) and (ii)
d)
(iii) and (iv)

|
Prisha Singh answered |
Option (b) and (d) are correct
∴ Correct choice : (a)
∴ Correct choice : (a)
The most convenient method to protect the bottom of ship made of iron is [2001]- a)Coating it with red lead oxide
- b)White tin plating
- c)Connecting it with Mg block
- d)Connecting it with Pb block
Correct answer is option 'C'. Can you explain this answer?
The most convenient method to protect the bottom of ship made of iron is [2001]
a)
Coating it with red lead oxide
b)
White tin plating
c)
Connecting it with Mg block
d)
Connecting it with Pb block

|
Mrinalini Bajaj answered |
For bottom of ship to be protected it is connected with more reactive metal than iron like magnesium. This technique is called cathodic protection.
Which of the following expressions correctly represents the equivalent conductance at infinite dilution of Al2 (SO4)3, Given that 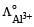 and
and 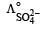 are the equivalent conductances at infinite dilution of the respective ions? [2010]
are the equivalent conductances at infinite dilution of the respective ions? [2010]- a)
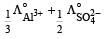
- b)
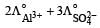
- c)
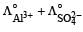
- d)
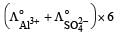
Correct answer is option 'C'. Can you explain this answer?
Which of the following expressions correctly represents the equivalent conductance at infinite dilution of Al2 (SO4)3, Given that 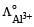 and
and 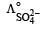 are the equivalent conductances at infinite dilution of the respective ions? [2010]
are the equivalent conductances at infinite dilution of the respective ions? [2010]
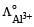 and
and 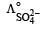 are the equivalent conductances at infinite dilution of the respective ions? [2010]
are the equivalent conductances at infinite dilution of the respective ions? [2010]a)
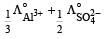
b)
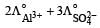
c)
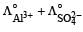
d)
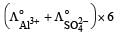

|
Nayanika Dasgupta answered |
Equivalent conductance of an electrolyte at infinite dilution is given by the sum of equivalent conductances of the respective ions at infinite dilution.
∴ Correct choice : (c)
∴ Correct choice : (c)
On heating one end of a piece of a metal, the other end becomes hot because of [1995]- a)resistance of the metal
- b)mobility of atoms in the metal
- c)energised electrons moving to the other end
- d)min or perturbation in the energy of atoms
Correct answer is option 'C'. Can you explain this answer?
On heating one end of a piece of a metal, the other end becomes hot because of [1995]
a)
resistance of the metal
b)
mobility of atoms in the metal
c)
energised electrons moving to the other end
d)
min or perturbation in the energy of atoms

|
Shanaya Rane answered |
When one end of a metal is heated, t he free electrons are energised and move to the other end. It heats up the other end of the metal.
How many grams of cobalt metal will be deposited when a solution of cobalt (II) chloride is electrolyzed with a current of 10 amperes for 109 minutes (1 Faraday = 96,500 C; Atomic mass of Co = 59 u) [NEET Kar. 2013]- a)0.66
- b)4.0
- c)20.0
- d)40.0
Correct answer is option 'C'. Can you explain this answer?
How many grams of cobalt metal will be deposited when a solution of cobalt (II) chloride is electrolyzed with a current of 10 amperes for 109 minutes (1 Faraday = 96,500 C; Atomic mass of Co = 59 u) [NEET Kar. 2013]
a)
0.66
b)
4.0
c)
20.0
d)
40.0

|
Ritika Khanna answered |
Applying,
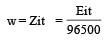
Equivalent weight of cobalt (II) = 59/2
I = 10 A
Time (t) = 109 min = 109 × 60 sec
Substituting these values we get,
I = 10 A
Time (t) = 109 min = 109 × 60 sec
Substituting these values we get,
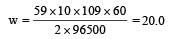
The standard e.m.f. of a galvanic cell involving cell reaction with n = 2 is found to be 0.295 V at 25°C. The equilibrium constant of the reaction would be (Given F = 96500 C mol–1; R = 8.314JK– 1mol–1)[2004]- a)2.0 x 1011
- b)4.0 x 1012
- c)1.0 x 102
- d)1.0 x 1010
Correct answer is option 'D'. Can you explain this answer?
The standard e.m.f. of a galvanic cell involving cell reaction with n = 2 is found to be 0.295 V at 25°C. The equilibrium constant of the reaction would be (Given F = 96500 C mol–1; R = 8.314JK– 1mol–1)[2004]
a)
2.0 x 1011
b)
4.0 x 1012
c)
1.0 x 102
d)
1.0 x 1010

|
Subham Chavan answered |
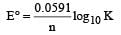
Here, n = 2, E° = 0.295
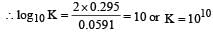
In the silver plating of copper, K[Ag(CN)2] is used instead of AgNO3. The reason is [2002]- a)A thin layer of Ag is formed on Cu
- b)More voltage is required
- c)Ag+ ion sare completely removed from solution
- d)Less availability of Ag+ ions, as Cu cannot displace Ag from [Ag(CN)2]– ion
Correct answer is option 'D'. Can you explain this answer?
In the silver plating of copper, K[Ag(CN)2] is used instead of AgNO3. The reason is [2002]
a)
A thin layer of Ag is formed on Cu
b)
More voltage is required
c)
Ag+ ion sare completely removed from solution
d)
Less availability of Ag+ ions, as Cu cannot displace Ag from [Ag(CN)2]– ion

|
Arindam Khanna answered |
In the silver plating of copper, K[Ag(CN)2] is used instead of AgNO3. Copper being more electropositive readily precipitate silver from their salt solution

whereas in K[Ag (CN)2] solution a complex anion [Ag(CN)2]– is formed and hence Ag+ are less available in the solution and therefore copper cannot displace Ag from its complex ion.
Without losing its concentration ZnCl2 solution cannot be kept in contact with [1998]- a)Au
- b)Al
- c)Pb
- d)Ag
Correct answer is option 'B'. Can you explain this answer?
Without losing its concentration ZnCl2 solution cannot be kept in contact with [1998]
a)
Au
b)
Al
c)
Pb
d)
Ag

|
Aman Sharma answered |
With out losing its concentrati on Zn Cl2 solution can not kept in contact with Al because Al is more reactive than Zn due to electrode (reduction) potential.
Standard electrode potential of three metals X, Y and Z are – 1.2 V, + 0.5 V and – 3.0 V, respectively. The reducing power of these metals will be : [2011]- a)Y > Z > X
- b)X > Y > Z
- c)Z > X > Y
- d)y > X > Z
Correct answer is option 'C'. Can you explain this answer?
Standard electrode potential of three metals X, Y and Z are – 1.2 V, + 0.5 V and – 3.0 V, respectively. The reducing power of these metals will be : [2011]
a)
Y > Z > X
b)
X > Y > Z
c)
Z > X > Y
d)
y > X > Z

|
Shounak Nair answered |
As the value of standard reduction potential decreases the reducing power increases i.e.,
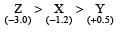
Standard electrode potential for Sn4+ / Sn2+ couple is + 0.15 V and that for the Cr3+ / Cr couple is – 0.74 V. These two couples in their standard state are connected to make a cell. The cell potential will be : [2011]- a)+ 1.19 V
- b)+ 0.89 V
- c)+ 0.18 V
- d)+ 1.83 V
Correct answer is option 'B'. Can you explain this answer?
Standard electrode potential for Sn4+ / Sn2+ couple is + 0.15 V and that for the Cr3+ / Cr couple is – 0.74 V. These two couples in their standard state are connected to make a cell. The cell potential will be : [2011]
a)
+ 1.19 V
b)
+ 0.89 V
c)
+ 0.18 V
d)
+ 1.83 V

|
Anirudh Datta answered |
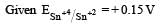
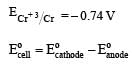
= 0.15 – (– 0.74)
= + 0.89 V
= + 0.89 V
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]- a)the temperature is constant
- b)e.m.f depends on molarities of acids used
- c)acids used in two compartments are different
- d)pH of 0.1 M HCl and 0.1 M CH3COOH is not same
Correct answer is option 'D'. Can you explain this answer?
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]
a)
the temperature is constant
b)
e.m.f depends on molarities of acids used
c)
acids used in two compartments are different
d)
pH of 0.1 M HCl and 0.1 M CH3COOH is not same

|
Bhargavi Choudhury answered |
For a concentration cell having different concentrations of ions.
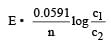
If all the concentrations are identical then obviously the cell voltage is zero. But as the pH of 0.1 M HCl (strong acid) & pH of 0.1M CH3 COOH is (weak acid) not same, therefore the cell voltage is not zero.
For the reduction of silver ions with copper metal, the standard cell potential was found to be + 0.46 V at 25°C. The value of standard Gibbs energy, ΔG0 will be (F = 96500 C mol –1)- a)– 89.0 kJ
- b)– 89.0 J [2010]
- c)– 44.5 kJ
- d)– 98.0 kJ
Correct answer is option 'A'. Can you explain this answer?
For the reduction of silver ions with copper metal, the standard cell potential was found to be + 0.46 V at 25°C. The value of standard Gibbs energy, ΔG0 will be (F = 96500 C mol –1)
a)
– 89.0 kJ
b)
– 89.0 J [2010]
c)
– 44.5 kJ
d)
– 98.0 kJ

|
Prasenjit Pillai answered |
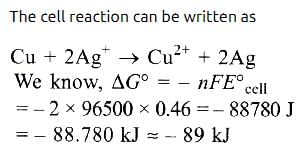
For the cell reaction, [1998] Cu2+ (C1, aq) + Zn(s) = Zn2+ (C2, aq) + Cu(s) of an electrochemical cell, the change in free energy, ΔG, at a given temperature is a function of- a)ln (C1)
- b)ln (C2/C1)
- c)ln (C2)
- d)ln (C1 + C2)
Correct answer is option 'B'. Can you explain this answer?
For the cell reaction, [1998] Cu2+ (C1, aq) + Zn(s) = Zn2+ (C2, aq) + Cu(s) of an electrochemical cell, the change in free energy, ΔG, at a given temperature is a function of
a)
ln (C1)
b)
ln (C2/C1)
c)
ln (C2)
d)
ln (C1 + C2)

|
Rhea Sarkar answered |
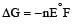
For concentration cell,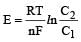
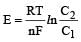
In it R, T, n and F are constant So E is based upon ln C2 / C1
Now 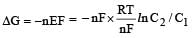
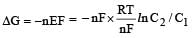
= –RTlnC2/C1
At constant temperature ΔG is based upon ln C2/C1.
The most durable metal plating on iron to protect against corrosion is [1994]- a)nickel plating
- b)copper plating
- c)tin plating
- d)zinc plating.
Correct answer is option 'D'. Can you explain this answer?
The most durable metal plating on iron to protect against corrosion is [1994]
a)
nickel plating
b)
copper plating
c)
tin plating
d)
zinc plating.

|
Dipanjan Chawla answered |
This is because zinc has higher oxidation potential than Ni, Cu and Sn. The process of coating of iron surface with zinc is known as galvanization. Galvanized iron sheets maintain their lustrue due to the formation of protective layer of basic zinc carbonate.
If the E°cell for a given reaction has a negative value, then which of the following gives the correct relationships for the values of ΔG° and Keq ?[2011]- a)ΔG° > 0 ; Keq > 1
- b)ΔG° < 0 ; Keq > 1
- c)ΔG° < 0 ; Keq < 1
- d)ΔG° > 0 ; Keq < 1
Correct answer is option 'D'. Can you explain this answer?
If the E°cell for a given reaction has a negative value, then which of the following gives the correct relationships for the values of ΔG° and Keq ?[2011]
a)
ΔG° > 0 ; Keq > 1
b)
ΔG° < 0 ; Keq > 1
c)
ΔG° < 0 ; Keq < 1
d)
ΔG° > 0 ; Keq < 1

|
Anu Bajaj answered |
Standard Gibbs free energy is given as ΔG°
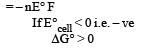
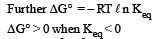
At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]- a)20.800%
- b)4.008%
- c)40.800%
- d)2.080%
Correct answer is option 'B'. Can you explain this answer?
At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]
a)
20.800%
b)
4.008%
c)
40.800%
d)
2.080%

|
Mehul Iyer answered |
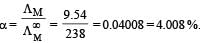
Th e ionic conductan ce of Ba2+ an d Cl– are respectively 127 and 76 ohm–1 cm2 at infinite dilution. The equivalent conductance (in ohm–1 cm2) of BaCl2 at infinite dilution will be : [2000]- a)139.5
- b)203
- c)279
- d)101.5
Correct answer is option 'A'. Can you explain this answer?
Th e ionic conductan ce of Ba2+ an d Cl– are respectively 127 and 76 ohm–1 cm2 at infinite dilution. The equivalent conductance (in ohm–1 cm2) of BaCl2 at infinite dilution will be : [2000]
a)
139.5
b)
203
c)
279
d)
101.5

|
Arindam Khanna answered |
The equivalent con ductance of BaCl2 at infinite dilution
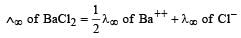
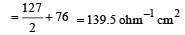
Kohlrausch’s law states that at : [2008]- a)finite dilution, each ion makes definite contribution to equivalent conductance of an electrolyte, whatever be the nature of the other ion of the electrolyte.
- b)infinite dilution each ion makes definite contribution to equivalent conductance of an electrolyte depending on the nature of the other ion of the electrolyte.
- c)infinite dilution, each ion makes definite contribution to conductance of an electrolyte whatever be the nature of the other ion of the electrolyte.
- d)infinite dilution, each ion makes definite contirubtion to equivalent conductance of an electrolyte, whatever be the nature of the other ion of the electrolyte.
Correct answer is option 'D'. Can you explain this answer?
Kohlrausch’s law states that at : [2008]
a)
finite dilution, each ion makes definite contribution to equivalent conductance of an electrolyte, whatever be the nature of the other ion of the electrolyte.
b)
infinite dilution each ion makes definite contribution to equivalent conductance of an electrolyte depending on the nature of the other ion of the electrolyte.
c)
infinite dilution, each ion makes definite contribution to conductance of an electrolyte whatever be the nature of the other ion of the electrolyte.
d)
infinite dilution, each ion makes definite contirubtion to equivalent conductance of an electrolyte, whatever be the nature of the other ion of the electrolyte.

|
Nayanika Dasgupta answered |
Kohlrausch ’s Law states that at infinite dilution, each ion migrates independently of its co-ion and contributes to the total equivalent conductance of an eletrolyte a definite share which depends only on its own nature.
From this definition we can see that option (d) is the correct answer.
From this definition we can see that option (d) is the correct answer.
Standard free energies of formation (in kJ/mol) at 298 K are – 237.2, – 394.4 and – 8.2 for H2O(l), CO2(g) and pentane (g), respectively. The value E°cell for the pentane-oxygen fuel cell is : [2008]- a)1.968 V
- b)2.0968 V
- c)1.0968 V
- d)0.0968 V
Correct answer is option 'C'. Can you explain this answer?
Standard free energies of formation (in kJ/mol) at 298 K are – 237.2, – 394.4 and – 8.2 for H2O(l), CO2(g) and pentane (g), respectively. The value E°cell for the pentane-oxygen fuel cell is : [2008]
a)
1.968 V
b)
2.0968 V
c)
1.0968 V
d)
0.0968 V

|
Akshat Chavan answered |

Al 2O3 is reduced by electrolysisat low potentials and high currents. If 4.0 × 104 amperes of current is passed through molten Al2O3 for 6 hours, what mass of aluminium is produced? (Assume 100% current efficiency. At. mass of Al = 27 g mol–1) [2009]- a)8.1 × 104 g
- b)2.4 × 105 g
- c)1.3 × 104 g
- d)9.0 × 103 g
Correct answer is option 'A'. Can you explain this answer?
Al 2O3 is reduced by electrolysisat low potentials and high currents. If 4.0 × 104 amperes of current is passed through molten Al2O3 for 6 hours, what mass of aluminium is produced? (Assume 100% current efficiency. At. mass of Al = 27 g mol–1) [2009]
a)
8.1 × 104 g
b)
2.4 × 105 g
c)
1.3 × 104 g
d)
9.0 × 103 g

|
Shivani Rane answered |
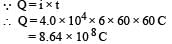
Now since 96500 C liberates 9 g of Al
8.64 × 108 C liberates 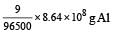
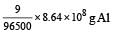
= 8.1 × 104 g of Al
Limiting molar conductivity of NH4OH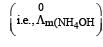 is equal to : [2012]
is equal to : [2012]- a)
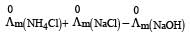
- b)
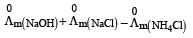
- c)
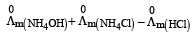
- d)
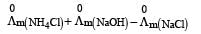
Correct answer is option 'D'. Can you explain this answer?
Limiting molar conductivity of NH4OH
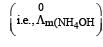 is equal to : [2012]
is equal to : [2012]a)
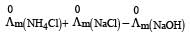
b)
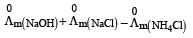
c)
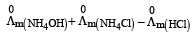
d)
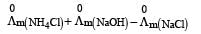

|
Smruti Sucharita answered |
Apply Kohlraausch's law....
If 0.5 amp current is passed through acidified silver nitrate solution for 100 minutes. The mass of silver deposited on cathode, is (eq.wt.of silver nitrate = 108) [1995]- a)2.3523 g
- b)3.3575 g
- c)5.3578 g
- d)6.3575 g
Correct answer is option 'B'. Can you explain this answer?
If 0.5 amp current is passed through acidified silver nitrate solution for 100 minutes. The mass of silver deposited on cathode, is (eq.wt.of silver nitrate = 108) [1995]
a)
2.3523 g
b)
3.3575 g
c)
5.3578 g
d)
6.3575 g

|
Diya Datta answered |
Given current (i) = 0.5 amp;
Time (t) = 100 minutes × 60 = 6000 sec
Equivalent weight of silver nitrate (E) = 108.
According to Faraday's first law of electrolysis
Time (t) = 100 minutes × 60 = 6000 sec
Equivalent weight of silver nitrate (E) = 108.
According to Faraday's first law of electrolysis
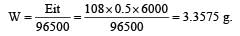
A hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH = 10 and by passing hydrogen gas around the platinum wire at one atm pressure. The oxidation potential of electrode would be ? [NEET 2013]- a)0.59 V
- b)0.118 V
- c)1.18 V
- d)0.059 V
Correct answer is option 'A'. Can you explain this answer?
A hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH = 10 and by passing hydrogen gas around the platinum wire at one atm pressure. The oxidation potential of electrode would be ? [NEET 2013]
a)
0.59 V
b)
0.118 V
c)
1.18 V
d)
0.059 V

|
Abhishek Desai answered |
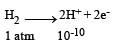
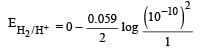
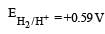
Consider the half-cell reduction reaction :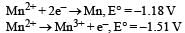 The E° for the reaction
The E° for the reaction 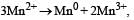 and possibility of the forward reaction are, respectively [NEET Kar. 2013]
and possibility of the forward reaction are, respectively [NEET Kar. 2013]- a)– 2.69 V and no
- b)– 4.18 V and yes
- c)+ 0.33 V and yes
- d)+ 2.69 V and no
Correct answer is option 'A'. Can you explain this answer?
Consider the half-cell reduction reaction :
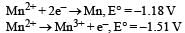
The E° for the reaction 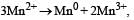 and possibility of the forward reaction are, respectively [NEET Kar. 2013]
and possibility of the forward reaction are, respectively [NEET Kar. 2013]
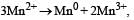 and possibility of the forward reaction are, respectively [NEET Kar. 2013]
and possibility of the forward reaction are, respectively [NEET Kar. 2013]a)
– 2.69 V and no
b)
– 4.18 V and yes
c)
+ 0.33 V and yes
d)
+ 2.69 V and no

|
Shalini Saha answered |
ΔE° = E°red + E°oxd = – 1.81 – 1.51
= – 2.69
Since ΔE° is negative
∴ ΔG = –nFE°, ΔG will have positive value so, forward reaction is not possible.
= – 2.69
Since ΔE° is negative
∴ ΔG = –nFE°, ΔG will have positive value so, forward reaction is not possible.
On passing a current of 1.0 ampere for 16 min and 5 sec through one litre solution of CuCl2, all copper of the solution was deposited at cathode. The strength of CuCl2 solution was (Molar mass of Cu= 63.5; Faraday constant = 96,500 Cmol–1) [1996]- a)0.01 N
- b)0.01 M
- c)0.02 M
- d)0.2 N
Correct answer is option 'A'. Can you explain this answer?
On passing a current of 1.0 ampere for 16 min and 5 sec through one litre solution of CuCl2, all copper of the solution was deposited at cathode. The strength of CuCl2 solution was (Molar mass of Cu= 63.5; Faraday constant = 96,500 Cmol–1) [1996]
a)
0.01 N
b)
0.01 M
c)
0.02 M
d)
0.2 N

|
Anirudh Datta answered |
By Faraday's Ist Law, 

(where q = it = charge of ion) we know that no of equivalent
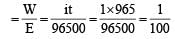
(where i= 1 A, t = 16×60+5 = 965 sec.) Since, we know that
Normality 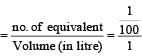
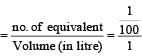
= 0.01 N
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to: [2010]- a)increase in ionic mobility of ions
- b)100% ionisation of electrolyte at normal dilution
- c)increase in both i.e. number of ions and ionic mobility of ions
- d)increase in number of ions
Correct answer is option 'A'. Can you explain this answer?
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to: [2010]
a)
increase in ionic mobility of ions
b)
100% ionisation of electrolyte at normal dilution
c)
increase in both i.e. number of ions and ionic mobility of ions
d)
increase in number of ions

|
Mehul Iyer answered |
Dilution of strong electrolytes increases ionisation, hence ionic mobility of ions which in turn increases equivalent conductance of the solution.
A solution contains Fe2+, Fe3+ and I– ions. This solution was treated with iodine at 35°C. E° for Fe3+ / Fe2+ is + 0.77 V and E° for I2/2I– = 0.536 V.The favourable redox reaction is : [2011 M]- a)I2 will be reduced to I–
- b)There will be no redox reaction
- c)I– will be oxidised to I2
- d)Fe2+ will be oxidised to Fe3+
Correct answer is option 'C'. Can you explain this answer?
A solution contains Fe2+, Fe3+ and I– ions. This solution was treated with iodine at 35°C. E° for Fe3+ / Fe2+ is + 0.77 V and E° for I2/2I– = 0.536 V.The favourable redox reaction is : [2011 M]
a)
I2 will be reduced to I–
b)
There will be no redox reaction
c)
I– will be oxidised to I2
d)
Fe2+ will be oxidised to Fe3+

|
Shounak Nair answered |
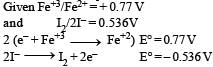
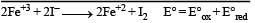
= 0.77 – 0.536
= 0.164 V
So, reaction will taken place.
= 0.164 V
So, reaction will taken place.
4.5 g of aluminium (at. mass 27 amu) is deposited at cathode from Al3+ solution by a certain quantity of electric charge. The volume of hydrogen produced at STP from H+ ions in solution by the same quantity of electric charge will be [2005]- a)44.8 L
- b)22.4 L
- c)11.2 L
- d)5.6 L
Correct answer is option 'D'. Can you explain this answer?
4.5 g of aluminium (at. mass 27 amu) is deposited at cathode from Al3+ solution by a certain quantity of electric charge. The volume of hydrogen produced at STP from H+ ions in solution by the same quantity of electric charge will be [2005]
a)
44.8 L
b)
22.4 L
c)
11.2 L
d)
5.6 L
|
|
Sahil Menon answered |
Calculation of Amount of Charge Required
To determine the volume of hydrogen produced at STP from H+ ions, we need to calculate the amount of charge required for the deposition of 4.5 g of aluminium from Al3+ solution.
1. Calculate the number of moles of aluminium deposited:
Number of moles of aluminium = Mass of aluminium / Atomic mass of aluminium
= 4.5 g / 27 g/mol
= 0.1667 mol
2. Calculate the amount of charge required using Faraday's laws of electrolysis:
Amount of charge = (Number of moles of metal deposited) x (Faraday constant)
= 0.1667 mol x 96500 C/mol
= 16041.5 C
Volume of Hydrogen Produced at STP
Now, we can use the amount of charge obtained above to determine the volume of hydrogen produced at STP from H+ ions in solution.
1. Calculate the number of moles of hydrogen gas produced:
Number of moles of hydrogen = Amount of charge / (2 x Faraday constant)
= 16041.5 C / (2 x 96500 C/mol)
= 0.0833 mol
2. Calculate the volume of hydrogen gas produced at STP using the ideal gas law:
PV = nRT
where P = pressure (1 atm), V = volume, n = number of moles (0.0833 mol), R = gas constant (0.0821 L atm/mol K), T = temperature (273 K)
V = nRT/P
= 0.0833 mol x 0.0821 L atm/mol K x 273 K / 1 atm
= 1.82 L
However, we need to remember that the question asks for the volume of hydrogen gas produced at STP. Therefore, we need to convert the volume to STP conditions (0°C and 1 atm).
Using the combined gas law, we can calculate the volume at STP:
(P1V1)/T1 = (P2V2)/T2
where P1 = 1 atm, V1 = 1.82 L, T1 = 273 K, P2 = 1 atm, V2 = ?, T2 = 273 K
V2 = (P1V1 x T2) / (P2 x T1)
= (1 atm x 1.82 L x 273 K) / (1 atm x 273 K)
= 5.6 L
Therefore, the volume of hydrogen gas produced at STP from H+ ions in solution by the same quantity of electric charge is 5.6 L. The correct answer is option D.
To determine the volume of hydrogen produced at STP from H+ ions, we need to calculate the amount of charge required for the deposition of 4.5 g of aluminium from Al3+ solution.
1. Calculate the number of moles of aluminium deposited:
Number of moles of aluminium = Mass of aluminium / Atomic mass of aluminium
= 4.5 g / 27 g/mol
= 0.1667 mol
2. Calculate the amount of charge required using Faraday's laws of electrolysis:
Amount of charge = (Number of moles of metal deposited) x (Faraday constant)
= 0.1667 mol x 96500 C/mol
= 16041.5 C
Volume of Hydrogen Produced at STP
Now, we can use the amount of charge obtained above to determine the volume of hydrogen produced at STP from H+ ions in solution.
1. Calculate the number of moles of hydrogen gas produced:
Number of moles of hydrogen = Amount of charge / (2 x Faraday constant)
= 16041.5 C / (2 x 96500 C/mol)
= 0.0833 mol
2. Calculate the volume of hydrogen gas produced at STP using the ideal gas law:
PV = nRT
where P = pressure (1 atm), V = volume, n = number of moles (0.0833 mol), R = gas constant (0.0821 L atm/mol K), T = temperature (273 K)
V = nRT/P
= 0.0833 mol x 0.0821 L atm/mol K x 273 K / 1 atm
= 1.82 L
However, we need to remember that the question asks for the volume of hydrogen gas produced at STP. Therefore, we need to convert the volume to STP conditions (0°C and 1 atm).
Using the combined gas law, we can calculate the volume at STP:
(P1V1)/T1 = (P2V2)/T2
where P1 = 1 atm, V1 = 1.82 L, T1 = 273 K, P2 = 1 atm, V2 = ?, T2 = 273 K
V2 = (P1V1 x T2) / (P2 x T1)
= (1 atm x 1.82 L x 273 K) / (1 atm x 273 K)
= 5.6 L
Therefore, the volume of hydrogen gas produced at STP from H+ ions in solution by the same quantity of electric charge is 5.6 L. The correct answer is option D.
Eº for the cell, 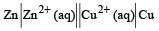 1.10 V at 25ºC. The equilibrium constant for the cell reaction:
1.10 V at 25ºC. The equilibrium constant for the cell reaction:
- a)10–18
- b)10–37
- c)1018
- d)1037
Correct answer is option 'D'. Can you explain this answer?
Eº for the cell, 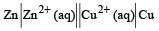
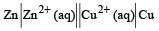
1.10 V at 25ºC. The equilibrium constant for the cell reaction:

a)
10–18
b)
10–37
c)
1018
d)
1037
|
|
Akshita Pillai answered |
Given for the reaction
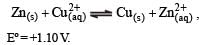
At equilibrium
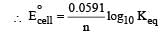
here (n → number of exchange of electrons)
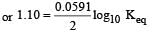
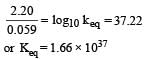
Specific conductance of a 0.1 N KCl solution at 23ºC is 0.012 ohm–1 cm–1. Resistance of cell containing the solution at same temperature was found to be 55 ohm. The cell constant is [2000]- a)0.918 cm–1
- b)0.66 cm–1
- c)1.142 cm–1
- d)1.12 cm–1
Correct answer is option 'B'. Can you explain this answer?
Specific conductance of a 0.1 N KCl solution at 23ºC is 0.012 ohm–1 cm–1. Resistance of cell containing the solution at same temperature was found to be 55 ohm. The cell constant is [2000]
a)
0.918 cm–1
b)
0.66 cm–1
c)
1.142 cm–1
d)
1.12 cm–1

|
Bhargavi Choudhury answered |
Given specific conductance of the solution (k) = 0.012 ohm–1 cm–1 and resistance (R) = 55 ohm. We know that cell constant = Specific conductance × Observed resistance = 0.012 × 55= 0.66cm–1.
Which reaction is not feasible? [2002]- a)
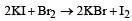
- b)
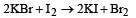
- c)
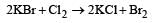
- d)
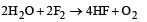
Correct answer is option 'B'. Can you explain this answer?
Which reaction is not feasible? [2002]
a)
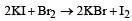
b)
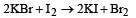
c)
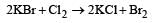
d)
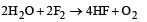
|
|
Yash Modi answered |
In feasible displacement reactions like those as shown, generally the more electronegative element displaces the less electronegative element. But reaction b is not feasible as Iodine which is less electronegative tries to displaces the more electronegative Bromine.
The standard reduction poten tials at 25°C of
Li+/ Li, Ba 2+ / Ba, Na+ / Na
and Mg 2+ / Mg are – 3.03, – 2.73, – 2.71 and – 2.37 volt respectively. Which one of the following is the strongest oxidising agent? [1994]
- a)Na+
- b)Li+
- c)Ba2+
- d)Mg2+
Correct answer is option 'D'. Can you explain this answer?
The standard reduction poten tials at 25°C of
Li+/ Li, Ba 2+ / Ba, Na+ / Na
and Mg 2+ / Mg are – 3.03, – 2.73, – 2.71 and – 2.37 volt respectively. Which one of the following is the strongest oxidising agent? [1994]
Li+/ Li, Ba 2+ / Ba, Na+ / Na
and Mg 2+ / Mg are – 3.03, – 2.73, – 2.71 and – 2.37 volt respectively. Which one of the following is the strongest oxidising agent? [1994]
a)
Na+
b)
Li+
c)
Ba2+
d)
Mg2+

|
Sneha Basak answered |

Given: [2009]
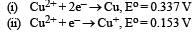
Electrode potential, Eo for the reaction, 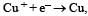 will be :
will be :- a)0.90 V
- b)0.30 V
- c)0.38 V
- d)0.52 V
Correct answer is option 'D'. Can you explain this answer?
Given: [2009]
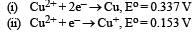
Electrode potential, Eo for the reaction,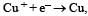 will be :
will be :
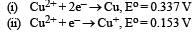
Electrode potential, Eo for the reaction,
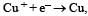 will be :
will be :a)
0.90 V
b)
0.30 V
c)
0.38 V
d)
0.52 V

|
Ayush Sengupta answered |
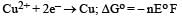
= – 2 × F × 0.337
= – 0.674 F ....(i)
= – 0.674 F ....(i)
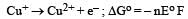
= – 1 × F × – 0.153
= 0.153 F ....(ii)
= 0.153 F ....(ii)
On adding eqn (i) & (ii)

Here n = 1 Δ Eo = + 0.52 V
Equivalent conductances of NaCl, HCl and CH3COONa at infinite dilution are 126.45, 426.16 and 91 ohm–1 cm2 respectively. The equivalent conductance of CH3COOH at infinite dilution would be [1997]- a)101.38 ohm–1 cm2
- b)253.62 ohm–1 cm2
- c)390.71 ohm–1 cm2
- d)678.90 ohm–1 cm2
Correct answer is option 'C'. Can you explain this answer?
Equivalent conductances of NaCl, HCl and CH3COONa at infinite dilution are 126.45, 426.16 and 91 ohm–1 cm2 respectively. The equivalent conductance of CH3COOH at infinite dilution would be [1997]
a)
101.38 ohm–1 cm2
b)
253.62 ohm–1 cm2
c)
390.71 ohm–1 cm2
d)
678.90 ohm–1 cm2
|
|
Ratan Durge answered |
C
Cu +aq is unstable in solution and undergoes simultaneous oxidation and reduction according to the reaction : [2000]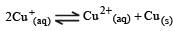 choose correct Eº for above reaction if Eº Cu2+/Cu = 0.34 V and Eº Cu2+/Cu+ = 0.15 V
choose correct Eº for above reaction if Eº Cu2+/Cu = 0.34 V and Eº Cu2+/Cu+ = 0.15 V- a)–0.38 V
- b)+0.49 V
- c)+0.38 V
- d)–0.19 V
Correct answer is option 'C'. Can you explain this answer?
Cu +aq is unstable in solution and undergoes simultaneous oxidation and reduction according to the reaction : [2000]
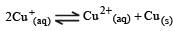
choose correct Eº for above reaction if Eº Cu2+/Cu = 0.34 V and Eº Cu2+/Cu+ = 0.15 V
a)
–0.38 V
b)
+0.49 V
c)
+0.38 V
d)
–0.19 V

|
Gowri Nair answered |
Ans.
Option (c)
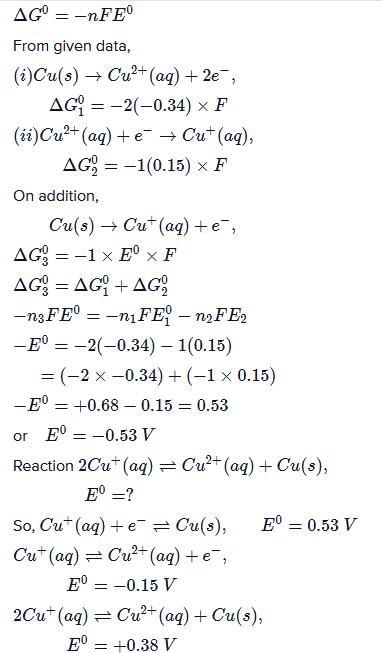
On the basis of the information available from the reaction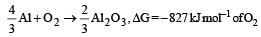 the minimum e.m.f required to carry out an electrolysis of Al2O3 is (F = 96500 C mol–1)
the minimum e.m.f required to carry out an electrolysis of Al2O3 is (F = 96500 C mol–1)- a)8.56 V
- b)2.14 V [2003]
- c)4.28 V
- d)6.42 V
Correct answer is option 'B'. Can you explain this answer?
On the basis of the information available from the reaction
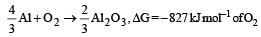
the minimum e.m.f required to carry out an electrolysis of Al2O3 is (F = 96500 C mol–1)
a)
8.56 V
b)
2.14 V [2003]
c)
4.28 V
d)
6.42 V

|
Anu Bajaj answered |
ΔG = –nEF
For 1 mol of Al, n = 3

According to question, 827 x1000 = 4 x E x 96500
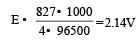
On the basis of the following E° values, the strongest oxidizing agent is : [2008]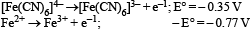
- a)[Fe(CN)6]4–
- b)Fe2+
- c)Fe3+
- d)[Fe(CN)6]3–
Correct answer is option 'C'. Can you explain this answer?
On the basis of the following E° values, the strongest oxidizing agent is : [2008]
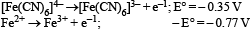
a)
[Fe(CN)6]4–
b)
Fe2+
c)
Fe3+
d)
[Fe(CN)6]3–

|
Snehal Shah answered |
From the given data we find Fe3+ is strongest oxideizing agent. More the positive value of E° OP, more is the tendency to get oxidized. Thus correct option is (c).
Standard electrode potentials are : Fe+2/Fe [ Eº = –0.44]; Fe+3/Fe+2 Eº = + 0.77 ; If Fe+2, Fe+3 and Fe blocks are kept together, then [2001]- a)Fe+2 increases
- b)Fe+3 decreases
- c)
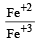 remains unchanged
remains unchanged - d)Fe+2 decreases
Correct answer is option 'B'. Can you explain this answer?
Standard electrode potentials are : Fe+2/Fe [ Eº = –0.44]; Fe+3/Fe+2 Eº = + 0.77 ; If Fe+2, Fe+3 and Fe blocks are kept together, then [2001]
a)
Fe+2 increases
b)
Fe+3 decreases
c)
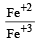 remains unchanged
remains unchangedd)
Fe+2 decreases

|
Harshitha Dey answered |
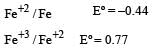
The metals having higher negative electrode potential can displaced metals having lower values of negative electrode potential from their salt solutions.
Standard potentials (Eº) for some half-reactions are given below :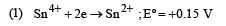
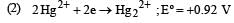
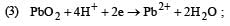 Eº = +1.45 VBased on the above, which one of the following statements is correct ? [1997]
Eº = +1.45 VBased on the above, which one of the following statements is correct ? [1997]- a)Sn4+ is a stronger oxidising agent than Pb4+
- b)Sn2+ is a stronger reducing agent than Hg22+
- c)Hg2+ is a stronger oxidising agent than Pb4+
- d)Pb2+ is a stronger reducing agent than Sn 2+
Correct answer is option 'B'. Can you explain this answer?
Standard potentials (Eº) for some half-reactions are given below :
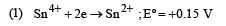
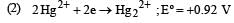
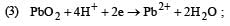 Eº = +1.45 V
Eº = +1.45 VBased on the above, which one of the following statements is correct ? [1997]
a)
Sn4+ is a stronger oxidising agent than Pb4+
b)
Sn2+ is a stronger reducing agent than Hg22+
c)
Hg2+ is a stronger oxidising agent than Pb4+
d)
Pb2+ is a stronger reducing agent than Sn 2+

|
Subham Chavan answered |
In elctrochemical series, Sn is above hydrogen and Hg below hydrogen. Elements above hyrogen are reducing in nature while below hydrogen are oxidising in nature.
A hypoth etical electrochemical cell is shown below 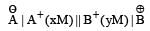 [2006] The emf measured is +0.20 V. The cell reaction is
[2006] The emf measured is +0.20 V. The cell reaction is - a)
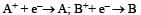
- b)The cell reaction cannot be predicted
- c)
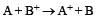
- d)
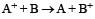
Correct answer is option 'C'. Can you explain this answer?
A hypoth etical electrochemical cell is shown below 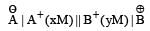 [2006] The emf measured is +0.20 V. The cell reaction is
[2006] The emf measured is +0.20 V. The cell reaction is
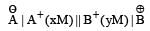 [2006] The emf measured is +0.20 V. The cell reaction is
[2006] The emf measured is +0.20 V. The cell reaction is a)
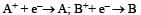
b)
The cell reaction cannot be predicted
c)
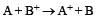
d)
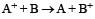

|
Arya Nair answered |
The cell reacton is as follows :

(c) is correct answer.
In electrolysis of NaCl when Pt electrode is taken then H2 is liberated at cathode while with Hg cathode it forms sodium amalgam. This is because [2002]- a)Hg is more inert than Pt
- b)More voltage is required to reduce H+ at Hg than at Pt
- c)Na is dissolved in Hg while it does not dissolve in Pt
- d)Conc. of H+ ions is larger when Pt electrode is taken
Correct answer is option 'B'. Can you explain this answer?
In electrolysis of NaCl when Pt electrode is taken then H2 is liberated at cathode while with Hg cathode it forms sodium amalgam. This is because [2002]
a)
Hg is more inert than Pt
b)
More voltage is required to reduce H+ at Hg than at Pt
c)
Na is dissolved in Hg while it does not dissolve in Pt
d)
Conc. of H+ ions is larger when Pt electrode is taken

|
Rhea Sarkar answered |
In electrolysis of NaCl when Pt electrode is taken then H2 liberated at cathode while with Hg cathode it forms sodium amalgam because more voltage is required to reduce H+ at Hg than Pt.z
Molar conductivities 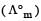 at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S cm2 mol–1 respectively.
at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S cm2 mol–1 respectively. 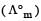 for CH3COOH will be : [2012 M]
for CH3COOH will be : [2012 M]- a)425.5 S cm2 mol–1
- b)180.5 S cm2 mol–1
- c)290.8 S cm2 mol–1
- d)390.5 S cm2 mol–1
Correct answer is option 'D'. Can you explain this answer?
Molar conductivities 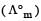 at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S cm2 mol–1 respectively.
at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S cm2 mol–1 respectively. 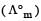 for CH3COOH will be : [2012 M]
for CH3COOH will be : [2012 M]
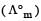 at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S cm2 mol–1 respectively.
at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S cm2 mol–1 respectively. 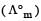 for CH3COOH will be : [2012 M]
for CH3COOH will be : [2012 M]a)
425.5 S cm2 mol–1
b)
180.5 S cm2 mol–1
c)
290.8 S cm2 mol–1
d)
390.5 S cm2 mol–1

|
Nayanika Reddy answered |
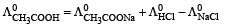
= 91 + 425.9 – 126.4 = 390.5
The equivalent conductance of 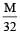 solution ofa weak monobasic acid is 8.0 mhos cm2 and at infinite dilution is 400 mhos cm2. The dissociation constant of this acid is: [2009]
solution ofa weak monobasic acid is 8.0 mhos cm2 and at infinite dilution is 400 mhos cm2. The dissociation constant of this acid is: [2009]- a)1.25 × 10–6
- b)6.25 × 10–4
- c)1.25 × 10–4
- d)1.25 × 10–5
Correct answer is option 'D'. Can you explain this answer?
The equivalent conductance of 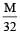 solution ofa weak monobasic acid is 8.0 mhos cm2 and at infinite dilution is 400 mhos cm2. The dissociation constant of this acid is: [2009]
solution ofa weak monobasic acid is 8.0 mhos cm2 and at infinite dilution is 400 mhos cm2. The dissociation constant of this acid is: [2009]
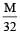 solution ofa weak monobasic acid is 8.0 mhos cm2 and at infinite dilution is 400 mhos cm2. The dissociation constant of this acid is: [2009]
solution ofa weak monobasic acid is 8.0 mhos cm2 and at infinite dilution is 400 mhos cm2. The dissociation constant of this acid is: [2009]a)
1.25 × 10–6
b)
6.25 × 10–4
c)
1.25 × 10–4
d)
1.25 × 10–5

|
Nayanika Reddy answered |
Degree of dissociation
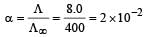
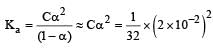
= 1.25 x 10-5
Chapter doubts & questions for Electrochemistry - NEET Past Year Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Electrochemistry - NEET Past Year Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup




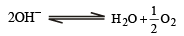
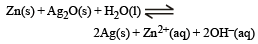
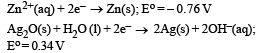

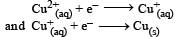
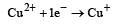
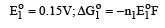
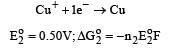
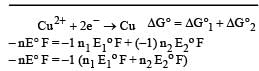
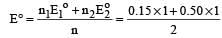
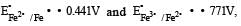 , the standard EMF of the reaction Fe + 2Fe3+ → 3Fe2+ will be [2006]
, the standard EMF of the reaction Fe + 2Fe3+ → 3Fe2+ will be [2006]