All Exams >
NEET >
Topic-wise MCQ Tests for NEET >
All Questions
All questions of Thermal Properties of Matter for NEET Exam
5 g of ice at 0° C is mixed with 10 g of water at 10° C. The temperature of the mixture is:- a)2°C
- b)0°C
- c)5°C
- d)2.5°C
Correct answer is option 'B'. Can you explain this answer?
5 g of ice at 0° C is mixed with 10 g of water at 10° C. The temperature of the mixture is:
a)
2°C
b)
0°C
c)
5°C
d)
2.5°C
|
|
Riya Banerjee answered |
Heat absorbed by 5g ice when it converted to at 0° C = 5 x 80 = 400 cal.
Heat liberated by 10g water at 10° C to 0° C = 100 cal
Hence there is 15g water at 0° C and 300 cal needs to be liberated , thus for some amount of water converts into ice, hence the temp of mixture is 0° C.
Heat liberated by 10g water at 10° C to 0° C = 100 cal
Hence there is 15g water at 0° C and 300 cal needs to be liberated , thus for some amount of water converts into ice, hence the temp of mixture is 0° C.
A steel tape is calibrated at 20° C. A piece of wood is being measured by steel tape at 10°C and reading is 30 cm on the tape. The real length of the wood is:
a)Equal to 30 cmb)less than 30 cmc)cannot be perdictedd)more than 30 cmCorrect answer is option 'B'. Can you explain this answer?
|
|
Preeti Khanna answered |
When heated the length between adjacent markings increases. Hence the actual length will be less than
30 cm.
30 cm.
The amount of heat required to raise the temperature of one mole of an ideal mono atomic gas through 2°C at constant pressure is (universal gas constant = R)- a)5R/2
- b)3 R
- c)2R
- d)5 R
Correct answer is option 'D'. Can you explain this answer?
The amount of heat required to raise the temperature of one mole of an ideal mono atomic gas through 2°C at constant pressure is (universal gas constant = R)
a)
5R/2
b)
3 R
c)
2R
d)
5 R
|
|
Hansa Sharma answered |
At constant pressure
dQ= nCpdT
=1*(5R/2)*2
=5R
dQ= nCpdT
=1*(5R/2)*2
=5R
Consider a compound slab consisting of two different materials having equal thicknesses and thermal conductivities K and 2K, respectively.
The equivalent thermal conductivity of the slab is [2003]- a)
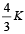
- b)
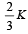
- c)
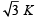
- d)3 K
Correct answer is option 'A'. Can you explain this answer?
Consider a compound slab consisting of two different materials having equal thicknesses and thermal conductivities K and 2K, respectively.
The equivalent thermal conductivity of the slab is [2003]
The equivalent thermal conductivity of the slab is [2003]
a)
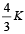
b)
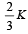
c)
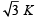
d)
3 K

|
Rajeev Sharma answered |
In series, equivalent thermal conductivity
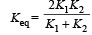
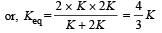
Can you explain the answer of this question below:Water is used as coolant in automobiles radiators because
- A:
it has high specific heat capacity
- B:
it is easily available
- C:
it is easy to carry
- D:
it is cheap
The answer is a.
Water is used as coolant in automobiles radiators because
it has high specific heat capacity
it is easily available
it is easy to carry
it is cheap
|
|
Lavanya Menon answered |
Water is used as a coolant in automobiles radiators because it has high specific heat capacity. So, it absorbs a large amount of heat for a degree rise in temperature.
The temperature range upto which Newton’s law of cooling holds good- a)30-35°C
- b)25-30°C
- c)30-40°C
- d)20-25°C
Correct answer is option 'D'. Can you explain this answer?
The temperature range upto which Newton’s law of cooling holds good
a)
30-35°C
b)
25-30°C
c)
30-40°C
d)
20-25°C
|
|
Hansa Sharma answered |
Newton’s law of cooling is to be used at temperatures around room temperature.
Equal masses of three liquids of specific heats C1, C2 and C3 at temperatures t1, t2 and t3 respectively are mixed. If there is no change of state, the temperature of the mixture is- a)(C1t1 + C2t2 + C3t3)/3(C1 + C2 + C1)
- b)(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
- c)(t1 + t2 +t3 )/3
- d)3(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
Correct answer is option 'B'. Can you explain this answer?
Equal masses of three liquids of specific heats C1, C2 and C3 at temperatures t1, t2 and t3 respectively are mixed. If there is no change of state, the temperature of the mixture is
a)
(C1t1 + C2t2 + C3t3)/3(C1 + C2 + C1)
b)
(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
c)
(t1 + t2 +t3 )/3
d)
3(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
|
|
Raghav Bansal answered |
For the composite system, energy conservation yields no net energy flow in or out of the system.
Let final temperature be T
Then, heat absorbed by A+heat absorbed by B+heat absorbed by C=0
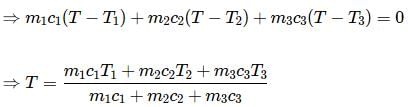
Here in the question m1=m2=m3
Let final temperature be T
Then, heat absorbed by A+heat absorbed by B+heat absorbed by C=0
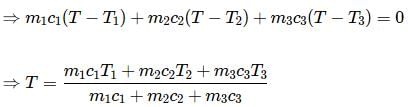
Here in the question m1=m2=m3
When water is heated from 0° C to 20° C its volume:- a)first decreases and then increases
- b)goes on increasing
- c)remains constant up to 15°C and then increases
- d)goes on decreasing
Correct answer is option 'A'. Can you explain this answer?
When water is heated from 0° C to 20° C its volume:
a)
first decreases and then increases
b)
goes on increasing
c)
remains constant up to 15°C and then increases
d)
goes on decreasing
|
|
Pooja Shah answered |
When water is heated from 0°C, its volume decreases because its density increases and you can see this effect upto 4°C. Since the density of ice is maximum at 4°C, afterwards as the density decreases the volume increases. The main reason for this is hydrogen bond in ice gets cleaved due to the melting of ice.
The heat of sun comes to us through:- a)convection
- b)conduction
- c)Sublimation
- d)Radiation
Correct answer is option 'D'. Can you explain this answer?
The heat of sun comes to us through:
a)
convection
b)
conduction
c)
Sublimation
d)
Radiation
|
|
Om Desai answered |
The sun heats the earth through radiation. Since there is no medium (like the gas in our atmosphere) in space, radiation is the primary way that heat travels in space. When the heat reaches the earth it warms the molecules of the atmosphere, and they warm other molecules and so on.
Water contract on heating between the temperatures- a)0°C to 4°C
- b)0°K to 4°K
- c)0o K to 273 K
- d)0°F to 4°F
Correct answer is option 'A'. Can you explain this answer?
Water contract on heating between the temperatures
a)
0°C to 4°C
b)
0°K to 4°K
c)
0o K to 273 K
d)
0°F to 4°F
|
|
Geetika Shah answered |
When water is heated from 0oC, its volume decreases because its density increases and you can see this effect upto 4oC. Since the density of ice is maximum at 4oC, afterwards as the density decreases the volume increases. The main reason for this is hydrogen bond in ice gets cleaved due to the melting of ice.
The process by which heat is transferred from one place to another without heating the intervening medium is known as- a)convection
- b)radiation
- c)sublimation
- d)fusion
Correct answer is option 'B'. Can you explain this answer?
The process by which heat is transferred from one place to another without heating the intervening medium is known as
a)
convection
b)
radiation
c)
sublimation
d)
fusion

|
Pioneer Academy answered |
Radiation alludes to the mechanism in which heat is transmitted without any physical contact between objects. It uses electromagnetic waves for transfer of heat.
Melting point of ice decreases with- a)Decrease in pressure
- b)Increase in temperature
- c)Decrease in temperature
- d)Increase in pressure
Correct answer is option 'D'. Can you explain this answer?
Melting point of ice decreases with
a)
Decrease in pressure
b)
Increase in temperature
c)
Decrease in temperature
d)
Increase in pressure
|
|
Preeti Iyer answered |
When ice melts the volume decreases and an increase in pressure will support this phenomena according to Le Chatalier’s rule.
A piece of iron of mass 100g is kept inside a furnace for a long time and Jthen put in a calorimeter of water equivalent 10g containing 240g of water at 20°C. The mixture attains an equilibrium temperature of 60°C. Find the temperature of the furnace. Specific heat capacity of iron = 470J/kg-°C.- a)500°C
- b)900°C
- c)953.6∘C
- d)706.80 °C
Correct answer is option 'C'. Can you explain this answer?
A piece of iron of mass 100g is kept inside a furnace for a long time and Jthen put in a calorimeter of water equivalent 10g containing 240g of water at 20°C. The mixture attains an equilibrium temperature of 60°C. Find the temperature of the furnace. Specific heat capacity of iron = 470J/kg-°C.
a)
500°C
b)
900°C
c)
953.6∘C
d)
706.80 °C
|
|
Gaurav Kumar answered |
Mass of Iron = 100g
Water Eq of caloriemeter = 10g
Mass of water = 240g
Let the Temp. of surface = 0ºC
Siron = 470J/kg°C
Water Eq of caloriemeter = 10g
Mass of water = 240g
Let the Temp. of surface = 0ºC
Siron = 470J/kg°C
Total heat gained = Total heat lost.
So,100/1000× 470 × (θ – 60) = 250/1000 × 4200 × (60 – 20)
⇒ 47θ – 47 × 60 = 25 × 42 × 40
⇒ θ = 4200 + 2820/47= 44820/47 =953.61°C
⇒ 47θ – 47 × 60 = 25 × 42 × 40
⇒ θ = 4200 + 2820/47= 44820/47 =953.61°C
What is the expression for temperature gradient?- a)
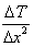
- b)
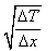
- c)
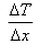
- d)
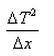
Correct answer is option 'C'. Can you explain this answer?
What is the expression for temperature gradient?
a)
b)
c)
d)
|
|
Krishna Iyer answered |
A temperature gradient is a physical quantity that describes in which direction and at what rate the temperature changes the most rapidly around a particular location.
The temperature gradient is a dimensional quantity expressed in units of degrees per unit length.
The SI unit is kelvin per meter.
Expression: ∆T/∆x, where ∆T = change in temperature and ∆x = change in distance
The temperature gradient is a dimensional quantity expressed in units of degrees per unit length.
The SI unit is kelvin per meter.
Expression: ∆T/∆x, where ∆T = change in temperature and ∆x = change in distance
According to law of calorimetry, which of the given relation is true?- a)Heat gained ≥ Heat lost
- b)Heat gained = Heat lost
- c)Heat gained > Heat lost
- d)Heat lost > Heat gained
Correct answer is option 'B'. Can you explain this answer?
According to law of calorimetry, which of the given relation is true?
a)
Heat gained ≥ Heat lost
b)
Heat gained = Heat lost
c)
Heat gained > Heat lost
d)
Heat lost > Heat gained
|
|
Anjali Iyer answered |
A principle of calorimetry states that if there is no loss of heat in surrounding the total heat lost by hot body equal to the total heat gained by a cold body.
i.e. heat loss = heat gain
The temperature for which the reading on Celsius and Fahrenheit scales are identical is- a)-273°C, -273 °F
- b)-30°C, -30 °F
- c)0 °C, 0 °F
- d)-40 °C, -40 °F
Correct answer is option 'D'. Can you explain this answer?
The temperature for which the reading on Celsius and Fahrenheit scales are identical is
a)
-273°C, -273 °F
b)
-30°C, -30 °F
c)
0 °C, 0 °F
d)
-40 °C, -40 °F
|
|
Preeti Iyer answered |
The Celsius and Fahrenheit are two important temperature scales. The Fahrenheit scale is used primarily in the United States, while Celsius is used throughout the world. The two scales have different zero points and the Celsius degree is bigger than the Fahrenheit one. There is one point on the Fahrenheit and Celsius scales where the temperatures in degrees are equal. This is -40 degree C and -40 degree F.
A device in which heat measurement can be made is called- a)Joule meter
- b)Calorimeter
- c)Thermal meter
- d)Gauge meter
Correct answer is option 'B'. Can you explain this answer?
A device in which heat measurement can be made is called
a)
Joule meter
b)
Calorimeter
c)
Thermal meter
d)
Gauge meter

|
Shanaya Tiwari answered |
Calorimeter:
The correct device for heat measurement is a calorimeter. Calorimeters are used to measure the heat involved in a chemical reaction or physical change. They work based on the principle of conservation of energy, where the heat released or absorbed during a reaction is measured by the change in temperature of a known mass of water.
How does a Calorimeter work?
- A known quantity of the substance undergoing the reaction is placed in the calorimeter along with a known quantity of water.
- The initial temperature of the water and the substance is recorded.
- The reaction takes place, and the heat released or absorbed causes a change in temperature of the water.
- By measuring the change in temperature, the heat exchanged can be calculated using the specific heat capacity of water.
- Calorimeters are designed to minimize heat loss to the surroundings to ensure accurate measurements.
Types of Calorimeters:
- There are different types of calorimeters such as bomb calorimeters, coffee cup calorimeters, and differential scanning calorimeters, each suitable for different applications.
- Bomb calorimeters are commonly used for measuring the heat of combustion of substances.
- Coffee cup calorimeters are simpler and are often used in educational settings to demonstrate heat exchange principles.
In conclusion, a calorimeter is the appropriate device for heat measurement as it allows for accurate determination of the heat involved in a reaction or process.
The correct device for heat measurement is a calorimeter. Calorimeters are used to measure the heat involved in a chemical reaction or physical change. They work based on the principle of conservation of energy, where the heat released or absorbed during a reaction is measured by the change in temperature of a known mass of water.
How does a Calorimeter work?
- A known quantity of the substance undergoing the reaction is placed in the calorimeter along with a known quantity of water.
- The initial temperature of the water and the substance is recorded.
- The reaction takes place, and the heat released or absorbed causes a change in temperature of the water.
- By measuring the change in temperature, the heat exchanged can be calculated using the specific heat capacity of water.
- Calorimeters are designed to minimize heat loss to the surroundings to ensure accurate measurements.
Types of Calorimeters:
- There are different types of calorimeters such as bomb calorimeters, coffee cup calorimeters, and differential scanning calorimeters, each suitable for different applications.
- Bomb calorimeters are commonly used for measuring the heat of combustion of substances.
- Coffee cup calorimeters are simpler and are often used in educational settings to demonstrate heat exchange principles.
In conclusion, a calorimeter is the appropriate device for heat measurement as it allows for accurate determination of the heat involved in a reaction or process.
Newton’s law of cooling states that the rate of cooling of a body is proportional to the _____________________.- a)temperature of the surroundings
- b)excess temperature of the body over the surroundings
- c)temperature of the body
- d)temperature of the body + temperature of the surroundings
Correct answer is option 'B'. Can you explain this answer?
Newton’s law of cooling states that the rate of cooling of a body is proportional to the _____________________.
a)
temperature of the surroundings
b)
excess temperature of the body over the surroundings
c)
temperature of the body
d)
temperature of the body + temperature of the surroundings
|
|
Geetika Shah answered |
Newton's law of cooling states that the heat released by a body with respect to time (or) the rate of heat released is directly proportional to the difference between the body's temperature and the surrounding temperature.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
Skating is possible on snow due to the formation of water below the skates. This water below skates comes as result of- a)Increase in temperature between skates and snow
- b)Increase in pressure between skates and snow
- c)Force due to weight of the skater
- d)Decrease in pressure between skates and snow
Correct answer is option 'B'. Can you explain this answer?
Skating is possible on snow due to the formation of water below the skates. This water below skates comes as result of
a)
Increase in temperature between skates and snow
b)
Increase in pressure between skates and snow
c)
Force due to weight of the skater
d)
Decrease in pressure between skates and snow
|
|
Naina Sharma answered |
As the pressure increases between the skates and ice, the ice starts melting below 0o C.
Can you explain the answer of this question below:Two identical rectangular strips, one of copper and the other of steel, are riveted as shown to form a bi-metal strip. On heating, the bi-metal strip will

- A:
bend with steel on the concave side
- B:
get twisted
- C:
remain straight
- D:
bend with steel on the convex side
The answer is a.
Two identical rectangular strips, one of copper and the other of steel, are riveted as shown to form a bi-metal strip. On heating, the bi-metal strip will

bend with steel on the concave side
get twisted
remain straight
bend with steel on the convex side
|
|
Nandini Patel answered |
On heating, the copper strip will suffer greater elongation and hence the bimetal strip will bend with the steel strip on the concave side.
[Bimetal strips are widely used in thermal switching applications such as automatic electric iron].
What should be the temperature of black body to emit radiant energy which is independent of the conditions in the surroundings?- a)temperature of black body should be less than zero
- b)temperature of black body should be more than zero
- c)temperature of black body should be equal to zero
- d)all of the above
Correct answer is option 'B'. Can you explain this answer?
What should be the temperature of black body to emit radiant energy which is independent of the conditions in the surroundings?
a)
temperature of black body should be less than zero
b)
temperature of black body should be more than zero
c)
temperature of black body should be equal to zero
d)
all of the above
|
|
Suresh Reddy answered |
Temperature of black body should be more than zero. Obvious answer. If the temperature is not greater than 0 then there will be no radiation.
A black body is at 727° C. It emits energy at a rate which is proportional to [2007]- a)(1000)4
- b)(1000)2
- c)727)4
- d)(727)2
Correct answer is option 'A'. Can you explain this answer?
A black body is at 727° C. It emits energy at a rate which is proportional to [2007]
a)
(1000)4
b)
(1000)2
c)
727)4
d)
(727)2

|
Ashwini Khanna answered |
According to Stefan's law, E ∝ T4
∝ (t + 273)4 K [727°C = (727 + 273)K]
∝ (727 + 273)4 K
∝ (1000)4 K
∝ (727 + 273)4 K
∝ (1000)4 K
What is the coefficient of thermal conductivity K?- a)ΔQt
- b)
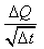
- c)
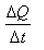
- d)
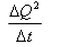
Correct answer is option 'C'. Can you explain this answer?
What is the coefficient of thermal conductivity K?
a)
ΔQt
b)
c)
d)

|
Ciel Knowledge answered |
K is rate of change of charge.
A body cools from 50.0°C to 48°C in 5s. How long will it take to cool from 40.0°C to 39°C?Assume the temperature of surroundings to be 30.0°C and Newton's law of cooling to be valid.
- a)2.5 sec
- b)10 sec
- c)20 sec
- d)9 sec
Correct answer is option 'B'. Can you explain this answer?
A body cools from 50.0°C to 48°C in 5s. How long will it take to cool from 40.0°C to 39°C?Assume the temperature of surroundings to be 30.0°C and Newton's law of cooling to be valid.
a)
2.5 sec
b)
10 sec
c)
20 sec
d)
9 sec

|
Maheshwar Saini answered |
Rate of cooling ∝ temperature difference between system and surrounding.
As the temperature difference is halved, so the rate of cooling will also be halved
As the temperature difference is halved, so the rate of cooling will also be halved
Radiation from which of the following sources, approximates black body radiation best? [2002]- a)A tungsten lamp
- b)Sodium flame
- c)Hot lamp black
- d)A hole in a cavity, maintained at constant temperature
Correct answer is option 'D'. Can you explain this answer?
Radiation from which of the following sources, approximates black body radiation best? [2002]
a)
A tungsten lamp
b)
Sodium flame
c)
Hot lamp black
d)
A hole in a cavity, maintained at constant temperature
|
|
Zaid Ali answered |
Option D this holed cavity is also called Fery's black body it is made such that when light enter the cavity it suffer multiple reflection inside it and since with every reflection some part is absorbed so almost all the radiant light get absorbed...
Mercury is a liquid which is heated by:- a)Convection
- b)Radiation
- c)Conduction
- d)transfusion
Correct answer is option 'C'. Can you explain this answer?
Mercury is a liquid which is heated by:
a)
Convection
b)
Radiation
c)
Conduction
d)
transfusion
|
|
Geetika Shah answered |
Hg expands on heat (thermometers) it acts as a good conducting metal.
The temperature and pressure at which all three phases of a substances coexist is called- a)Fusion point
- b)Triple point
- c)Sublimation point
- d)Melting Point
Correct answer is option 'B'. Can you explain this answer?
The temperature and pressure at which all three phases of a substances coexist is called
a)
Fusion point
b)
Triple point
c)
Sublimation point
d)
Melting Point
|
|
Rajeev Saxena answered |
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. It is that temperature and pressure at which the sublimation curve, fusion curve and the vaporisation curve meet.
A piece of iron is heated in a flame. It first becomes dull red then becomes reddish yellow and finally turns to white hot. The correct explanation for the above observation is possible by using [NEET 2013]- a)Wien’s displacement law
- b)Kirchoff’s law
- c)Newton’s law of cooling
- d)Stefan’s law
Correct answer is option 'A'. Can you explain this answer?
A piece of iron is heated in a flame. It first becomes dull red then becomes reddish yellow and finally turns to white hot. The correct explanation for the above observation is possible by using [NEET 2013]
a)
Wien’s displacement law
b)
Kirchoff’s law
c)
Newton’s law of cooling
d)
Stefan’s law

|
Srishti Sen answered |
Wein’s displacement law According to this law
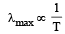
or, λmax × T = constant
So, as the temperature increases λ decreases.
So, as the temperature increases λ decreases.
Which phase of matter has maximum value of temperature coefficient of cubical expansion?- a)Gaseous
- b)Solid
- c)Liquid
- d)none of these
Correct answer is option 'A'. Can you explain this answer?
Which phase of matter has maximum value of temperature coefficient of cubical expansion?
a)
Gaseous
b)
Solid
c)
Liquid
d)
none of these
|
|
Pooja Shah answered |
Gas expands more than solid and liquid because gas particles are far apart hence freely move.
If the radius of a star is R and it acts as a black body, what would be the temperature of the star, in which the rate of energy production is Q ? [2012](σ stands for Stefan’S constant)- a)Q/4πR2σ
- b)(Q/4πR2σ)–1/2
- c)(4πR2Q/σ)1/4
- d)(Q/4πR2σ)1/4
Correct answer is option 'D'. Can you explain this answer?
If the radius of a star is R and it acts as a black body, what would be the temperature of the star, in which the rate of energy production is Q ? [2012]
(σ stands for Stefan’S constant)
a)
Q/4πR2σ
b)
(Q/4πR2σ)–1/2
c)
(4πR2Q/σ)1/4
d)
(Q/4πR2σ)1/4

|
Ashwini Khanna answered |
Stefan’s law for black body radiation Q = σe AT4
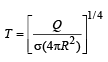
Here e = 1
A = 4πR2
A = 4πR2
Value of coefficient of thermal coefficient is:- a)same incase of conductors and insulators
- b)Good incase of conductors and small incase of insulators
- c)Does not depend on insulators and conductors
- d)Small incase of insulators and good incase of insulators
Correct answer is option 'B'. Can you explain this answer?
Value of coefficient of thermal coefficient is:
a)
same incase of conductors and insulators
b)
Good incase of conductors and small incase of insulators
c)
Does not depend on insulators and conductors
d)
Small incase of insulators and good incase of insulators
|
|
Raghav Bansal answered |
Conductors have free electrons in them whereas insulators don’t have any. Therefore, conductors conduct heat and electricity better than insulators. Therefore the value of thermal coefficient is good in case of conductors and less in case in insulators.
Which of the given phenomenon is not related to convection?- a)In winter metallic handles appear colder than wooden door
- b)Maintaining comfortable room temperature in cold countries
- c)Formation of trade winds
- d)Formation of land and sea breezes
Correct answer is option 'A'. Can you explain this answer?
Which of the given phenomenon is not related to convection?
a)
In winter metallic handles appear colder than wooden door
b)
Maintaining comfortable room temperature in cold countries
c)
Formation of trade winds
d)
Formation of land and sea breezes
|
|
Stuti Joshi answered |
Introduction:
Convection is a mode of heat transfer that occurs due to the movement of a fluid. It involves the transfer of heat energy through the bulk movement of molecules within a fluid such as air or water. Convection can occur in various scenarios, but not all phenomena are related to convection.
Explanation:
Let's analyze each option to determine which one is not related to convection:
a) In winter metallic handles appear colder than wooden door:
This phenomenon is not related to convection. It is actually related to the thermal conductivity of different materials. Metals, such as metallic handles, have higher thermal conductivity compared to wood. Therefore, when touched, metallic handles conduct heat away from our hands more rapidly than wooden doors, making them feel colder.
b) Maintaining comfortable room temperature in cold countries:
This phenomenon is related to convection. In cold countries, the temperature inside a room can be maintained at a comfortable level by using heating systems such as radiators or heaters. These devices heat the air in the room, causing it to expand and rise. As the warm air rises, it creates a convection current, distributing the heat throughout the room.
c) Formation of trade winds:
This phenomenon is related to convection. Trade winds are a pattern of prevailing winds that blow from east to west in the equatorial region. They are formed due to the convectional heating of air near the equator. The intense solar radiation at the equator heats the air, causing it to expand and rise. As the air rises, it creates a low-pressure zone, and the cooler air from higher latitudes flows in to replace it, resulting in the formation of trade winds.
d) Formation of land and sea breezes:
This phenomenon is related to convection. Land and sea breezes are local winds that occur near coastal areas. During the day, the land heats up more quickly than the sea. The warm air over the land rises, creating a low-pressure zone, and the cooler air from the sea flows in to replace it, resulting in the formation of a sea breeze. At night, the land cools down more rapidly than the sea, causing the warm air to rise over the sea and the cooler air from the land to flow in, resulting in the formation of a land breeze.
Conclusion:
Out of the given options, the phenomenon of metallic handles appearing colder than wooden doors in winter is not related to convection. It is primarily influenced by the thermal conductivity of different materials. Convection, on the other hand, is involved in maintaining room temperature, formation of trade winds, and land and sea breezes.
Convection is a mode of heat transfer that occurs due to the movement of a fluid. It involves the transfer of heat energy through the bulk movement of molecules within a fluid such as air or water. Convection can occur in various scenarios, but not all phenomena are related to convection.
Explanation:
Let's analyze each option to determine which one is not related to convection:
a) In winter metallic handles appear colder than wooden door:
This phenomenon is not related to convection. It is actually related to the thermal conductivity of different materials. Metals, such as metallic handles, have higher thermal conductivity compared to wood. Therefore, when touched, metallic handles conduct heat away from our hands more rapidly than wooden doors, making them feel colder.
b) Maintaining comfortable room temperature in cold countries:
This phenomenon is related to convection. In cold countries, the temperature inside a room can be maintained at a comfortable level by using heating systems such as radiators or heaters. These devices heat the air in the room, causing it to expand and rise. As the warm air rises, it creates a convection current, distributing the heat throughout the room.
c) Formation of trade winds:
This phenomenon is related to convection. Trade winds are a pattern of prevailing winds that blow from east to west in the equatorial region. They are formed due to the convectional heating of air near the equator. The intense solar radiation at the equator heats the air, causing it to expand and rise. As the air rises, it creates a low-pressure zone, and the cooler air from higher latitudes flows in to replace it, resulting in the formation of trade winds.
d) Formation of land and sea breezes:
This phenomenon is related to convection. Land and sea breezes are local winds that occur near coastal areas. During the day, the land heats up more quickly than the sea. The warm air over the land rises, creating a low-pressure zone, and the cooler air from the sea flows in to replace it, resulting in the formation of a sea breeze. At night, the land cools down more rapidly than the sea, causing the warm air to rise over the sea and the cooler air from the land to flow in, resulting in the formation of a land breeze.
Conclusion:
Out of the given options, the phenomenon of metallic handles appearing colder than wooden doors in winter is not related to convection. It is primarily influenced by the thermal conductivity of different materials. Convection, on the other hand, is involved in maintaining room temperature, formation of trade winds, and land and sea breezes.
An electric kettle takes 4A current at 220 V. How much time will it take to boil 1 kg of water from temperature 20° C? The temperature of boiling water is 100° C. [2008]- a)6.3 min
- b)8.4 min
- c)12.6 min
- d)4.2 min
Correct answer is option 'A'. Can you explain this answer?
An electric kettle takes 4A current at 220 V. How much time will it take to boil 1 kg of water from temperature 20° C? The temperature of boiling water is 100° C. [2008]
a)
6.3 min
b)
8.4 min
c)
12.6 min
d)
4.2 min

|
Ishaan Menon answered |
Heat required to raise the temperature of 1kg water from 20°C to 100°C is given by Q = msΔθ = (1× 4200 × 80) J
Power of kettle (P) = VI = (220 × 4)W
Power of kettle (P) = VI = (220 × 4)W
∴ Time taken =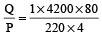
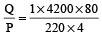
= 381.81 sec = 6.36 min
The density of water at 20°C is 998 kg/m3 and at 40°C 992 kg/m3. The coefficient of volume expansion of water is [NEET Kar. 2013]- a)10–4/°C
- b)3 × 10–4/°C
- c)2 × 10–4/°C
- d)6 × 10–4/°C
Correct answer is option 'B'. Can you explain this answer?
The density of water at 20°C is 998 kg/m3 and at 40°C 992 kg/m3. The coefficient of volume expansion of water is [NEET Kar. 2013]
a)
10–4/°C
b)
3 × 10–4/°C
c)
2 × 10–4/°C
d)
6 × 10–4/°C
|
|
Priya Menon answered |
From question, Δρ = (998 – 992) kg/m3 = 6 kg/m3
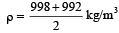 = 995 kg/m3
= 995 kg/m3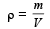
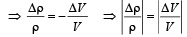
∴ Coefficient of volume expansion of water,
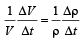
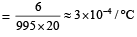
N molecules each of mass m of a gas A and 2N molecules each of mass 2m of gas B are contained in the same vessel which is maintained at temperature T. The mean square velocity of molecules of B type is v2 and the mean square rectangular component of the velocity of A type is denoted by ω2. Then ω2/v2 [1991]- a)2
- b)1
- c)1/3
- d)2/3
Correct answer is option 'D'. Can you explain this answer?
N molecules each of mass m of a gas A and 2N molecules each of mass 2m of gas B are contained in the same vessel which is maintained at temperature T. The mean square velocity of molecules of B type is v2 and the mean square rectangular component of the velocity of A type is denoted by ω2. Then ω2/v2 [1991]
a)
2
b)
1
c)
1/3
d)
2/3

|
Aashna Mukherjee answered |
Mean kinetic energy of the two types of molecules should be equal. The mean square velocity of A type molecules = ω2 + ω2 + ω2 = 3ω2
Therefore, 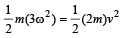
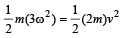
This gives ω2 / v2 = 2/3
The presence of gravitational field is required for the heat transfer by [2000]- a)con duction
- b)stirring of liquids
- c)natural convection
- d)radiation
Correct answer is option 'C'. Can you explain this answer?
The presence of gravitational field is required for the heat transfer by [2000]
a)
con duction
b)
stirring of liquids
c)
natural convection
d)
radiation

|
Shruti Chauhan answered |
In convection, the temperature gradient exists in the vertical direction and not in the horizontal direction. So, up and down movement of particles takes place which depends on the weight and gravity.
Two rods of thermal conductivities K1 and K2, cross-sections A1 and A2 and specific heats S1 and S2 are of equal lengths. The temperatures of two ends of each rod are T1 and T2. The rate of flow of heat at the steady state will be equal if [2002]- a)

- b)K1A1 = K2A2
- c)K1S1 = K2S2
- d)A1S1 = A2S2
Correct answer is option 'B'. Can you explain this answer?
Two rods of thermal conductivities K1 and K2, cross-sections A1 and A2 and specific heats S1 and S2 are of equal lengths. The temperatures of two ends of each rod are T1 and T2. The rate of flow of heat at the steady state will be equal if [2002]
a)

b)
K1A1 = K2A2
c)
K1S1 = K2S2
d)
A1S1 = A2S2
|
|
Hansa Sharma answered |
Rate of heat flow for one rod
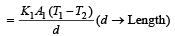
.Rate of heat flow for other rod
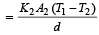
In steady state, 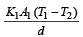
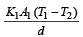
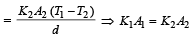
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as- a)conduction
- b)convection
- c)Sublimation
- d)Fusion
Correct answer is option 'A'. Can you explain this answer?
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as
a)
conduction
b)
convection
c)
Sublimation
d)
Fusion
|
|
Geetika Shah answered |
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as conduction. This is the definition of conduction.
An increase in temperature in a liquid would cause a phase change to which of the following?- a)Gas
- b)liquid
- c)solid
- d)plasma
Correct answer is option 'A'. Can you explain this answer?
An increase in temperature in a liquid would cause a phase change to which of the following?
a)
Gas
b)
liquid
c)
solid
d)
plasma
|
|
Neha Sharma answered |
Increasing the temperature of liquid, increases the average K.E. of the molecules. The molecules start moving vigorously in all the directions, thereby increasing and the inter-molecular space between them. Thus, the liquid changes into gas.
A cylindrical metallic rod in therrnal contact with two reservoirs of heat at its two ends conducts an amount of heat Q in time t. The metallic rod is melted and the material is formed into a rod of half the radius of the original rod. What is the amount of heat conducted by the new rod, when placed in thermal contact with the two reservoirs in time t? [2010]- a)
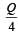
- b)
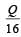
- c)2Q
- d)
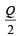
Correct answer is option 'B'. Can you explain this answer?
A cylindrical metallic rod in therrnal contact with two reservoirs of heat at its two ends conducts an amount of heat Q in time t. The metallic rod is melted and the material is formed into a rod of half the radius of the original rod. What is the amount of heat conducted by the new rod, when placed in thermal contact with the two reservoirs in time t? [2010]
a)
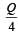
b)
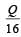
c)
2Q
d)
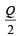
|
|
Yash Modi answered |
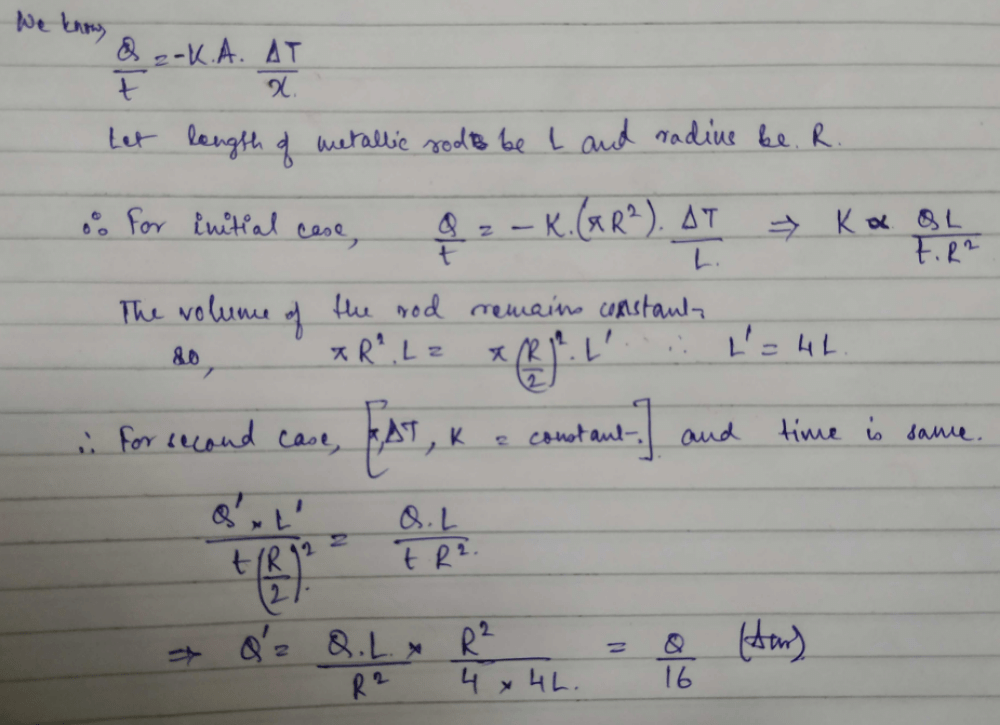
The process by which heat flows from the region of higher temperature to the region of lower temperature by actual movement of material particles is called- a)Sublimation
- b)radiaton
- c)Conduction
- d)Convection
Correct answer is option 'D'. Can you explain this answer?
The process by which heat flows from the region of higher temperature to the region of lower temperature by actual movement of material particles is called
a)
Sublimation
b)
radiaton
c)
Conduction
d)
Convection
|
|
Rajat Patel answered |
In this process, heat is transferred in the liquid and gases from a region of higher temperature to a region of lower temperature. Convection heat transfer occurs partly due to the actual movement of molecules or due to the mass transfer.
SI unit of thermal conductivity is- a)Js K
- b)Js-1 K
- c)Js K-1
- d)Jm-1s-1 K-1
Correct answer is option 'D'. Can you explain this answer?
SI unit of thermal conductivity is
a)
Js K
b)
Js-1 K
c)
Js K-1
d)
Jm-1s-1 K-1
|
|
Pooja Shah answered |
K = power × thickness (m) ÷ area × ∆T
K = (J s-1) (m) ÷ (m2) (K)
K = J m-1 s-1 k-1
K = (J s-1) (m) ÷ (m2) (K)
K = J m-1 s-1 k-1
If the temperature difference between the body and surrounding is small, then the rate of loss of heat is directly proportional to- a)Temperature of two bodies
- b)Nature of two bodies
- c)Pressure difference of the body
- d)Size of the two bodies
Correct answer is option 'A'. Can you explain this answer?
If the temperature difference between the body and surrounding is small, then the rate of loss of heat is directly proportional to
a)
Temperature of two bodies
b)
Nature of two bodies
c)
Pressure difference of the body
d)
Size of the two bodies

|
Mahendra Pal Sunda answered |
Where are the two bodies and rate of loss of heat also depends on sigma( property of material)
If 1 g of steam is mixed with 1 g of ice, then the resultant temperature of the mixture is [1999]- a)270ºC
- b)230ºC
- c)100ºC
- d)50ºC
Correct answer is option 'C'. Can you explain this answer?
If 1 g of steam is mixed with 1 g of ice, then the resultant temperature of the mixture is [1999]
a)
270ºC
b)
230ºC
c)
100ºC
d)
50ºC

|
Pooja Choudhary answered |
Heat required by ice a t 0° C to reach a temperature of 100°C = mL + mcΔθ = 1 × 80 + 1 × 1 × (100 – 0) = 180 cal
Heat available with 1 g steam to condense into 1 g of water at 100°C = 536 cal.
Obviously the whole steam will not be condensed and ice will attain a temperature of 100°C; so the temperature of mixture = 100°C.
Heat available with 1 g steam to condense into 1 g of water at 100°C = 536 cal.
Obviously the whole steam will not be condensed and ice will attain a temperature of 100°C; so the temperature of mixture = 100°C.
A student added a drop of red food coloring to 4 beakers of water.Each beaker contained 100 ml of a different temperature water. The student recorded how long it took each beaker to mix completely (without stirring). The following table shows her results:
 What conclusion should the student make from this experiment? Particles are moving
What conclusion should the student make from this experiment? Particles are moving - a)faster in warm water so they mix faster
- b)slowly in warm water so they mix faster
- c)faster in warm water so they mix slowly
- d)slowly in warm water so they mix slowly
Correct answer is option 'A'. Can you explain this answer?
A student added a drop of red food coloring to 4 beakers of water.Each beaker contained 100 ml of a different temperature water. The student recorded how long it took each beaker to mix completely (without stirring). The following table shows her results:


What conclusion should the student make from this experiment? Particles are moving
a)
faster in warm water so they mix faster
b)
slowly in warm water so they mix faster
c)
faster in warm water so they mix slowly
d)
slowly in warm water so they mix slowly
|
|
Preeti Iyer answered |
Adding heat energy to water increases the motion of the water molecules and the molecules of food coloring. The faster moving molecules cause the food coloring to mix into the water faster. The water molecules and food coloring molecules in cold water don't move as quickly as in hot water.
Chapter doubts & questions for Thermal Properties of Matter - Topic-wise MCQ Tests for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermal Properties of Matter - Topic-wise MCQ Tests for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily