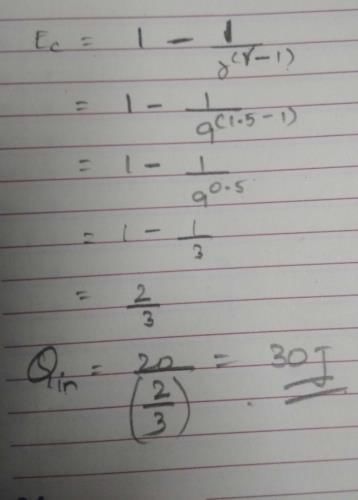All Exams >
NEET >
Topic-wise MCQ Tests for NEET >
All Questions
All questions of Thermodynamics for NEET Exam
Which wall would allow the flow of thermal energy between systems A and B to achieve thermal equilibrium?
- a)Diathermic wall
- b)Adiabatic wall
- c)Diadiabatic wall
- d)Thermal wall
Correct answer is option 'A'. Can you explain this answer?
Which wall would allow the flow of thermal energy between systems A and B to achieve thermal equilibrium?

a)
Diathermic wall
b)
Adiabatic wall
c)
Diadiabatic wall
d)
Thermal wall
|
|
Rajesh Gupta answered |
Wall that permits *heat" to flow through them,such as engine block is called diathermic wall.
wall Perfectly insulating ball that doesn't allow the flow heat to them are called adiabatic walls.
wall Perfectly insulating ball that doesn't allow the flow heat to them are called adiabatic walls.
What is not true for a cyclic process?a) System returns to its initial state
b) ΔU = 0
c) ΔW= 0
d) ΔQ = -ΔW
Correct answer is option 'C'. Can you explain this answer?
b) ΔU = 0
c) ΔW= 0
d) ΔQ = -ΔW
Correct answer is option 'C'. Can you explain this answer?
|
|
Neha Joshi answered |
As work is a path function rather than a state function, we can easily say that work can often be graphically represented as the area under the PV graph. And as cyclic processes are represented as closed shapes on PV graph it is obvious that they have non zero area and thus work done is non zero.
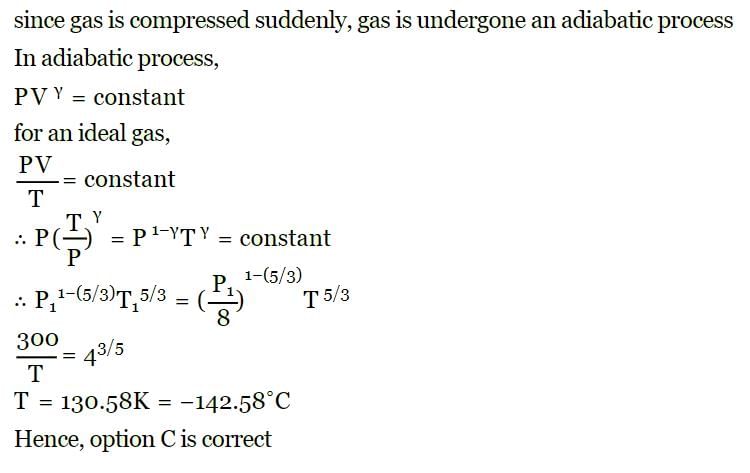
A mass of diatomic gas (γ= 1.4) at a pressure of 2 atmospheres is compressed adiabatically so that its temperature rises from 27°C to 927°C.The pressure of the gas in final state is [2011M]- a)28 atm
- b)68.7 atm
- c)256 atm
- d)8 atm
Correct answer is option 'C'. Can you explain this answer?
A mass of diatomic gas (γ= 1.4) at a pressure of 2 atmospheres is compressed adiabatically so that its temperature rises from 27°C to 927°C.The pressure of the gas in final state is [2011M]
a)
28 atm
b)
68.7 atm
c)
256 atm
d)
8 atm
|
|
Surendra Bishnoi answered |
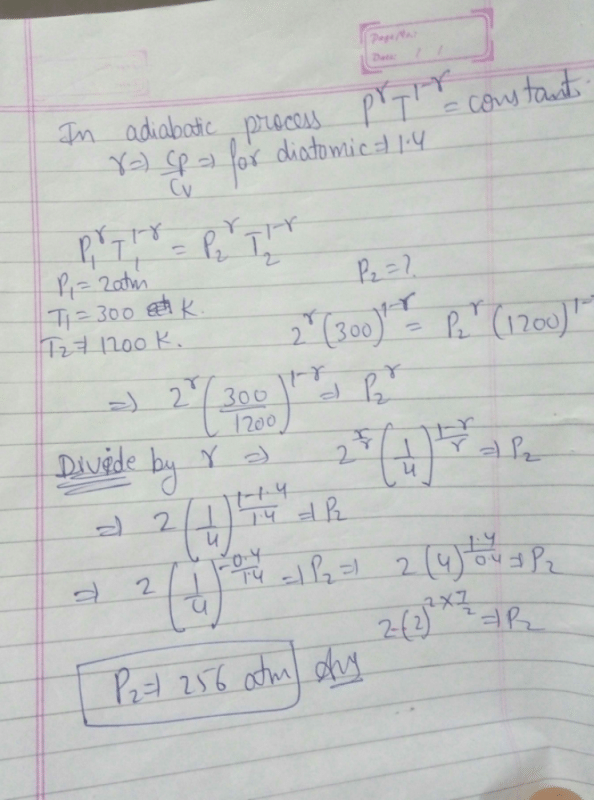
The temperature inside a refrigerator is 4°C and the room temperature is 27°C. How many joules of heat will be delivered to the room for each joule of electricity consumed by the refrigerator?( Treat the refrigerator as ideal).- a)1 J
- b)12 J
- c)8.3 J
- d)13 J
Correct answer is option 'D'. Can you explain this answer?
The temperature inside a refrigerator is 4°C and the room temperature is 27°C. How many joules of heat will be delivered to the room for each joule of electricity consumed by the refrigerator?( Treat the refrigerator as ideal).
a)
1 J
b)
12 J
c)
8.3 J
d)
13 J
|
|
Krishna Iyer answered |
Coefficient of performance(cop) of a refrigerator,
β= Q2/Q1-Q2=T2/T1-T2=277/300-277=277/23≈12
OR, Q2/W=12. Therefore, W=1J.Q2=12J
Also, Q1=Q2+W
=(12+1) J
=13J
β= Q2/Q1-Q2=T2/T1-T2=277/300-277=277/23≈12
OR, Q2/W=12. Therefore, W=1J.Q2=12J
Also, Q1=Q2+W
=(12+1) J
=13J
In a Carnot engine 800 J of heat is absorbed from a source at 400 K and 640 J of heat is rejected to the sink. The temperature of the sink is:- a)273 K
- b)100 K
- c)320 K
- d)240 K
Correct answer is option 'C'. Can you explain this answer?
In a Carnot engine 800 J of heat is absorbed from a source at 400 K and 640 J of heat is rejected to the sink. The temperature of the sink is:
a)
273 K
b)
100 K
c)
320 K
d)
240 K
|
|
Nandini Patel answered |
In a Carnot engine, Q1/T1 = Q2/T2
so that the temperature of the sink,
T2 = T1Q2/Q1 = 400x640/800 = 320 K.
The internal energy and the work done by a system decreases by same amount then- a)The temperature must decrease
- b)The process must be adiabatic
- c)The process must be isothermal
- d)both a and b
Correct answer is option 'D'. Can you explain this answer?
The internal energy and the work done by a system decreases by same amount then
a)
The temperature must decrease
b)
The process must be adiabatic
c)
The process must be isothermal
d)
both a and b
|
|
Gaurav Kumar answered |
The internal energy of a system decreases by the same amount as the work done by the system.
Change in internal energy=work done+heat exchange
change in internal energy=work done if process has no heat exchange, i.e. it's adiabatic and the temperature must decrease
Change in internal energy=work done+heat exchange
change in internal energy=work done if process has no heat exchange, i.e. it's adiabatic and the temperature must decrease
What would be the horse power of a steam engine with average pressure of steam 9x 104 Nm-2 , the area of cross section of the piston is 0.2 m2, length of stroke is 0.6 m and piston makes 5 revolutions per second?- a)150 h.p.
- b)140.7 h.p.
- c)104.7 h.p.
- d)144.77 h.p.
Correct answer is option 'D'. Can you explain this answer?
What would be the horse power of a steam engine with average pressure of steam 9x 104 Nm-2 , the area of cross section of the piston is 0.2 m2, length of stroke is 0.6 m and piston makes 5 revolutions per second?
a)
150 h.p.
b)
140.7 h.p.
c)
104.7 h.p.
d)
144.77 h.p.
|
|
Pooja Shah answered |
Energy in 1 revolution=2 x P x a x l=2 x 9 x104 x 0.2 x0.6 j=21600J
Energy in n revolutions= 21600nJ
Power=E/t=21600n/t=21600 x 5=18000 J/s= 108000/746 h.p=144.77 h.p.
Energy in n revolutions= 21600nJ
Power=E/t=21600n/t=21600 x 5=18000 J/s= 108000/746 h.p=144.77 h.p.
An ideal gas undergoes four different processes from the same initial state (Fig.). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic.
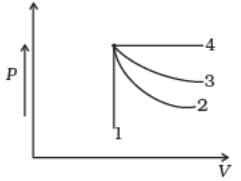
- a)4
- b)3
- c)1
- d)2
Correct answer is option 'D'. Can you explain this answer?
An ideal gas undergoes four different processes from the same initial state (Fig.). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic.


a)
4
b)
3
c)
1
d)
2
|
|
Raghav Bansal answered |
4 is isobaric process, 1 is isochoric. Out of 3 and 2, 3 has the smaller slope (magnitude) hence is isothermal. Remaining process 2 is adiabatic.
First law of thermodynamics tells us:- a)the nature of the process taking place
- b)the direction in which a given process can take place
- c)to what extent the process takes place
- d)that heat supplied is used to carry out the process
Correct answer is option 'D'. Can you explain this answer?
First law of thermodynamics tells us:
a)
the nature of the process taking place
b)
the direction in which a given process can take place
c)
to what extent the process takes place
d)
that heat supplied is used to carry out the process
|
|
Hansa Sharma answered |
First law of thermodynamics is based on law of conservation of energy i.e. energy supplied to a system has to be used in raising the internal energy of the system. So heat supplied is used to carry out the process.
∆U = Q + W.
∆U = Q + W.
Which of the following are the extensive variables?- a)Internal energy, pressure and volume
- b)Pressure, temperature and density
- c)Internal energy, volume, total mass
- d)Pressure, temperature and volume
Correct answer is option 'C'. Can you explain this answer?
Which of the following are the extensive variables?
a)
Internal energy, pressure and volume
b)
Pressure, temperature and density
c)
Internal energy, volume, total mass
d)
Pressure, temperature and volume
|
|
Anjali Iyer answered |
An extensive variable is one which depends on system size (like mass or volume). ... An intensive variable is one which does not depend on system size (like temperature, pressure, or density).
The Zeroth law leads to the concept of- a)temprature
- b)heat
- c)internal energy
- d)work
Correct answer is option 'A'. Can you explain this answer?
The Zeroth law leads to the concept of
a)
temprature
b)
heat
c)
internal energy
d)
work
|
|
Gaurav Kumar answered |
Zeroth law states that if two thermodynamic systems are each in thermal equilibrium with a third one, then they are in thermal equilibrium with each other. Thus as it deals with thermal equilibrium it is very clear that it is a concept of temperature.
The First Law of Thermodynamics states that:- a)ΔQ - W = ΔU
- b)ΔQ - ΔW = U
- c)ΔQ - ΔU = ΔW
- d)Q - W = U
Correct answer is option 'C'. Can you explain this answer?
The First Law of Thermodynamics states that:
a)
ΔQ - W = ΔU
b)
ΔQ - ΔW = U
c)
ΔQ - ΔU = ΔW
d)
Q - W = U
|
|
Preeti Iyer answered |
The first law of thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.
According to this law, some of the heat given to system is used to change the internal energy while the rest in doing work by the system. Mathematically,
ΔQ=ΔU+ΔW
where,
ΔQ = Heat supplied to the system
ΔW= Work done by the system.
ΔU = Change in the internal energy of the system.
If Q is positive, then there is a net heat transfer into the system, if W is positive, then there is work done by the system. So positive Q adds energy to the system and positive W takes energy from the system.
It can also be represented as ΔU=ΔQ-ΔW
We can say that internal energy tends to increase when heat is given to the system and vice versa.
If the door of refrigerator is left open inside a closed room, what would happen to the temperature of the room?- a)Room temperature would decrease
- b)Room temperature would increase
- c)Room temperature would be same as the temperature inside the refrigerator
- d)Room temperature would not be effected
Correct answer is option 'B'. Can you explain this answer?
If the door of refrigerator is left open inside a closed room, what would happen to the temperature of the room?
a)
Room temperature would decrease
b)
Room temperature would increase
c)
Room temperature would be same as the temperature inside the refrigerator
d)
Room temperature would not be effected
|
|
Riya Banerjee answered |
If you leave the door open, heat is merely recycled from the room into therefrigerator, then back into the room. A net room temperature increase wouldresult from the heat of the motor that would be constantly running to move energy around in a circle.
Find the final temperature of one mole of an ideal gas at an initial temperature to t K.The gas does 9 R joules of work adiabatically. The ratio of specific heats of this gas at constant pressure and at constant volume is 4/3.- a)(t-9)K
- b)(t - 4/3)K
- c)t + 3K
- d)(t - 3)K
Correct answer is option 'D'. Can you explain this answer?
Find the final temperature of one mole of an ideal gas at an initial temperature to t K.The gas does 9 R joules of work adiabatically. The ratio of specific heats of this gas at constant pressure and at constant volume is 4/3.
a)
(t-9)K
b)
(t - 4/3)K
c)
t + 3K
d)
(t - 3)K
|
|
Gaurav Kumar answered |
TInitial = t K
Work, W = 9R
Ratio of specific heats, γ = Cp / Cv = 4/3
In an adiabatic process, we have
W = R(TFinal – Tinitial) / (1-γ)
9R = R (TFinal – t) / (1 – 4/3)
TFinal – t = 9 (-1/3) = -3
TFinal = (t-3) K
Work, W = 9R
Ratio of specific heats, γ = Cp / Cv = 4/3
In an adiabatic process, we have
W = R(TFinal – Tinitial) / (1-γ)
9R = R (TFinal – t) / (1 – 4/3)
TFinal – t = 9 (-1/3) = -3
TFinal = (t-3) K
Which of the following statement is true for a thermodynamical system where ∆U is the increase in internal energy and ∆W work done respectively?- a)∆U = ∆W in isothermal process
- b)∆U = ∆W in a adiabatic process
- c)∆U= -∆W in case of ideal gas
- d)∆U= -∆W in an adiabatic process
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is true for a thermodynamical system where ∆U is the increase in internal energy and ∆W work done respectively?
a)
∆U = ∆W in isothermal process
b)
∆U = ∆W in a adiabatic process
c)
∆U= -∆W in case of ideal gas
d)
∆U= -∆W in an adiabatic process
|
|
Hansa Sharma answered |
∆ U = ∆ Q - ∆ W
In an adiabatic process ∆Q is zero, therefore
∆ U = - ∆ W
In an adiabatic process ∆Q is zero, therefore
∆ U = - ∆ W
Which of the following is not thermodynamical function ? [1993]- a)Enthalpy
- b)Work done
- c)Gibb's energy
- d)Internal energy
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not thermodynamical function ? [1993]
a)
Enthalpy
b)
Work done
c)
Gibb's energy
d)
Internal energy

|
Anirudh Datta answered |
Work done is not a thermodynamical function.
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the- a)coefficient of performance
- b)principle of heat engine
- c)efficiency of heat engine
- d)efficiency of refrigerator
Correct answer is option 'A'. Can you explain this answer?
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the
a)
coefficient of performance
b)
principle of heat engine
c)
efficiency of heat engine
d)
efficiency of refrigerator
|
|
Lavanya Menon answered |
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the coefficient of performance. It is the definition of coefficient of performance.
One mole of an ideal gas goes from an initial state A to final state B via two processes : It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is : [2012]- a)

- b)

- c)
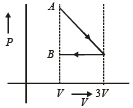
- d)
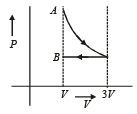
Correct answer is option 'D'. Can you explain this answer?
One mole of an ideal gas goes from an initial state A to final state B via two processes : It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is : [2012]
a)

b)

c)

d)


|
Shivani Tiwari answered |
1st process is isothermal expansion which is only correct shown in option (d) 2nd process is isobaric compression which is correctly shown in option (d)
Isothermal process can be represented by which law?- a)Charle’s law
- b)Boyle’s law
- c)Gay-Lussac’s law
- d)2nd law of thermodynamics
Correct answer is option 'B'. Can you explain this answer?
Isothermal process can be represented by which law?
a)
Charle’s law
b)
Boyle’s law
c)
Gay-Lussac’s law
d)
2nd law of thermodynamics
|
|
Janhavi Choudhury answered |
's law
b)Boyle's law
c)Gay-Lussac's law
d)Joule's law
d) Joule's law.
b)Boyle's law
c)Gay-Lussac's law
d)Joule's law
d) Joule's law.
Refrigerators X and Y are removing 1000 J of heat from the freezer. Refrigerator X is working between -5° C and 25° C and refrigerator Y is working between -20° C and 20 °C. Find efficiency of refrigerator X and Y?- a)20,2
- b)7.9,6.5
- c)9.8,7.3
- d)8.9,7.3
Correct answer is option 'D'. Can you explain this answer?
Refrigerators X and Y are removing 1000 J of heat from the freezer. Refrigerator X is working between -5° C and 25° C and refrigerator Y is working between -20° C and 20 °C. Find efficiency of refrigerator X and Y?
a)
20,2
b)
7.9,6.5
c)
9.8,7.3
d)
8.9,7.3
|
|
Neha Joshi answered |
We know that the efficiency of refrigeration for a refrigerator is T2 / T1 + T2
Where T1 is source temperature and T2 is sink temperature
For refrigerator X we have T1 = 298K and T2 = 268K
Hence the efficiency of refrigeration = 268 / 298 - 268
= 268 / 30
= 8.93
For refrigerator Y we have T1 = 293K and T2 = 253K
Hence the efficiency of refrigeration = 253 / 293 - 253
= 253 / 40
= 6.35
Where T1 is source temperature and T2 is sink temperature
For refrigerator X we have T1 = 298K and T2 = 268K
Hence the efficiency of refrigeration = 268 / 298 - 268
= 268 / 30
= 8.93
For refrigerator Y we have T1 = 293K and T2 = 253K
Hence the efficiency of refrigeration = 253 / 293 - 253
= 253 / 40
= 6.35
The second law of thermodynamics says- a)Coefficient of performance can never be infinite for refrigerator
- b)Heat released to the cold reservoir can be zero
- c)Ideal gas can expand infinitely
- d)Efficiency of a heat engine can be 100%.
Correct answer is option 'A'. Can you explain this answer?
The second law of thermodynamics says
a)
Coefficient of performance can never be infinite for refrigerator
b)
Heat released to the cold reservoir can be zero
c)
Ideal gas can expand infinitely
d)
Efficiency of a heat engine can be 100%.
|
|
Raghav Bansal answered |
The second law of thermodynamics gives a fundamental limitation to the efficiency of a heat engine and the coefficient of performance of a refrigerator. It says that the efficiency of a heat engine can never be unity or 100%, this implies that the heat released to the cold reservoir can never be made zero.
For a refrigerator the second law says that the coefficient through performance can never be infinite, this implies that the external work can never be zero.
For a refrigerator the second law says that the coefficient through performance can never be infinite, this implies that the external work can never be zero.
Which word is defined by this statement: A measure of this disorder, or randomness?- a)energy
- b)enthalpy
- c)mass
- d)entropy
Correct answer is option 'D'. Can you explain this answer?
Which word is defined by this statement: A measure of this disorder, or randomness?
a)
energy
b)
enthalpy
c)
mass
d)
entropy
|
|
Janhavi Rane answered |
Understanding Entropy
Entropy is a fundamental concept in thermodynamics and statistical mechanics that quantifies the level of disorder or randomness in a system. Let's explore this in detail.
Definition of Entropy
- Measure of Disorder: Entropy can be understood as a measure of how spread out or dispersed the energy in a system is.
- Randomness: A higher entropy value indicates greater randomness and less predictability in the arrangement of particles in a system.
Importance of Entropy
- Second Law of Thermodynamics: This law states that in any energy transfer or transformation, the total entropy of a closed system will either increase or remain constant. This is a reflection of natural processes moving towards a state of maximum disorder.
- Spontaneous Processes: Entropy helps to explain why certain processes occur spontaneously. For example, ice melting in a warm room increases the entropy of the system, as the structured ice molecules become more disordered in liquid form.
Comparison with Other Options
- Energy: While energy is related to the ability to do work, it does not directly measure disorder.
- Enthalpy: This is a measure of total heat content in a system, not specifically disorder.
- Mass: Mass refers to the amount of matter in a system and does not provide information about the arrangement or randomness of that matter.
Conclusion
In summary, the correct answer is option 'D' – Entropy – because it specifically defines a measure of disorder or randomness within a system, making it essential for understanding the behavior of thermodynamic processes.
Entropy is a fundamental concept in thermodynamics and statistical mechanics that quantifies the level of disorder or randomness in a system. Let's explore this in detail.
Definition of Entropy
- Measure of Disorder: Entropy can be understood as a measure of how spread out or dispersed the energy in a system is.
- Randomness: A higher entropy value indicates greater randomness and less predictability in the arrangement of particles in a system.
Importance of Entropy
- Second Law of Thermodynamics: This law states that in any energy transfer or transformation, the total entropy of a closed system will either increase or remain constant. This is a reflection of natural processes moving towards a state of maximum disorder.
- Spontaneous Processes: Entropy helps to explain why certain processes occur spontaneously. For example, ice melting in a warm room increases the entropy of the system, as the structured ice molecules become more disordered in liquid form.
Comparison with Other Options
- Energy: While energy is related to the ability to do work, it does not directly measure disorder.
- Enthalpy: This is a measure of total heat content in a system, not specifically disorder.
- Mass: Mass refers to the amount of matter in a system and does not provide information about the arrangement or randomness of that matter.
Conclusion
In summary, the correct answer is option 'D' – Entropy – because it specifically defines a measure of disorder or randomness within a system, making it essential for understanding the behavior of thermodynamic processes.
For proper utilization of exergy, it is desirable to make first law efficiency ____ and the source and use temperatures should ____- a)as close to unity, be different
- b)as close to unity, match
- c)as close to zero, match
- d)as close to zero, be different
Correct answer is option 'B'. Can you explain this answer?
For proper utilization of exergy, it is desirable to make first law efficiency ____ and the source and use temperatures should ____
a)
as close to unity, be different
b)
as close to unity, match
c)
as close to zero, match
d)
as close to zero, be different
|
|
Raghav Bansal answered |
If first law efficiency is close to unity, the all the energy carried in by heat transfer is used and no heat is lost to the surroundings.
During an isothermal expansion, a confined ideal gas does –150 J of work against its surroundings. This implies that [2011]- a)150 J heat has been removed from the gas
- b)300 J of heat has been added to the gas
- c)no heat is transferred because the process is isothermal
- d)150 J of heat has been added to the gas
Correct answer is option 'D'. Can you explain this answer?
During an isothermal expansion, a confined ideal gas does –150 J of work against its surroundings. This implies that [2011]
a)
150 J heat has been removed from the gas
b)
300 J of heat has been added to the gas
c)
no heat is transferred because the process is isothermal
d)
150 J of heat has been added to the gas

|
Vaibhav Basu answered |
From the first law of thermodynamics
ΔU = Q + W
For isothermal process, ΔU = 0
therefore, Q = - W
Given W = - 150
Therefore, Q = + 150
When Q is positive, the heat is added to the gas.
Suppose we have a box filled with gas and a piston is also attached at the top of the box.What are the ways of changing the state of gas (and hence its internal energy)? Answer could be more than one choice.- a)Bring box in contact with a body with higher temperature
- b)Move the box so that it has kinetic energy
- c)Pushing the piston down so as to do work on the system
- d)both a and c
Correct answer is option 'D'. Can you explain this answer?
Suppose we have a box filled with gas and a piston is also attached at the top of the box.What are the ways of changing the state of gas (and hence its internal energy)? Answer could be more than one choice.
a)
Bring box in contact with a body with higher temperature
b)
Move the box so that it has kinetic energy
c)
Pushing the piston down so as to do work on the system
d)
both a and c
|
|
Shail Majumdar answered |
**Explanation:**
To understand why the correct answer is option 'D', let's analyze each option one by one:
**a) Bring box in contact with a body with higher temperature:**
When a box filled with gas is brought in contact with a body at a higher temperature, heat flows from the higher temperature body to the gas inside the box. This increases the temperature and hence the internal energy of the gas. Therefore, this option is valid for changing the state of the gas.
**b) Move the box so that it has kinetic energy:**
Moving the box so that it has kinetic energy does not directly change the state of the gas. It only changes the position and motion of the box. However, if the box is connected to the piston, and the piston is not fixed, the kinetic energy of the box can be transferred to the gas by pushing the piston down. This will do work on the system and change the state of the gas. Therefore, this option indirectly allows for changing the state of the gas.
**c) Pushing the piston down so as to do work on the system:**
Pushing the piston down compresses the gas inside the box, reducing its volume. This work is done on the system, and as a result, the internal energy of the gas increases. Therefore, this option is valid for changing the state of the gas.
**d) Both a and c:**
From the explanations above, it is clear that both options a and c allow for changing the state of the gas. Bringing the box in contact with a body at a higher temperature increases the internal energy of the gas, and pushing the piston down to do work on the system also increases the internal energy of the gas. Therefore, the correct answer is option 'D' - both a and c.
By using both options a and c, we can effectively change the state of the gas by increasing its internal energy through heat transfer and work done on the system.
To understand why the correct answer is option 'D', let's analyze each option one by one:
**a) Bring box in contact with a body with higher temperature:**
When a box filled with gas is brought in contact with a body at a higher temperature, heat flows from the higher temperature body to the gas inside the box. This increases the temperature and hence the internal energy of the gas. Therefore, this option is valid for changing the state of the gas.
**b) Move the box so that it has kinetic energy:**
Moving the box so that it has kinetic energy does not directly change the state of the gas. It only changes the position and motion of the box. However, if the box is connected to the piston, and the piston is not fixed, the kinetic energy of the box can be transferred to the gas by pushing the piston down. This will do work on the system and change the state of the gas. Therefore, this option indirectly allows for changing the state of the gas.
**c) Pushing the piston down so as to do work on the system:**
Pushing the piston down compresses the gas inside the box, reducing its volume. This work is done on the system, and as a result, the internal energy of the gas increases. Therefore, this option is valid for changing the state of the gas.
**d) Both a and c:**
From the explanations above, it is clear that both options a and c allow for changing the state of the gas. Bringing the box in contact with a body at a higher temperature increases the internal energy of the gas, and pushing the piston down to do work on the system also increases the internal energy of the gas. Therefore, the correct answer is option 'D' - both a and c.
By using both options a and c, we can effectively change the state of the gas by increasing its internal energy through heat transfer and work done on the system.
Refrigerator transfers heat from the cold cooling coils to warm surroundings, which law of thermodynamics favour this process- a)Zeroth law of thermodynamics
- b)Third law of thermodynamics
- c)First law of thermodynamics
- d)Second law of thermodynamics
Correct answer is option 'D'. Can you explain this answer?
Refrigerator transfers heat from the cold cooling coils to warm surroundings, which law of thermodynamics favour this process
a)
Zeroth law of thermodynamics
b)
Third law of thermodynamics
c)
First law of thermodynamics
d)
Second law of thermodynamics
|
|
Rajat Patel answered |
Refrigerator follows the principle of clausius statement of second law of thermodynamics. It does not violate second law of thermodynamics because it takes energy to transfer heat from low temperature body to high temperature body. Electrical work is given to refrigerator to extract heat from low temperature body and to transfer it to higher temperature body. If any refrigerator is transferring heat from low temperature body to higher temperature body without any external energy then we can say that it violates second law of thermodynamics.But in actual it takes energy to do.
Which of the following is an example of heat pump?- a)Internal combustion engine
- b)Blower heater
- c)Refrigerator
- d)Carnot engine
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of heat pump?
a)
Internal combustion engine
b)
Blower heater
c)
Refrigerator
d)
Carnot engine
|
|
Om Desai answered |
A heat pump is an electrical device that heats a building by pumping heat in from the cold outside. In other words, it’s the same as a refrigerator, but its purpose is to warm the hot reservoir rather than to cool the cold reservoir (even though it does both).
The internal energy change in a system that has absorbed 2 kcals of heat and done 500 J of work is: [2009]- a)6400 J
- b)5400 J
- c)7900 J
- d)8900 J
Correct answer is option 'C'. Can you explain this answer?
The internal energy change in a system that has absorbed 2 kcals of heat and done 500 J of work is: [2009]
a)
6400 J
b)
5400 J
c)
7900 J
d)
8900 J

|
Shivani Rane answered |
According to first law of thermodynamics Q = ΔU + W
ΔU = Q – W
= 2 × 4.2 × 1000 – 500 = 8400 –500
= 7900 J
ΔU = Q – W
= 2 × 4.2 × 1000 – 500 = 8400 –500
= 7900 J
Hot coffee in a thermos flask is shaken vigorously, considering it as a system which of the statement is not true?- a)Temperature of the system rises
- b)Internal energy of the coffee increased
- c)Heat energy has been added to coffee
- d)Work is done on the system
Correct answer is option 'C'. Can you explain this answer?
Hot coffee in a thermos flask is shaken vigorously, considering it as a system which of the statement is not true?
a)
Temperature of the system rises
b)
Internal energy of the coffee increased
c)
Heat energy has been added to coffee
d)
Work is done on the system
|
|
Preeti Iyer answered |
No, heat is not transferred as the flask is insulated from the surroundings ∴dQ=0
Internal energy of a system increases by 60 J when 140 Jof heat is added to the gaseous system. The amount of work done would be:- a)80 J
- b)100 J
- c)200 J
- d)140 J
Correct answer is option 'A'. Can you explain this answer?
Internal energy of a system increases by 60 J when 140 Jof heat is added to the gaseous system. The amount of work done would be:
a)
80 J
b)
100 J
c)
200 J
d)
140 J
|
|
Lavanya Menon answered |
We know that dq = dU + dW
And as dU = 60 and dq = 140J
We get dW = 140 - 60 = 80J
And as dU = 60 and dq = 140J
We get dW = 140 - 60 = 80J
An engine has an efficiency of 1/6. When the temperature of sink is reduced by 62°C, its efficiency is doubled. Temperature of the source is- a)37°C
- b)62°C [2007]
- c)99°C
- d)124°C
Correct answer is option 'C'. Can you explain this answer?
An engine has an efficiency of 1/6. When the temperature of sink is reduced by 62°C, its efficiency is doubled. Temperature of the source is
a)
37°C
b)
62°C [2007]
c)
99°C
d)
124°C
|
|
Jaya Singh answered |
°F, its efficiency becomes 1/4. Find the initial and final temperatures of the sink.
We can use the formula for the efficiency of a heat engine:
efficiency = 1 - (temperature of sink / temperature of source)
Let's call the initial temperature of the source Ts and the initial temperature of the sink T0. We can set up two equations based on the given information:
1/6 = 1 - (T0 / Ts)
1/4 = 1 - ((T0 - 62) / Ts)
We can solve this system of equations for Ts and T0. One way to do this is to solve for one variable in terms of the other, and then substitute into one of the equations to solve for the remaining variable. Here's one possible method:
1/6 = 1 - (T0 / Ts)
T0 / Ts = 5/6
T0 = (5/6)Ts
1/4 = 1 - ((T0 - 62) / Ts)
(T0 - 62) / Ts = 3/4
T0 - 62 = (3/4)Ts
Substituting T0 = (5/6)Ts into the second equation:
(5/6)Ts - 62 = (3/4)Ts
(1/12)Ts = 62
Ts = 744
Substituting Ts = 744 into T0 = (5/6)Ts:
T0 = (5/6)(744) = 620
Therefore, the initial temperature of the source was 744°F and the initial temperature of the sink was 620°F. When the temperature of the sink was reduced by 62°F, its final temperature was 558°F.
We can use the formula for the efficiency of a heat engine:
efficiency = 1 - (temperature of sink / temperature of source)
Let's call the initial temperature of the source Ts and the initial temperature of the sink T0. We can set up two equations based on the given information:
1/6 = 1 - (T0 / Ts)
1/4 = 1 - ((T0 - 62) / Ts)
We can solve this system of equations for Ts and T0. One way to do this is to solve for one variable in terms of the other, and then substitute into one of the equations to solve for the remaining variable. Here's one possible method:
1/6 = 1 - (T0 / Ts)
T0 / Ts = 5/6
T0 = (5/6)Ts
1/4 = 1 - ((T0 - 62) / Ts)
(T0 - 62) / Ts = 3/4
T0 - 62 = (3/4)Ts
Substituting T0 = (5/6)Ts into the second equation:
(5/6)Ts - 62 = (3/4)Ts
(1/12)Ts = 62
Ts = 744
Substituting Ts = 744 into T0 = (5/6)Ts:
T0 = (5/6)(744) = 620
Therefore, the initial temperature of the source was 744°F and the initial temperature of the sink was 620°F. When the temperature of the sink was reduced by 62°F, its final temperature was 558°F.
A sample of gas expands from volume V1 to V2.The amount of work done by the gas is greatest, when the expansion is [1997]- a)adiabatic
- b)isobaric
- c)isothermal
- d)equal in all cases
Correct answer is option 'B'. Can you explain this answer?
A sample of gas expands from volume V1 to V2.The amount of work done by the gas is greatest, when the expansion is [1997]
a)
adiabatic
b)
isobaric
c)
isothermal
d)
equal in all cases

|
Shanaya Rane answered |
In thermodynamics for same change in volume, the work done is maximum in isobaric process because in P – V graph, area enclosed by curve and volume axis is maximum in isobaric process.
So, the choice (b) is correct.
So, the choice (b) is correct.
The temperature of source and sink of a heat engine are 127ºC and 27ºC respectively. An inventor claims its efficiency to be 26%, then:- a)it is impossible [2001]
- b)it is possible with high probability
- c)it is possible with low probability
- d)data are insufficient.
Correct answer is option 'A'. Can you explain this answer?
The temperature of source and sink of a heat engine are 127ºC and 27ºC respectively. An inventor claims its efficiency to be 26%, then:
a)
it is impossible [2001]
b)
it is possible with high probability
c)
it is possible with low probability
d)
data are insufficient.

|
Arnab Iyer answered |
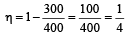
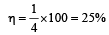
Hence, it is not possible to have efficiency more than 25%.
First law of thermodynamics is consequence of conservation of [1988]- a)work
- b)energy
- c)heat
- d)all of these
Correct answer is option 'B'. Can you explain this answer?
First law of thermodynamics is consequence of conservation of [1988]
a)
work
b)
energy
c)
heat
d)
all of these

|
Krish Patel answered |
The first law of thermodynamics is just a conservation of energy.
A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of source be increased so as to increase, its efficiency by 50% of original efficiency ? [2006]- a)325 K
- b)250 K
- c)380 K
- d)275 K
Correct answer is option 'B'. Can you explain this answer?
A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of source be increased so as to increase, its efficiency by 50% of original efficiency ? [2006]
a)
325 K
b)
250 K
c)
380 K
d)
275 K
|
|
Tejas Kumar answered |
We know that efficiency of Carnot Engine
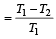
where, T1 is temp. of source & T2 is temp. of sink
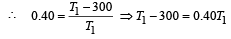
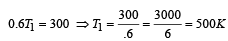
Now efficiency to be increased by 50%
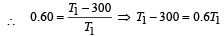
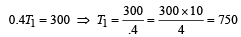
Increase in temp = 750 – 500 = 250 K
A body of mass 2kg is dragged on a horizontal surface with a constant speed of 2 m/s. If the coefficient of friction between the body and the surface is 0.2, then find the heat generated in 5 sec.- a)18.66 cal
- b)10 cal
- c)8.71 cal
- d)9.33 cal
Correct answer is option 'D'. Can you explain this answer?
A body of mass 2kg is dragged on a horizontal surface with a constant speed of 2 m/s. If the coefficient of friction between the body and the surface is 0.2, then find the heat generated in 5 sec.
a)
18.66 cal
b)
10 cal
c)
8.71 cal
d)
9.33 cal
|
|
Pallavi Pillai answered |
Understanding the Problem
A body with a mass of 2 kg is being dragged on a horizontal surface at a constant speed of 2 m/s. The coefficient of friction between the body and the surface is 0.2. We need to find the heat generated due to friction over a period of 5 seconds.
Calculating the Force of Friction
- The formula for the force of friction (F_friction) is given by:
- F_friction = coefficient of friction * normal force
- For a horizontal surface, the normal force (N) equals the weight of the body:
- N = mass * g (where g is approximately 9.81 m/s²)
- Therefore:
- N = 2 kg * 9.81 m/s² = 19.62 N
- F_friction = 0.2 * 19.62 N = 3.924 N
Calculating Work Done Against Friction
- Work done (W) against friction is given by:
- W = F_friction * distance
- The distance traveled in 5 seconds at 2 m/s:
- Distance = speed * time = 2 m/s * 5 s = 10 m
- Thus, the work done:
- W = 3.924 N * 10 m = 39.24 J
Converting Work to Heat
- The heat generated (Q) is equal to the work done against friction.
- To convert joules to calories, use the conversion factor:
- 1 cal = 4.184 J
- Converting the work done to calories:
- Q = 39.24 J / 4.184 J/cal ≈ 9.38 cal
Final Answer
- Rounding off gives approximately 9.33 cal, which corresponds to option D.
In conclusion, the heat generated due to friction in this scenario is approximately 9.33 calories.
A body with a mass of 2 kg is being dragged on a horizontal surface at a constant speed of 2 m/s. The coefficient of friction between the body and the surface is 0.2. We need to find the heat generated due to friction over a period of 5 seconds.
Calculating the Force of Friction
- The formula for the force of friction (F_friction) is given by:
- F_friction = coefficient of friction * normal force
- For a horizontal surface, the normal force (N) equals the weight of the body:
- N = mass * g (where g is approximately 9.81 m/s²)
- Therefore:
- N = 2 kg * 9.81 m/s² = 19.62 N
- F_friction = 0.2 * 19.62 N = 3.924 N
Calculating Work Done Against Friction
- Work done (W) against friction is given by:
- W = F_friction * distance
- The distance traveled in 5 seconds at 2 m/s:
- Distance = speed * time = 2 m/s * 5 s = 10 m
- Thus, the work done:
- W = 3.924 N * 10 m = 39.24 J
Converting Work to Heat
- The heat generated (Q) is equal to the work done against friction.
- To convert joules to calories, use the conversion factor:
- 1 cal = 4.184 J
- Converting the work done to calories:
- Q = 39.24 J / 4.184 J/cal ≈ 9.38 cal
Final Answer
- Rounding off gives approximately 9.33 cal, which corresponds to option D.
In conclusion, the heat generated due to friction in this scenario is approximately 9.33 calories.
Kelvin- Planck statement states that- a)The process whose sole result is transfer of heat from a colder object to a hotter object is not possible
- b)Irreversible processes can be made reversible under certain conditions
- c)No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work
- d)Heat flows from colder body to hotter body
Correct answer is option 'C'. Can you explain this answer?
Kelvin- Planck statement states that
a)
The process whose sole result is transfer of heat from a colder object to a hotter object is not possible
b)
Irreversible processes can be made reversible under certain conditions
c)
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work
d)
Heat flows from colder body to hotter body
|
|
Jyoti Kumar answered |
The Kelvin-Planck statement is a fundamental principle of thermodynamics that is applicable to all heat engines. It states that:
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work.
This statement implies that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. In other words, it is impossible to have a 100% efficient heat engine.
Explanation:
To understand the Kelvin-Planck statement, we need to have a basic understanding of heat engines. A heat engine is a device that converts heat into work. It operates on the principle of the Carnot cycle, which involves four processes: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression. The efficiency of a heat engine is defined as the ratio of the work output to the heat input. According to the second law of thermodynamics, the efficiency of a heat engine cannot exceed the efficiency of a reversible heat engine operating between the same two reservoirs.
The Kelvin-Planck statement is based on the fact that any heat engine must reject some heat to a low-temperature reservoir in order to operate. This means that not all of the heat energy can be converted into useful work. The statement implies that there must always be some waste heat that cannot be utilized to produce work. This is because all natural processes tend to move towards a state of maximum entropy, and the conversion of heat into work is a process that results in a decrease in entropy. Therefore, it is impossible to have a heat engine that can convert all of the heat energy it absorbs into useful work.
Conclusion:
In conclusion, the Kelvin-Planck statement is a fundamental principle of thermodynamics that states that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. This statement is based on the second law of thermodynamics, which states that all natural processes tend to move towards a state of maximum entropy. The Kelvin-Planck statement has important implications for the design and operation of heat engines, and it sets a fundamental limit on the efficiency of such devices.
No process is possible whose sole result is absorption of heat from a reservoir and all the heat is converted to work.
This statement implies that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. In other words, it is impossible to have a 100% efficient heat engine.
Explanation:
To understand the Kelvin-Planck statement, we need to have a basic understanding of heat engines. A heat engine is a device that converts heat into work. It operates on the principle of the Carnot cycle, which involves four processes: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression. The efficiency of a heat engine is defined as the ratio of the work output to the heat input. According to the second law of thermodynamics, the efficiency of a heat engine cannot exceed the efficiency of a reversible heat engine operating between the same two reservoirs.
The Kelvin-Planck statement is based on the fact that any heat engine must reject some heat to a low-temperature reservoir in order to operate. This means that not all of the heat energy can be converted into useful work. The statement implies that there must always be some waste heat that cannot be utilized to produce work. This is because all natural processes tend to move towards a state of maximum entropy, and the conversion of heat into work is a process that results in a decrease in entropy. Therefore, it is impossible to have a heat engine that can convert all of the heat energy it absorbs into useful work.
Conclusion:
In conclusion, the Kelvin-Planck statement is a fundamental principle of thermodynamics that states that it is impossible to construct a heat engine that can extract heat from a single thermal reservoir and convert it completely into work. This statement is based on the second law of thermodynamics, which states that all natural processes tend to move towards a state of maximum entropy. The Kelvin-Planck statement has important implications for the design and operation of heat engines, and it sets a fundamental limit on the efficiency of such devices.
A reversible engine converts one-sixth of the heat input into work. When the temperature of the sink is reduced by 62ºC, the efficiency of the engine is doubled. The temperatures of the source and sink are [2000]- a)99ºC, 37ºC
- b)80ºC, 37ºC
- c)95ºC, 37ºC
- d)90ºC, 37ºC
Correct answer is option 'A'. Can you explain this answer?
A reversible engine converts one-sixth of the heat input into work. When the temperature of the sink is reduced by 62ºC, the efficiency of the engine is doubled. The temperatures of the source and sink are [2000]
a)
99ºC, 37ºC
b)
80ºC, 37ºC
c)
95ºC, 37ºC
d)
90ºC, 37ºC
|
|
Aarav Shah answered |
Initially the efficiency of the engine was 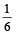 which increases to
which increases to 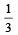 when the sinktemperature reduces by 62º C.
when the sinktemperature reduces by 62º C.
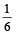 which increases to
which increases to 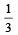 when the sinktemperature reduces by 62º C.
when the sinktemperature reduces by 62º C. when T2 = sink temperature T1 = source temperature
when T2 = sink temperature T1 = source temperature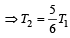
Secondly,
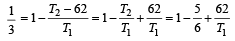
or, T1 = 62 × 6 = 372K =372– 273 = 99ºC
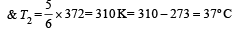
Two Carnot engines A and B are operated in series. The engine A receives heat from the source at temperature T1 and rejects the heat to the sink at temperature T. The second engine B receives the heat at temperature T and rejects to its sink at temperature T2. For what value of T the efficiencies of the two engines are equal? [NEET Kar. 2013]- a)
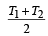
- b)
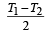
- c)T1T2
- d)
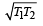
Correct answer is option 'D'. Can you explain this answer?
Two Carnot engines A and B are operated in series. The engine A receives heat from the source at temperature T1 and rejects the heat to the sink at temperature T. The second engine B receives the heat at temperature T and rejects to its sink at temperature T2. For what value of T the efficiencies of the two engines are equal? [NEET Kar. 2013]
a)
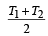
b)
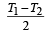
c)
T1T2
d)
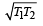

|
Abhijeet Goyal answered |
Efficiency of engine A, 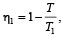
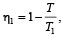
Efficiency of engine B, 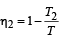
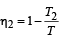
Here, η1 = η2
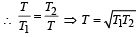
If for a gas, 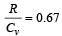 , the gas is made up of molecules which are [1992]
, the gas is made up of molecules which are [1992]- a)diatomic
- b)mixture of diatomic and polyatomic molecules
- c)monoatomic
- d)polyatomic
Correct answer is option 'C'. Can you explain this answer?
If for a gas, 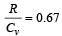 , the gas is made up of molecules which are [1992]
, the gas is made up of molecules which are [1992]
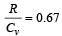 , the gas is made up of molecules which are [1992]
, the gas is made up of molecules which are [1992]a)
diatomic
b)
mixture of diatomic and polyatomic molecules
c)
monoatomic
d)
polyatomic
|
|
Yash Modi answered |
Given, Cv / R = 3/2. ( 0.67 = 2/3)
So, Cv = 3R/2 , hence the gas is monoatomic.
So, Cv = 3R/2 , hence the gas is monoatomic.
In actual home refrigerator vapours of Freon ( which is dichlorodifluoro methane CCl2F2) act as- a)Sink
- b)working substance
- c)source
- d)Insulating pad
Correct answer is option 'B'. Can you explain this answer?
In actual home refrigerator vapours of Freon ( which is dichlorodifluoro methane CCl2F2) act as
a)
Sink
b)
working substance
c)
source
d)
Insulating pad
|
|
Vijay Bansal answered |
Dichlorodifluoromethane (R-12) is a colorless gas usually sold under the brand name Freon-12, and a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in developed countries (non-article 5 countries) in 1996, and developing countries (article 5 countries) in 2010 due to concerns about its damaging impact to the ozone layer.[better source needed] Its only allowed usage is as fire retardant in submarines and aircraft. It is soluble in many organic solvents. Dichlorodifluoromethane was one of the original propellants for Silly String. R-12 cylinders are colored white.
The first law of thermodynamics1. Is a restatement of the principle of conservation of energy as applied to heat energy
2. Is the basis for the definition of internal energy
3. Is basis for the definition of temperature
4. asserts the impossibility of achieving an absolute zero temperature.- a)1 and 2
- b)only 1
- c)1 and 3
- d)1,2 and 4
Correct answer is option 'A'. Can you explain this answer?
The first law of thermodynamics
1. Is a restatement of the principle of conservation of energy as applied to heat energy
2. Is the basis for the definition of internal energy
3. Is basis for the definition of temperature
4. asserts the impossibility of achieving an absolute zero temperature.
2. Is the basis for the definition of internal energy
3. Is basis for the definition of temperature
4. asserts the impossibility of achieving an absolute zero temperature.
a)
1 and 2
b)
only 1
c)
1 and 3
d)
1,2 and 4
|
|
Stuti Joshi answered |
The change is internal energy if the system is equal to the difference between the heat added to the system and work done by the system.


The molar specific heat at constant pressure of an ideal gas is (7/2) R. The ratio of specific heat at constant pressure to that at constant volume is- a)8/7
- b)5/7
- c)9/7
- d)7/5 [2006]
Correct answer is option 'D'. Can you explain this answer?
The molar specific heat at constant pressure of an ideal gas is (7/2) R. The ratio of specific heat at constant pressure to that at constant volume is
a)
8/7
b)
5/7
c)
9/7
d)
7/5 [2006]
|
|
Yash Modi answered |
Cp = 7R/2 so Cv = 5R/2 (Cp-Cv = R)
So Cp/Cv = 7/5
So Cp/Cv = 7/5
Chapter doubts & questions for Thermodynamics - Topic-wise MCQ Tests for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermodynamics - Topic-wise MCQ Tests for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily