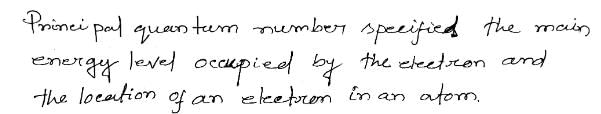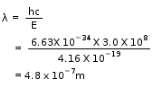All Exams >
NEET >
Physical Chemistry for NEET >
All Questions
All questions of Structure of Atom for NEET Exam
Direction (Q. Nos. 12 and 13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)A hypothetical electromagnetic wave is pictured here.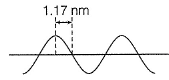 Q. Energy associated with this wave is
Q. Energy associated with this wave is- a)4.24 x 10-19J
- b)2.12 x 10-19J
- c)1.06 x 10-19J
- d)8.49 x 10-19J
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 12 and 13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
A hypothetical electromagnetic wave is pictured here.
Q. Energy associated with this wave is
a)
4.24 x 10-19J
b)
2.12 x 10-19J
c)
1.06 x 10-19J
d)
8.49 x 10-19J
|
|
Hansa Sharma answered |
A to E makes one complete wave.
The number of radial nodes for 3p orbital is __________.- a)3
- b)4
- c)2
- d)1
Correct answer is 'D'. Can you explain this answer?
The number of radial nodes for 3p orbital is __________.
a)
3
b)
4
c)
2
d)
1

|
Amrita Kumar answered |
Number of radial nodes = n-1 – 1
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
A proton accelerated from rest through a potential difference of 'V' volts has a wavelength λ associated with it. An alpha particle in order to have the same wavelength must be accelerated from rest through a potential difference of - a)V volt
- b)4V volt
- c)2V volt
- d)V/8 volt
Correct answer is option 'D'. Can you explain this answer?
A proton accelerated from rest through a potential difference of 'V' volts has a wavelength λ associated with it. An alpha particle in order to have the same wavelength must be accelerated from rest through a potential difference of
a)
V volt
b)
4V volt
c)
2V volt
d)
V/8 volt
|
|
Sonal Dey answered |
Values of e/m (charge/mass) in the categories alpha particle (α), electron (e) and protons (p) increase in the order:
- a)α < e < p
- b)p < e < α
- c)e < α < p
- d)α< p < e
Correct answer is option 'D'. Can you explain this answer?
Values of e/m (charge/mass) in the categories alpha particle (α), electron (e) and protons (p) increase in the order:
a)
α < e < p
b)
p < e < α
c)
e < α < p
d)
α< p < e
|
|
Hansa Sharma answered |
E/m values of particle (α), electron (e) and protons (p) increase in the order α< p < e
The spectrum of He is expected to be similar to that[1988]- a)H
- b)Li+
- c)Na
- d)He+
Correct answer is option 'B'. Can you explain this answer?
The spectrum of He is expected to be similar to that[1988]
a)
H
b)
Li+
c)
Na
d)
He+

|
Arindam Khanna answered |
Both He and Li+ contain 2 electrons each therefore their spectrum will be similar.
Isobars are the atoms with- a)same atomic number but different number of neutrons
- b)same mass number but different atomic number
- c)same atomic number but different mass number
- d)same number of neutrons but different mass number
Correct answer is option 'B'. Can you explain this answer?
Isobars are the atoms with
a)
same atomic number but different number of neutrons
b)
same mass number but different atomic number
c)
same atomic number but different mass number
d)
same number of neutrons but different mass number
|
|
Nandini Patel answered |
Isobars are atoms (nuclides) of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number (or number of protons) but have the same mass number.
The nature of positive rays depends on?
- a)The nature of discharge tube.
- b)The nature of residual gas.
- c)The nature of electrode.
- d)All of above
Correct answer is option 'B'. Can you explain this answer?
The nature of positive rays depends on?
a)
The nature of discharge tube.
b)
The nature of residual gas.
c)
The nature of electrode.
d)
All of above
|
|
Om Desai answered |
- The nature of positive rays produced in a vacuum discharge tube depends upon the nature of the gas-filled.
- The positive rays consist of positive ions obtained by removing one or more electrons from gas molecules.
How much energy is needed to ionize a hydrogen atom if electron is present in n=1 orbit?- a)13.6 eV
- b)10.2 eV
- c)3.4eV
- d)1 eV
Correct answer is option 'A'. Can you explain this answer?
How much energy is needed to ionize a hydrogen atom if electron is present in n=1 orbit?
a)
13.6 eV
b)
10.2 eV
c)
3.4eV
d)
1 eV
|
|
Pooja Shah answered |
For hydrogen Z=1,
and n is given as 1,
Then
E= -13.6 × n power 2/ Z.
E= - 13.6 × 1 power 2/ 1.
E= -13.6e.v.
and n is given as 1,
Then
E= -13.6 × n power 2/ Z.
E= - 13.6 × 1 power 2/ 1.
E= -13.6e.v.
The energy of a photon of light has what kind of proportionality to its frequency and its wavelength.- a)directly, inversely
- b)inversely, directly
- c)inversely, inversely
- d)directly, directly
Correct answer is option 'A'. Can you explain this answer?
The energy of a photon of light has what kind of proportionality to its frequency and its wavelength.
a)
directly, inversely
b)
inversely, directly
c)
inversely, inversely
d)
directly, directly
|
|
Pooja Shah answered |
As E=h(frequency) E is directly proportional to frequency and as frequency = speed of light / wavelength frequency is inversely proportional to wavelength.
Most penetrating radiation of radioactive element is:- a) β — rays
- b) α — rays
- c) x — rays
- d) γ — rays
Correct answer is option 'D'. Can you explain this answer?
Most penetrating radiation of radioactive element is:
a)
β — rays
b)
α — rays
c)
x — rays
d)
γ — rays
|
|
Anjana Sharma answered |
Alpha rays as the least penetrating, followed by beta rays, followed by gamma rays as the most penetrating.
Calculate the wavelength of light that corresponds to the radiation that is given off during the transition of an electron from the n = 5 to n = 2 state of the hydrogen atom.- a)434 nm
- b)275 nm
- c)305 nm
- d)183 nm
Correct answer is option 'A'. Can you explain this answer?
Calculate the wavelength of light that corresponds to the radiation that is given off during the transition of an electron from the n = 5 to n = 2 state of the hydrogen atom.
a)
434 nm
b)
275 nm
c)
305 nm
d)
183 nm
|
|
Hansa Sharma answered |
1/wavelength =RH x z2 x (1/22-1/52) =109677 x 1 x (1/4-1/25) =109677 x 21/100 =2303.2m wavelength=1/2303.2m =1/2303.2 x 107nm =434.1nm~434nm
Increasing order of magnetic moment among the following species is __________ .Na+, Fe+3, Co2+, Cr+2- a)Na+<Fe+3<Co2+ <Cr+2
- b)Na+<Co2+<Cr2+ <Fe+3
- c)Na+<Cr2+<Co2+ <Fe+3
- d)Na+<Fe3+<Cr2+ <Co+2
Correct answer is option 'B'. Can you explain this answer?
Increasing order of magnetic moment among the following species is __________ .
Na+, Fe+3, Co2+, Cr+2
a)
Na+<Fe+3<Co2+ <Cr+2
b)
Na+<Co2+<Cr2+ <Fe+3
c)
Na+<Cr2+<Co2+ <Fe+3
d)
Na+<Fe3+<Cr2+ <Co+2
|
|
Nitin Khanna answered |
Na+, Co+2, Cr2+, Fe+3
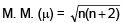
we get Na+, Co+2, Cr2+, Fe+3
we get Na+, Co+2, Cr2+, Fe+3
The de Broglie wavelength of a particle is given by.- a)h/mv
- b)h + mv
- c)hmv
- d)mv/c
Correct answer is option 'A'. Can you explain this answer?
The de Broglie wavelength of a particle is given by.
a)
h/mv
b)
h + mv
c)
hmv
d)
mv/c

|
Mrunal Korde answered |
The formula is h/p = wavelength. where p = mv
The de – Broglie wavelength of an electron is 600 nm. The velocity of the electron having the mass 9.1 X 10-31 Kg is- a)0.0012 x 10+4 m/s
- b)0.0012 x 10+3 m/s
- c)0.0012 x 10+6 m/s
- d)0.0012 x 10+2 m/s
Correct answer is option 'C'. Can you explain this answer?
The de – Broglie wavelength of an electron is 600 nm. The velocity of the electron having the mass 9.1 X 10-31 Kg is
a)
0.0012 x 10+4 m/s
b)
0.0012 x 10+3 m/s
c)
0.0012 x 10+6 m/s
d)
0.0012 x 10+2 m/s
|
|
Arjun Gupta answered |
1nm= 10^-9m.
wavelength= 600nm= 600 ×10-9m
wavelength= h/p.
wavelength= h/mv
v= h/ m wavelength
v=6.625×10^-34/9.1×10^-31×600× 10-9.
v= 6.625 × 10+4/9.1 ×6.
v= 6.625× 10+4/9.1×6.
v= 6.625 ×104/54.6.
v= 0.12 × 104.
v= 0.0012 × 10+6 .
wavelength= 600nm= 600 ×10-9m
wavelength= h/p.
wavelength= h/mv
v= h/ m wavelength
v=6.625×10^-34/9.1×10^-31×600× 10-9.
v= 6.625 × 10+4/9.1 ×6.
v= 6.625× 10+4/9.1×6.
v= 6.625 ×104/54.6.
v= 0.12 × 104.
v= 0.0012 × 10+6 .
The total energy of an electron in the nth orbit of a hydrogen atom is given by the formula
En = -13.6 eV/n2. What does the negative energy for an electron indicate?- a)Energy of electron in an atom is lower than energy of an electron far away from nucleus
- b)The electron has a negative charge.
- c)The electron is far away from nucleus
- d)Electrons have both wave and particle-like properties.
Correct answer is option 'A'. Can you explain this answer?
The total energy of an electron in the nth orbit of a hydrogen atom is given by the formula
En = -13.6 eV/n2. What does the negative energy for an electron indicate?
En = -13.6 eV/n2. What does the negative energy for an electron indicate?
a)
Energy of electron in an atom is lower than energy of an electron far away from nucleus
b)
The electron has a negative charge.
c)
The electron is far away from nucleus
d)
Electrons have both wave and particle-like properties.
|
|
Gaurav Kumar answered |
The negative sign means that the energy of the electron in the atom is lower than the energy of a free electron at rest. A free electron at rest is an electron that is infinitely far away from the nucleus and is assigned the energy value zero.
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?- a)Rutherford Model
- b)Bohr’s Model
- c)J.J Thomson Model
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?
a)
Rutherford Model
b)
Bohr’s Model
c)
J.J Thomson Model
d)
None of the above
|
|
Suresh Reddy answered |
Bohr Model of atom:
- An atom is made up of three particles: Electrons, neutrons and protons.
- The protons and neutrons are located in a small nucleus at the centre of the atom.
- The electrons revolve rapidly around the nucleus at the centre of the atom.
- There is a limit to the number of electrons that each energy level can hold.
- Each energy level is associated with a fixed amount of energy.
- There is no change in the energy of electrons as long as they keep revolving in the same energy level.
Bohr explained the stability through the concept of revolution of electrons in different energy levels.
The change in the energy of an electron occurs when it jumps from lower to higher energy levels. When it gains energy, it excites from lower to higher and vice versa.
Thus energy is not lost and the atom remains stable.
The change in the energy of an electron occurs when it jumps from lower to higher energy levels. When it gains energy, it excites from lower to higher and vice versa.
Thus energy is not lost and the atom remains stable.
The energy associated with the first orbit in the hydrogen atom is -2.18 x 10−18 J/atom. What is the energy associated with the fifth orbit?
a) -7.72 ×10−20 J/atom
b) -5.72 ×10−20 J/atom
c) -3.72 ×10−20 J/atom
d) -8.72 ×10−20 J/atom
Correct answer is 'D'. Can you explain this answer?
|
|
Geetika Shah answered |
Energy for any n shell = -2.18 x 10−18 )/n'2 (') stands for raise to the power of therefore, E associated with 5 orbit = -2.18 x 10−18/52 = -2.18 × 10-18/25 = -218 × 10-20/25 = -8.72 x 10-20J/atom
Chapter doubts & questions for Structure of Atom - Physical Chemistry for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Structure of Atom - Physical Chemistry for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Physical Chemistry for NEET
117 videos|226 docs|237 tests
|