All Exams >
NEET >
NCERT Based Tests for NEET >
All Questions
All questions of Alcohols, Phenols and Ethers for NEET Exam
Identify the alcohol or phenol having a stronger acidic nature from the following.- a)CH3CH2OH
- b)C6H5OH
- c)CH3CHOHCH2CH3
- d)CH3CH2CH2CH2OH
Correct answer is option 'B'. Can you explain this answer?
Identify the alcohol or phenol having a stronger acidic nature from the following.
a)
CH3CH2OH
b)
C6H5OH
c)
CH3CHOHCH2CH3
d)
CH3CH2CH2CH2OH

|
Mohit Rajpoot answered |
- Phenol (C₆H₅OH) is more acidic than typical alcohols.
- This is due to the resonance stabilization of the phenoxide ion formed when phenol loses a hydrogen ion (H⁺).
- The negative charge on the oxygen atom in phenoxide ion is delocalized over the aromatic ring, increasing stability and acidity.
- In contrast, alcohols like ethanol (CH₃CH₂OH) do not have such resonance stabilization, making them less acidic.
- Therefore, phenol is the strongest acid among the given options.
- This is due to the resonance stabilization of the phenoxide ion formed when phenol loses a hydrogen ion (H⁺).
- The negative charge on the oxygen atom in phenoxide ion is delocalized over the aromatic ring, increasing stability and acidity.
- In contrast, alcohols like ethanol (CH₃CH₂OH) do not have such resonance stabilization, making them less acidic.
- Therefore, phenol is the strongest acid among the given options.
Which of the following statement is correct?- a)The -OH group in phenol is meta directing.
- b)The electron releasing groups increase the acidic character of phenols.
- c)Phenols are less acidic than aromatic alcohols.
- d)Boiling point of phenol is higher than that of toluene of comparable molecular mass.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is correct?
a)
The -OH group in phenol is meta directing.
b)
The electron releasing groups increase the acidic character of phenols.
c)
Phenols are less acidic than aromatic alcohols.
d)
Boiling point of phenol is higher than that of toluene of comparable molecular mass.

|
Divey Sethi answered |
The correct answer is Option D.
Phenols have higher boiling point than toluene due to the presence of intermolecular hydrogen bonding in phenols. The formation of hydrogen bonds increases the intermolecular force of attraction between the phenol molecules and thereby increases its boiling point.
Can you explain the answer of this question below:The acidity of phenols is due to
- A:
Oxidation process
- B:
Resonance stabilization of its ions.
- C:
Hybridisation
- D:
Presence of O-H group
The answer is b.
The acidity of phenols is due to
Oxidation process
Resonance stabilization of its ions.
Hybridisation
Presence of O-H group
|
|
Rahul Bansal answered |
The acidity of phenols is due to its ability to lose hydrogen ion to form phenoxide ions. In a phenol molecule, the sp2hybridised carbon atom of benzene ring attached directly to the hydroxyl group acts as an electron withdrawing group. This sp2 hybridized carbon atom of benzene ring attached directly to the hydroxyl group has higher electronegativity in comparison to hydroxyl group. Due to the higher electronegativity of this carbon atom in comparison to the hydroxyl group attached, electron density decreases on oxygen atom. The decrease in electron density increases the polarity of O-H bond and results in the increase in ionization of phenols. Thus, the phenoxide ion is formed. The phenoxide ion formed is stabilized by the delocalization of negative charge due to the resonance in benzene ring. Phenoxide ion has greater stability than phenols, as in case of phenol charge separation takes place during resonance.The resonance structures of phenoxide ions explain the delocalization of negative charge. In case of substituted phenols, acidity of phenols increases in the presence of electron withdrawing group. This is due to the stability of the phenoxide ion generated. The acidity of phenols further increases if these groups are attached at ortho and para positions. This is due to the fact that the negative charge in phenoxide ion is mainly delocalized at ortho and para positions of the attached benzene ring. On the other hand, the acidity of phenols decreases in presence of electron donating groups as they prohibit the formation of phenoxide ion.
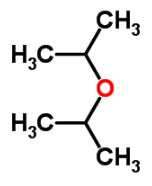
What is the IUPAC name of di-isopropyl ethera)Salicyaldehydeb)Ethyl acetatec)Iso propoxy propaned)Ethyl methyl etherCorrect answer is option 'C'. Can you explain this answer?
|
|
Shreya Gupta answered |
2-Iso propoxy propane
- Molecular Formula: C6H14O
- Average mass: 102.174797 Da
- Monoisotopic mass: 102.104462 Da

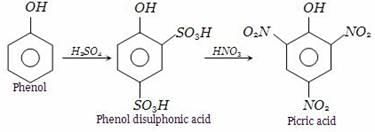
We can obtain picric acid from phenol by:- a)Sulphonation of phenol
- b)By Reimer Tiemann reaction
- c)Nitration of phenol
- d)Halogenation of phenol
Correct answer is option 'C'. Can you explain this answer?
We can obtain picric acid from phenol by:
a)
Sulphonation of phenol
b)
By Reimer Tiemann reaction
c)
Nitration of phenol
d)
Halogenation of phenol
|
|
Shreya Gupta answered |
Phenol heated with sulphuric acid gives phenol disulphonic acid, which further on reaction with nitric acid forms picric acid (2,4,6-trinitrophenol).
IUPAC name of m-cresol is ___________.- a)3-methylphenol
- b)3-chlorophenol
- c) 3-methoxyphenol
- d)benzene-1,3-diol
Correct answer is option 'A'. Can you explain this answer?
IUPAC name of m-cresol is ___________.
a)
3-methylphenol
b)
3-chlorophenol
c)
3-methoxyphenol
d)
benzene-1,3-diol
|
|
Preeti Iyer answered |
Meta-Cresol, also 3-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless, viscous liquid that is used as an intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and o-cresol.
One or More than One Options Correct TypeDirection (Q. Nos. 9-14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correctConsider the following reaction,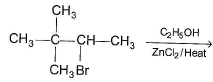 Q. The expected substitution product(s) is/are
Q. The expected substitution product(s) is/are- a)
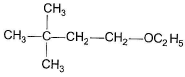
- b)
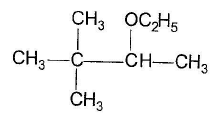
- c)
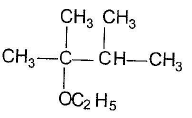
- d)
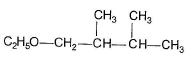
Correct answer is option 'B,C'. Can you explain this answer?
One or More than One Options Correct Type
Direction (Q. Nos. 9-14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct
Consider the following reaction,
Q.
The expected substitution product(s) is/are
a)
b)

c)

d)
|
|
Om Desai answered |
Reaction proceeds by SN1 mechanism.
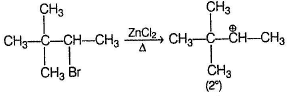
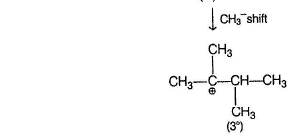
Above carbocations give the desired products.
Above carbocations give the desired products.
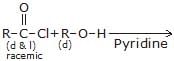 Product of above reaction is -
Product of above reaction is -- a)Enantiomer
- b)Racemic
- c)Diastereomers
- d)Meso
Correct answer is option 'C'. Can you explain this answer?
Product of above reaction is -
a)
Enantiomer
b)
Racemic
c)
Diastereomers
d)
Meso
|
|
Krishna Iyer answered |

HCl is formed in above reaction and react with pyridine and gives
Which catalyst is used in Fischer-Speier esterification?
- a)Concentrated H2SO4
- b)Dry HCl gas
- c)Concentrated HNO3
- d)Pyridine
Correct answer is option 'A'. Can you explain this answer?
Which catalyst is used in Fischer-Speier esterification?
a)
Concentrated H2SO4
b)
Dry HCl gas
c)
Concentrated HNO3
d)
Pyridine
|
|
Pooja Mehta answered |
Esterification is a relatively slow process at room temperature and does not proceed to completion. Concentrated sulfuric acid is used as a catalyst, and has a dual role: Speeds up the reaction. Acts as a dehydrating agent, forcing the equilibrium to the right and resulting in a greater yield of ester.
Williamsons synthesis is an example of :- a)Electrophilic substitution
- b)Electrophilic addition
- c)Nucleophilic substitution reaction
- d)Nucleophilic addition
Correct answer is option 'C'. Can you explain this answer?
Williamsons synthesis is an example of :
a)
Electrophilic substitution
b)
Electrophilic addition
c)
Nucleophilic substitution reaction
d)
Nucleophilic addition
|
|
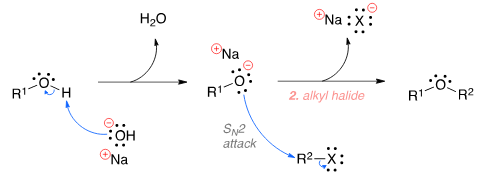
Shreya Gupta answered |
The Williamson ether synthesis is an organic reaction used to convert an alcohol and an alkyl halide to an ether using a base such as NaOH. The mechanism begins with the base abstracting the proton from the alcohol to form an alkoxide intermediate. The alkoxide then attacks the alkyl halide in a nucleophilic substi-tution reaction (SN2), which results in the formation of the final ether product and a metal halide by-product.

Phenols do not respond to which of these tests?- a)Schiff’s reagent test
- b)FeCl3 test
- c)Br2 water test
- d)Litmus test
Correct answer is option 'A'. Can you explain this answer?
Phenols do not respond to which of these tests?
a)
Schiff’s reagent test
b)
FeCl3 test
c)
Br2 water test
d)
Litmus test
|
|
Rajeev Saxena answered |
Phenols respond to all the above mentioned tests except Schiff’s reagent test, which is shown by aldehydes.
Which of the following compound reduces by NaBH4 ?- a)

- b)CH3–NO2
- c)
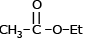
- d)
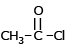
Correct answer is option 'D'. Can you explain this answer?
Which of the following compound reduces by NaBH4 ?
a)
b)
CH3–NO2
c)
d)
|
|
Suresh Iyer answered |
NaBH4 are weak reducing agent so only acid chloride are reduced in alcohal.
Phenol on treatment with Phthalic anhydride gives:- a)Phenolphthalein
- b)Salicylaldehyde
- c)Phthalic acid
- d)Salicylic acid
Correct answer is option 'A'. Can you explain this answer?
Phenol on treatment with Phthalic anhydride gives:
a)
Phenolphthalein
b)
Salicylaldehyde
c)
Phthalic acid
d)
Salicylic acid

|
Infinity Academy answered |
The correct answer is option A
Phenolphthalein gives pink colour with alkali.
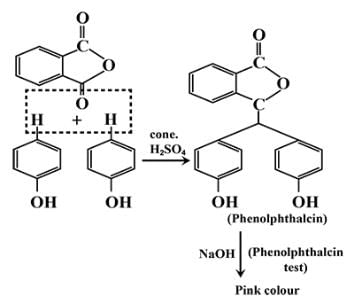
Phenolphthalein gives pink colour with alkali.

Phenol can be distinguished from ethanol by the reactions with _________.
- a)Br2/water
- b)Na
- c)Neutral FeCl3
- d)A and C
Correct answer is option 'D'. Can you explain this answer?
Phenol can be distinguished from ethanol by the reactions with _________.
a)
Br2/water
b)
Na
c)
Neutral FeCl3
d)
A and C

|
Nisha Banerjee answered |
- Phenol can be distinguished from ethanol using Br2/water and Neutral FeCl3.
- Br2/water: Phenol reacts with bromine water to give a white precipitate of 2,4,6-tribromophenol, while ethanol does not react.
- Neutral FeCl3: Phenol forms a violet complex with neutral ferric chloride, whereas ethanol shows no color change.
- Therefore, options A and C are correct for distinguishing phenol from ethanol.
Ethers may be used as solvents because they react only with which of the following reactants?- a)Acids
- b)Bases
- c)Oxidising agent
- d)Reducing agents
Correct answer is option 'A'. Can you explain this answer?
Ethers may be used as solvents because they react only with which of the following reactants?
a)
Acids
b)
Bases
c)
Oxidising agent
d)
Reducing agents
|
|
Geetika Shah answered |
Ethers resist the attack of nucleophiles and bases. However, they are very good solvents in many organic reactions due to their ability to solvate cations by donating the electron pair from oxygen atom. Ethers are generally less reactive and react only with acids.
Isopropyl alcohol on oxidation forms:- a)Acetone
- b)Ether
- c)Acetaldehyde
- d)Methane
Correct answer is option 'A'. Can you explain this answer?
Isopropyl alcohol on oxidation forms:
a)
Acetone
b)
Ether
c)
Acetaldehyde
d)
Methane
|
|
Rahul Bansal answered |
The oxidation of isopropyl alcohol by potassium dichromate (K 2Cr 2O 7) gives acetone, the simplest ketone: Unlike aldehydes, ketones are relatively resistant to further oxidation, so no special precautions are required to isolate them as they form.
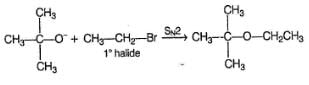
To prepare tert-butyl ethyl ether, the reagents required are: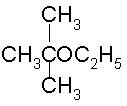
- a)Sodium ethoxide and tert-butyl bromide
- b)Sodium tert butoxide and ethyl bromide
- c)Dimethyl ketone, ethylbromide and sodium
- d)Sodium propoxide and propyl bromide
Correct answer is option 'B'. Can you explain this answer?
To prepare tert-butyl ethyl ether, the reagents required are:
a)
Sodium ethoxide and tert-butyl bromide
b)
Sodium tert butoxide and ethyl bromide
c)
Dimethyl ketone, ethylbromide and sodium
d)
Sodium propoxide and propyl bromide

|
Ayush Joshi answered |
Because 3degree haloalkanes like tert-butyl bromide (option a) give alkenes and not ethers when treated with a strong base like sodium ethoxide. So the exact answer is (b).
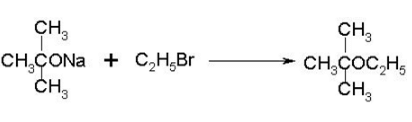
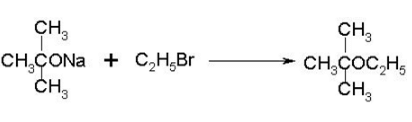
Which of the following compounds will react with sodium hydroxide solution in water?- a)C6H5OH
- b)C6H5CH2OH
- c)(CH3)3COH
- d)C2H5OH
Correct answer is option 'A'. Can you explain this answer?
Which of the following compounds will react with sodium hydroxide solution in water?
a)
C6H5OH
b)
C6H5CH2OH
c)
(CH3)3COH
d)
C2H5OH
|
|
Vijay Bansal answered |
When phenol reacts with sodium hydroxide solution it gives a colourless solution containing sodium phenoxide.
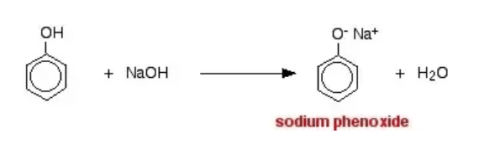
In this reaction, the hydrogen ion has been removed by the strongly basic hydroxide ion in the sodium hydroxide solution.
How many alcohols with molecular formula C4H10O are chiral in nature?a)2b)1c)4d)3Correct answer is option 'B'. Can you explain this answer?
|
|
Mansi Nair answered |

Here, again carbon is not chiral in nature.
So, only one alcohol is chiral in nature
So, only one alcohol is chiral in nature
An organic compound X is oxidised by using acidified K2Cr2O7. The product obtained reacts with Phenyl hydrazine but does not answer silver mirror test. The possible structure of X is- a)CH3CH2OH
- b)CH3CHO
- c)(CH3)2CHOH
- d)None of the these
Correct answer is option 'C'. Can you explain this answer?
An organic compound X is oxidised by using acidified K2Cr2O7. The product obtained reacts with Phenyl hydrazine but does not answer silver mirror test. The possible structure of X is
a)
CH3CH2OH
b)
CH3CHO
c)
(CH3)2CHOH
d)
None of the these
|
|
Baby Ghosh answered |
Yup...it will be propan-2-ol..by oxidation it..it will convert into acetone and it reacts with phenyl hydrazine,it produces acetone phenyl hydrazone and there is no aldehyde grp in acetone. so it doesn't react in silver mirror test means with tollen's reagent..
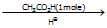 (A)product (A) is -
(A)product (A) is -- a)
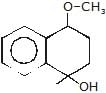
- b)
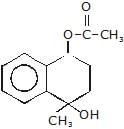
- c)

- d)

Correct answer is option 'B'. Can you explain this answer?
product (A) is -
a)
b)
c)
d)
|
|
Baby Ghosh answered |
According to Frice rearrangement...I think...
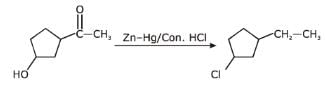
An alcohol has molecular formula C6H12O X and it gives immediate turbidity with cold, concentrated HCI even in the absence of ZnCI2. X can also be obtained by treatment of an ether with excess of CH3MgBr followed by acid hydrolysis. Hence, the correct statement regarding X is- a)It is 3-methyl-3-pentanol
- b)It is 2-methyl-3-pentanol
- c)It is 2-methyl-2-pentanol
- d)Either (b) or (c)
Correct answer is option 'C'. Can you explain this answer?
An alcohol has molecular formula C6H12O X and it gives immediate turbidity with cold, concentrated HCI even in the absence of ZnCI2. X can also be obtained by treatment of an ether with excess of CH3MgBr followed by acid hydrolysis. Hence, the correct statement regarding X is
a)
It is 3-methyl-3-pentanol
b)
It is 2-methyl-3-pentanol
c)
It is 2-methyl-2-pentanol
d)
Either (b) or (c)

|
Arpita Nair answered |
Only One Option Correct TypeDirection (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. Identify the final major product of the reaction sequence.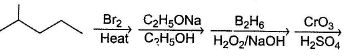
- a)

- b)

- c)

- d)

Correct answer is option 'C'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Identify the final major product of the reaction sequence.
a)
b)
c)
d)

|
Dr Manju Sen answered |
One Integer Value Correct TypeDirection (Q. Nos. 19-22) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).In the following reaction,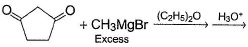 Q. How many different diols are formed as a result of nucleophilic addition reaction?
Q. How many different diols are formed as a result of nucleophilic addition reaction?
Correct answer is '3'. Can you explain this answer?
One Integer Value Correct Type
Direction (Q. Nos. 19-22) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
In the following reaction,
Q.
How many different diols are formed as a result of nucleophilic addition reaction?
|
|
Ritu Singh answered |
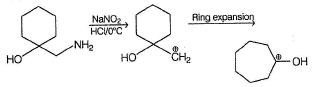
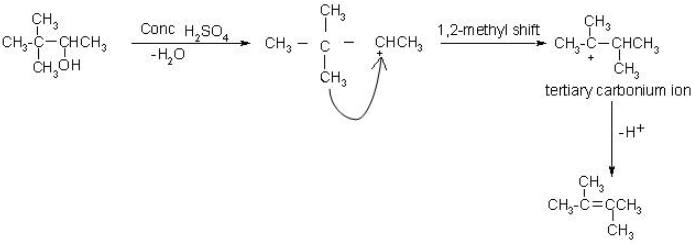
What is the correct structure for the major compound produced by the following reaction sequence?
- a)

- b)

- c)

- d)

Correct answer is option 'B'. Can you explain this answer?
What is the correct structure for the major compound produced by the following reaction sequence?
a)
b)
c)
d)

|
Gunjan Lakhani answered |
Reaction involves rearrangement of carbocation
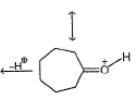


Which of the following compound reduces by DIBAL-H ?- a)CH3–
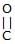 –OH
–OH - b)
- c)CH3–
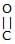 –O–Et
–O–Et - d)All
Correct answer is option 'D'. Can you explain this answer?
Which of the following compound reduces by DIBAL-H ?
a)
CH3–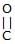 –OH
–OH
b)
c)
CH3–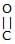 –O–Et
–O–Et
d)
All

|
Kiran Sanodiya answered |
Yes it can reduce ester ,acid n cyanide to corresponding aldehydes.
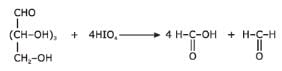
Which reaction(s) given below gives dicarbonyl?
- a)
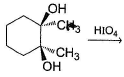
- b)
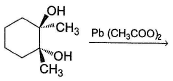
- c)

- d)
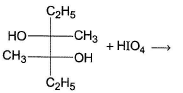
Correct answer is option 'A,D'. Can you explain this answer?
Which reaction(s) given below gives dicarbonyl?
a)
b)
c)
d)

|
Gunjan Lakhani answered |
Syn vicinal diols undergo oxidative cleavage with HIO4 giving diols, Option (b) and option (c) are anti diol and have restricted rotation.
Ph–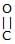 –O–CH3
–O–CH3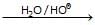 Above reaction is known as -
Above reaction is known as -- a)Esterification
- b)saponification
- c)Transesterification
- d)Acidic hydrolysis
Correct answer is option 'B'. Can you explain this answer?
Ph–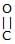 –O–CH3
–O–CH3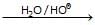
Above reaction is known as -
a)
Esterification
b)
saponification
c)
Transesterification
d)
Acidic hydrolysis
|
|
Nikita Singh answered |
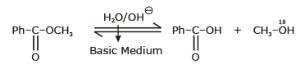
Hydrolysis of ester in basic Medium is known as saponification.
Chapter doubts & questions for Alcohols, Phenols and Ethers - NCERT Based Tests for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Alcohols, Phenols and Ethers - NCERT Based Tests for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
NCERT Based Tests for NEET
684 tests
|








 +CH3–O18H
+CH3–O18H