All Exams >
BPSC (Bihar) >
Science & Technology for State PSC Exams >
All Questions
All questions of Chemistry for BPSC (Bihar) Exam
Consider the following statements :
1. Diamond is hard and graphite is soft.
2. Diamond is soft and graphite is hard.
3. Diamond is a bad conductor but graphite is a good conductor.
4. Diamond is a good conductor but graphite is a bad conductor.Q. Which of the statements given above is/are correct ?- a)1 and 3
- b)1 only
- c)2 and 3
- d)1 and 4
Correct answer is option 'A'. Can you explain this answer?
Consider the following statements :
1. Diamond is hard and graphite is soft.
2. Diamond is soft and graphite is hard.
3. Diamond is a bad conductor but graphite is a good conductor.
4. Diamond is a good conductor but graphite is a bad conductor.
1. Diamond is hard and graphite is soft.
2. Diamond is soft and graphite is hard.
3. Diamond is a bad conductor but graphite is a good conductor.
4. Diamond is a good conductor but graphite is a bad conductor.
Q. Which of the statements given above is/are correct ?
a)
1 and 3
b)
1 only
c)
2 and 3
d)
1 and 4

|
Aashna Singh answered |
Diamond is hard and bad conductor but Graphite is soft and good conductor.
Consider the following statements: Hard water is not suitable for
1. Drinking
2. Washing clothes with soap
3. Use in boilers
4. Irrigating cropsQ. Which of these statements are correct?- a)1 and 3
- b)2 and 3
- c)1, 2 and 4
- d)1, 2, 3 and 4
Correct answer is option 'B'. Can you explain this answer?
Consider the following statements: Hard water is not suitable for
1. Drinking
2. Washing clothes with soap
3. Use in boilers
4. Irrigating crops
1. Drinking
2. Washing clothes with soap
3. Use in boilers
4. Irrigating crops
Q. Which of these statements are correct?
a)
1 and 3
b)
2 and 3
c)
1, 2 and 4
d)
1, 2, 3 and 4

|
Aravind Chatterjee answered |
Hard water is not suitable for washing clothes with soap and use in boilers.
H2O is liquid and H2S is a gas because- a)oxygen forms stronger hydrogen level than sulphur.
- b)oxygen is less electronegative than sulphur.
- c)atomic radius of oxygen is less than that of sulphur.
- d)atomic radius of oxygen is greater than that of sulphur.
Correct answer is option 'A'. Can you explain this answer?
H2O is liquid and H2S is a gas because
a)
oxygen forms stronger hydrogen level than sulphur.
b)
oxygen is less electronegative than sulphur.
c)
atomic radius of oxygen is less than that of sulphur.
d)
atomic radius of oxygen is greater than that of sulphur.
|
|
Rahul Rathore answered |
In h2o there is hydrogen bonding because oxygen has a high electronegativity(only second to flourine). so, the hydrogen atoms from other molecules of water forms a hydrogen bonds with oxygen resulting in intermolecular hydrogen bonding. ... therefore at room temperature h2o is aliquid and h2s is a gas.
Consider the following statements regarding diamond:
1. It is an allotrope of silicon.
2. It is a bad conductor of heat and electricity.
3. It is the hardest substance.
4. It burns to produce carbon dioxide.
Q. Which of the statements given above are correct?- a)1, 2, 3 and 4
- b)2, 3, and 4
- c)1 and 2
- d)1, 3 and 4
Correct answer is option 'B'. Can you explain this answer?
Consider the following statements regarding diamond:
1. It is an allotrope of silicon.
2. It is a bad conductor of heat and electricity.
3. It is the hardest substance.
4. It burns to produce carbon dioxide.
Q. Which of the statements given above are correct?
1. It is an allotrope of silicon.
2. It is a bad conductor of heat and electricity.
3. It is the hardest substance.
4. It burns to produce carbon dioxide.
Q. Which of the statements given above are correct?
a)
1, 2, 3 and 4
b)
2, 3, and 4
c)
1 and 2
d)
1, 3 and 4

|
Shreya Desai answered |
Diamond is an allotrope of carbon not silicon
Which one of the following sets of elements was primarily responsible for the origin of life on the Earth?- a)Hydrogen, oxygen, sodium
- b)Carbon, hydrogen, nitrogen
- c)Oxygen, calcium, phosphorus
- d)Carbon, hydrogen, potassium
Correct answer is option 'B'. Can you explain this answer?
Which one of the following sets of elements was primarily responsible for the origin of life on the Earth?
a)
Hydrogen, oxygen, sodium
b)
Carbon, hydrogen, nitrogen
c)
Oxygen, calcium, phosphorus
d)
Carbon, hydrogen, potassium

|
Arnab Saha answered |
Carbon, hydrogen and nitrogen were primarily responsible for the origin of life on the earth.
When iron is left exposed in open air, it gets rusted.
Which constituent(s) of air is /are responsible for rusting iron?
1. Oxygen gas present in air
2. Moisture present in air
3. Carbon dioxide gas present in air
Q. Select the correct answer using the codes given below :- a)1 only
- b)2 only
- c)1 and 2
- d)2 and 3
Correct answer is option 'C'. Can you explain this answer?
When iron is left exposed in open air, it gets rusted.
Which constituent(s) of air is /are responsible for rusting iron?
1. Oxygen gas present in air
2. Moisture present in air
3. Carbon dioxide gas present in air
Q. Select the correct answer using the codes given below :
Which constituent(s) of air is /are responsible for rusting iron?
1. Oxygen gas present in air
2. Moisture present in air
3. Carbon dioxide gas present in air
Q. Select the correct answer using the codes given below :
a)
1 only
b)
2 only
c)
1 and 2
d)
2 and 3

|
Akshat Jain answered |
Both oxygen and moisture present in air cause rusting of iron.
Hydrogen bomb is based on the principle of- a)controlled fusion reaction
- b)uncontrolled fusion reaction
- c)controlled fission reaction
- d)uncontrolled fission reaction
Correct answer is option 'B'. Can you explain this answer?
Hydrogen bomb is based on the principle of
a)
controlled fusion reaction
b)
uncontrolled fusion reaction
c)
controlled fission reaction
d)
uncontrolled fission reaction

|
Rithika Menon answered |
Thermonuclear fusion, or hydrogen bombs explode with enormous power using uncontrolled self-sustaining chain fusion reactions. Deuterium and tritium, under extremely high temperatures, form helium providing the energy. D + T → 4He + n In principle, a mixture of D, T and 6Li heated to very high temperature and confined to a high density will start a chain fusion reaction, liberating an enormous amount of energy.
Which one of the following pairs is correctly matched?- a)Silver iodide — Horn silver
- b)Silver chloride — Artificial rain
- c)Zinc phosphide — Rat poison
- d)Zinc sulphide — Philosopher’s wool
Correct answer is option 'C'. Can you explain this answer?
Which one of the following pairs is correctly matched?
a)
Silver iodide — Horn silver
b)
Silver chloride — Artificial rain
c)
Zinc phosphide — Rat poison
d)
Zinc sulphide — Philosopher’s wool

|
Kunal Roy answered |
Cerargyrite, also called Horn Silver, gray, very heavy halide mineral composed of silver chloride (AgCl); it is an ore of silver. Tiny particles of silver iodide are sprayed on a cloud from an aeroplane. The particles attract water drops from the cloud. When they form a drop that is large enough, it starts raining. Zinc phosphide is an inorganic compound that is used in pesticide products as a rodenticide.. Zinc oxide is also known as philosopher's wool.
The difference between a nuclear reactor and atomic bomb is that- a)no chain reaction takes place in nuclear reactor while in the atomic bomb there is a chain reaction.
- b)the chain reaction in nuclear reactor is controlled.
- c)the chain reaction in nuclear reactor is not controlled.
- d)no chain reaction takes place in atomic bomb while it takes place in nuclear reactor.
Correct answer is option 'B'. Can you explain this answer?
The difference between a nuclear reactor and atomic bomb is that
a)
no chain reaction takes place in nuclear reactor while in the atomic bomb there is a chain reaction.
b)
the chain reaction in nuclear reactor is controlled.
c)
the chain reaction in nuclear reactor is not controlled.
d)
no chain reaction takes place in atomic bomb while it takes place in nuclear reactor.

|
Pallavi Chakraborty answered |
There are two main fundamental differences between the design of an atomic bomb, and the design of a nuclear reactor. One difference is the way the fission reactions are controlled and the second difference stems from the enrichment of the fuel.
Enriched uranium is- a)Uranium rods kept under special shield
- b)Natural uranium in which the component of radioactive isotope U235 is artificial increased
- c)Natural uranium mixed with thorium
- d)Uranium rods coated with chromium
Correct answer is option 'B'. Can you explain this answer?
Enriched uranium is
a)
Uranium rods kept under special shield
b)
Natural uranium in which the component of radioactive isotope U235 is artificial increased
c)
Natural uranium mixed with thorium
d)
Uranium rods coated with chromium

|
Palak Nambiar answered |
Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. The International Atomic Energy Agency attempts to monitor and control enriched uranium supplies and processes in its efforts to ensure nuclear power generation safety and curb nuclear weapons proliferation.
Living in the atmosphere of CO is dangerous because it- a)Reduces organic matter of tissues
- b)Dries up the blood
- c)Combined with O2 present inside to form CO2
- d)Combines with haemoglobin and makes it incapable of absorbing oxygen
Correct answer is option 'D'. Can you explain this answer?
Living in the atmosphere of CO is dangerous because it
a)
Reduces organic matter of tissues
b)
Dries up the blood
c)
Combined with O2 present inside to form CO2
d)
Combines with haemoglobin and makes it incapable of absorbing oxygen

|
Devanshi Chavan answered |
When CO is not ventilated it binds to haemoglobin, which is the principal oxygencarrying compound in blood; this produces a compound known as carboxyhaemoglobin. The traditional belief is that carbon monoxide toxicity arises from the formation of carboxyhaemoglobin, which decreases the oxygen-carrying capacity of the blood and inhibits the transport, delivery, and utilization of oxygen by the body.
Consider the following statements :
1. An alloy is a mixture of two or more metals.
2. An alloy is a mixture of a metal or metals with a non-metal.Q. Which of the statements given above is/are correct ?- a)1 only
- b)2 only
- c)Both 1 and 2
- d)Neither 1 nor 2
Correct answer is option 'A'. Can you explain this answer?
Consider the following statements :
1. An alloy is a mixture of two or more metals.
2. An alloy is a mixture of a metal or metals with a non-metal.
1. An alloy is a mixture of two or more metals.
2. An alloy is a mixture of a metal or metals with a non-metal.
Q. Which of the statements given above is/are correct ?
a)
1 only
b)
2 only
c)
Both 1 and 2
d)
Neither 1 nor 2

|
Janhavi Bajaj answered |
A homogeneous mixture of two or more metals is known as alloys.
Which of the following pairs is/are correctly matched?
1. Isotopes : Atoms with same atomic number but different atomic mass.
2. Isobars : Atoms with same number of neutrons but different atomic number.
3. Isotones : Atoms with same mass number but different atomic number.
Q. Select the correct answer using the codes given below :- a)1, 2 and 3
- b)1 only
- c)1 and 2 only
- d)2 only
Correct answer is option 'B'. Can you explain this answer?
Which of the following pairs is/are correctly matched?
1. Isotopes : Atoms with same atomic number but different atomic mass.
2. Isobars : Atoms with same number of neutrons but different atomic number.
3. Isotones : Atoms with same mass number but different atomic number.
Q. Select the correct answer using the codes given below :
1. Isotopes : Atoms with same atomic number but different atomic mass.
2. Isobars : Atoms with same number of neutrons but different atomic number.
3. Isotones : Atoms with same mass number but different atomic number.
Q. Select the correct answer using the codes given below :
a)
1, 2 and 3
b)
1 only
c)
1 and 2 only
d)
2 only

|
Aarya Choudhary answered |
Isotopes are variants of a particular chemical element: while all isotopes of a given element share the same number of protons and electrons, each isotope differs from the others in its number of neutrons. For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively
Which one of the following statements is not true about cosmic rays?
- a)They are electromagnetic waves
- b)They have very high wavelength
- c)They are made of highly energetic charged particles
- d)They originated from the sun
Correct answer is option 'B'. Can you explain this answer?
Which one of the following statements is not true about cosmic rays?
a)
They are electromagnetic waves
b)
They have very high wavelength
c)
They are made of highly energetic charged particles
d)
They originated from the sun

|
Sreemoyee Shah answered |
Correct option is B. They have very high wavelength
Acid rains is caused due to emission of which of the following into the atmosphere?- a)Oxides of nitrogen and sulphur
- b)Carbon dioxide and carbon monoxide
- c)Ozone and carbon dioxide
- d)Carbon monoxide and nitrogen
Correct answer is option 'A'. Can you explain this answer?
Acid rains is caused due to emission of which of the following into the atmosphere?
a)
Oxides of nitrogen and sulphur
b)
Carbon dioxide and carbon monoxide
c)
Ozone and carbon dioxide
d)
Carbon monoxide and nitrogen

|
Srestha Bajaj answered |
Acid rain is caused by emissions of sulphur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids.
Domestic cooking gas consists of mostly- a)Methane and ethane
- b)Liquified butane and isobutane
- c)Ethylene and carbon monoxide
- d)Hydrogen and acetylene
Correct answer is option 'B'. Can you explain this answer?
Domestic cooking gas consists of mostly
a)
Methane and ethane
b)
Liquified butane and isobutane
c)
Ethylene and carbon monoxide
d)
Hydrogen and acetylene
|
|
Nikhil Ghosh answered |
Introduction to Domestic Cooking Gas
Domestic cooking gas primarily refers to the fuel used in household cooking appliances. This gas is commonly supplied in a liquefied form for ease of storage and transportation.
Composition of Domestic Cooking Gas
- Domestic cooking gas mainly consists of liquefied petroleum gas (LPG), which is a mixture of:
- Butane: A four-carbon alkane that is a key component of LPG.
- Isobutane: The branched-chain isomer of butane, also present in significant amounts.
Why Option B is Correct
- Efficiency: Butane and isobutane are efficient fuels that produce a high energy output with relatively low emissions when burned.
- Storage and Transportation: These gases can be liquefied at moderate pressures, making them easy to store in cylinders.
- Safety and Convenience: LPG is odorized for safety, allowing users to detect leaks through a distinct smell.
Comparison with Other Options
- Option A (Methane and Ethane): Although methane is a significant component of natural gas, domestic cooking gas is primarily LPG, not a mix of methane and ethane.
- Option C (Ethylene and Carbon Monoxide): These gases are not typically used for cooking due to their toxic nature and are not part of domestic cooking gas.
- Option D (Hydrogen and Acetylene): While hydrogen is a clean fuel, acetylene is not used for cooking due to its instability and safety concerns.
Conclusion
In summary, the correct answer is option B because domestic cooking gas primarily consists of liquefied butane and isobutane, which are safe, efficient, and practical for household cooking purposes.
Domestic cooking gas primarily refers to the fuel used in household cooking appliances. This gas is commonly supplied in a liquefied form for ease of storage and transportation.
Composition of Domestic Cooking Gas
- Domestic cooking gas mainly consists of liquefied petroleum gas (LPG), which is a mixture of:
- Butane: A four-carbon alkane that is a key component of LPG.
- Isobutane: The branched-chain isomer of butane, also present in significant amounts.
Why Option B is Correct
- Efficiency: Butane and isobutane are efficient fuels that produce a high energy output with relatively low emissions when burned.
- Storage and Transportation: These gases can be liquefied at moderate pressures, making them easy to store in cylinders.
- Safety and Convenience: LPG is odorized for safety, allowing users to detect leaks through a distinct smell.
Comparison with Other Options
- Option A (Methane and Ethane): Although methane is a significant component of natural gas, domestic cooking gas is primarily LPG, not a mix of methane and ethane.
- Option C (Ethylene and Carbon Monoxide): These gases are not typically used for cooking due to their toxic nature and are not part of domestic cooking gas.
- Option D (Hydrogen and Acetylene): While hydrogen is a clean fuel, acetylene is not used for cooking due to its instability and safety concerns.
Conclusion
In summary, the correct answer is option B because domestic cooking gas primarily consists of liquefied butane and isobutane, which are safe, efficient, and practical for household cooking purposes.
A sample of chloroform before using as an anaesthetic, is tested by- a)Fehling’s solution
- b)Ammonical cuprous chloride
- c)Ammonical silver nitrate solution
- d)Silver nitrate solution after boiling with alcoholic KOH
Correct answer is option 'C'. Can you explain this answer?
A sample of chloroform before using as an anaesthetic, is tested by
a)
Fehling’s solution
b)
Ammonical cuprous chloride
c)
Ammonical silver nitrate solution
d)
Silver nitrate solution after boiling with alcoholic KOH

|
Gauri Bose answered |
It is tested by ammonical silver nitrate solution.
Which one among the following statements regarding the properties of mixtures and compounds is not correct?- a)A mixture shows the properties of its constituents but the properties of a compound are entirely different from its constituents
- b)A mixture may be homogeneous or heterogeneous but a compound is a homogeneous substance
- c)The constituents of a mixture can be separated by physical methods but those of a compound cannot be separated by physical methods
- d)Energy is either absorbed or evolved during the preparation of a mixture but not in the preparation of a compound
Correct answer is option 'D'. Can you explain this answer?
Which one among the following statements regarding the properties of mixtures and compounds is not correct?
a)
A mixture shows the properties of its constituents but the properties of a compound are entirely different from its constituents
b)
A mixture may be homogeneous or heterogeneous but a compound is a homogeneous substance
c)
The constituents of a mixture can be separated by physical methods but those of a compound cannot be separated by physical methods
d)
Energy is either absorbed or evolved during the preparation of a mixture but not in the preparation of a compound

|
Dipika Gupta answered |
The compound is always formed by absorption or evolution of energy but no energy is released or absorbed during the formation of mixture.
Consider the following statements:
Nitrogen is an essential constituent of
1. soils
2. animals
3. plants
Q. Which of the statements given above is/are correct ?- a)3 only
- b)1 and 3 only
- c)1, 2 and 3
- d)1 and 2 only
Correct answer is option 'C'. Can you explain this answer?
Consider the following statements:
Nitrogen is an essential constituent of
1. soils
2. animals
3. plants
Q. Which of the statements given above is/are correct ?
Nitrogen is an essential constituent of
1. soils
2. animals
3. plants
Q. Which of the statements given above is/are correct ?
a)
3 only
b)
1 and 3 only
c)
1, 2 and 3
d)
1 and 2 only
|
|
Aarav Saini answered |
Understanding the Importance of Nitrogen
Nitrogen is a crucial element that plays a significant role in the ecosystem. The statements regarding its presence in soils, animals, and plants highlight its essential nature. Let's break down each statement to understand why the correct answer is option 'C'.
1. Nitrogen in Soils
- Nitrogen is a vital nutrient found in soils, primarily in the form of organic matter and various nitrogenous compounds.
- It is a key component for the growth of crops, as it contributes to soil fertility and health.
2. Nitrogen in Animals
- Animals require nitrogen for the synthesis of amino acids, which are the building blocks of proteins and essential for various bodily functions.
- Nitrogen is present in the form of proteins and nucleic acids in animals, making it an essential constituent.
3. Nitrogen in Plants
- Plants absorb nitrogen from the soil in the form of nitrates and ammonium, which are critical for their growth and development.
- Nitrogen is a key element in chlorophyll, the molecule responsible for photosynthesis, thus influencing plant health and productivity.
Conclusion
Given that nitrogen is indeed an essential constituent of soils, animals, and plants, all three statements are correct. Therefore, the correct answer is:
c) 1, 2 and 3
This highlights the interconnectedness of nitrogen in various forms of life and its importance in maintaining ecological balance.
Nitrogen is a crucial element that plays a significant role in the ecosystem. The statements regarding its presence in soils, animals, and plants highlight its essential nature. Let's break down each statement to understand why the correct answer is option 'C'.
1. Nitrogen in Soils
- Nitrogen is a vital nutrient found in soils, primarily in the form of organic matter and various nitrogenous compounds.
- It is a key component for the growth of crops, as it contributes to soil fertility and health.
2. Nitrogen in Animals
- Animals require nitrogen for the synthesis of amino acids, which are the building blocks of proteins and essential for various bodily functions.
- Nitrogen is present in the form of proteins and nucleic acids in animals, making it an essential constituent.
3. Nitrogen in Plants
- Plants absorb nitrogen from the soil in the form of nitrates and ammonium, which are critical for their growth and development.
- Nitrogen is a key element in chlorophyll, the molecule responsible for photosynthesis, thus influencing plant health and productivity.
Conclusion
Given that nitrogen is indeed an essential constituent of soils, animals, and plants, all three statements are correct. Therefore, the correct answer is:
c) 1, 2 and 3
This highlights the interconnectedness of nitrogen in various forms of life and its importance in maintaining ecological balance.
The order of appearance of the following with increasing temperature during the refining of crude oil is __________- a)Kerosene, gasoline, diesel
- b)Diesel, gasoline, kerosene
- c)Gasoline, kerosene, diesel
- d)Gasoline, diesel, kerosene
Correct answer is option 'C'. Can you explain this answer?
The order of appearance of the following with increasing temperature during the refining of crude oil is __________
a)
Kerosene, gasoline, diesel
b)
Diesel, gasoline, kerosene
c)
Gasoline, kerosene, diesel
d)
Gasoline, diesel, kerosene

|
Saanvi Reddy answered |
Petroleum products are usually grouped into three categories: light distillates (LPG, gasoline, naphtha), middle distillates (kerosene, diesel), heavy distillates and residuum (heavy fuel oil, lubricating oils, wax, asphalt). Hence, the correct option would be: Gasoline, kerosene, diesel.
The blue colour of water in the sea. What is the reason behind the phenomenon?- a)Refraction of the blue light by the impurities in sea water.
- b)Scattering of blue light by water molecules.
- c)Refraction of blue sky by sea water.
- d)Absorption of other colours except the blue colour by water molecules.
Correct answer is option 'B'. Can you explain this answer?
The blue colour of water in the sea. What is the reason behind the phenomenon?
a)
Refraction of the blue light by the impurities in sea water.
b)
Scattering of blue light by water molecules.
c)
Refraction of blue sky by sea water.
d)
Absorption of other colours except the blue colour by water molecules.

|
Anjana Nair answered |
Water molecules scatter blue wavelengths by absorbing the light waves, and then rapidly remitting the light waves in different directions. That is why there are mostly blue wavelengths that are reflected back to our eyes.
Helium is preferred to hydrogen in air balloons because it- a)is cheaper
- b)is less dense
- c)has greater lifting power
- d)does not form an explosive mixture with air
Correct answer is option 'D'. Can you explain this answer?
Helium is preferred to hydrogen in air balloons because it
a)
is cheaper
b)
is less dense
c)
has greater lifting power
d)
does not form an explosive mixture with air

|
Anshul Ghosh answered |
Hydrogen combines with oxygen with explosive force in the presence of a spark.Helium is an inert gas that will not burn or explode, so is much safer to use in balloons instead of hydrogen.
In the process of electroplating a utensil with zinc,- a)the utensil is made the cathode
- b)pure zinc is made the anode
- c)the utensil is made the cathode and pure zinc is made the anode
- d)the utensil is made the anode and pure zinc is made the cathode
Correct answer is option 'C'. Can you explain this answer?
In the process of electroplating a utensil with zinc,
a)
the utensil is made the cathode
b)
pure zinc is made the anode
c)
the utensil is made the cathode and pure zinc is made the anode
d)
the utensil is made the anode and pure zinc is made the cathode

|
Meghana Shah answered |
The zinc serves as a sacrificial anode, so that it cathodically protects exposed steel.
What is the role of ultraviolet (UV) radiation in the water purification system?
1. It inactivates / kills the harmful microorganisms in water.
2. It removes all the undesirable odours from the water.
3. It quickens the sedimentation of solid particles and improves the clarity of water.Q. Which of the statements given above is/are correct?- a)1 only
- b)2 and 3 only
- c)1 and 3 only
- d)1, 2 and 3
Correct answer is option 'A'. Can you explain this answer?
What is the role of ultraviolet (UV) radiation in the water purification system?
1. It inactivates / kills the harmful microorganisms in water.
2. It removes all the undesirable odours from the water.
3. It quickens the sedimentation of solid particles and improves the clarity of water.
1. It inactivates / kills the harmful microorganisms in water.
2. It removes all the undesirable odours from the water.
3. It quickens the sedimentation of solid particles and improves the clarity of water.
Q. Which of the statements given above is/are correct?
a)
1 only
b)
2 and 3 only
c)
1 and 3 only
d)
1, 2 and 3

|
Bijoy Kumar answered |
It inactivates/kills the harmful microorganisms in water.
Which one of the following pairs is not correctly matched?- a)Dry ice : Solid carbon dioxide
- b)Mustard gas : Poisonous liquid used in chemical warfare
- c)Teflon : Polymer containing fluorine
- d)Fullerene : Organic compounds containing fluorine
Correct answer is option 'D'. Can you explain this answer?
Which one of the following pairs is not correctly matched?
a)
Dry ice : Solid carbon dioxide
b)
Mustard gas : Poisonous liquid used in chemical warfare
c)
Teflon : Polymer containing fluorine
d)
Fullerene : Organic compounds containing fluorine

|
Shanaya Bajaj answered |
Fullerene is a pure carbon molecule composed of at least 60 atoms of carbon.
What happened when a hard boiled egg after shelling is immersed in saturated brine?- a)It shrinks
- b)It grows in size
- c)Its size remains unchanged
- d)it initially grows in size and then shrinks.
Correct answer is option 'C'. Can you explain this answer?
What happened when a hard boiled egg after shelling is immersed in saturated brine?
a)
It shrinks
b)
It grows in size
c)
Its size remains unchanged
d)
it initially grows in size and then shrinks.

|
Nidhi Pillai answered |
When hard boiled egg after shelling is immersed in saturated brine, its size remains same. Due to coagulation of inner liquid there is no flow of solvent molecules across the membrane.
Consider the following statements : The purpose of adding sodium sulphate and sodium silicate to washing powder is -
1. To keep washing powder dry
2. To maintain the alkalinity of the powder
Q. which of these statements is/are correct ?- a)Only 1
- b)Only 2
- c)Both 1 and 2
- d)Neither 1 nor 2
Correct answer is option 'A'. Can you explain this answer?
Consider the following statements : The purpose of adding sodium sulphate and sodium silicate to washing powder is -
1. To keep washing powder dry
2. To maintain the alkalinity of the powder
Q. which of these statements is/are correct ?
1. To keep washing powder dry
2. To maintain the alkalinity of the powder
Q. which of these statements is/are correct ?
a)
Only 1
b)
Only 2
c)
Both 1 and 2
d)
Neither 1 nor 2

|
Avantika Mukherjee answered |
Sodium sulphate and sodium silicate are added to keep the washing powder dry. Sodium triphosphate or sodium carbonate is added to washing powder to maintain the alkalinity.
Which of the statements given below is/are correct?
Permanent hardness of water is due to the presence of soluble.
1. chloride of calcium
2. bicarbonate of calcium
3. sulphate of magnesium
4. bicarbonate of magnesiumQ. Select the correct answer using the codes given below.- a)1 only
- b)1 and 3
- c)2 and 4
- d)1, 2 and 3
Correct answer is option 'B'. Can you explain this answer?
Which of the statements given below is/are correct?
Permanent hardness of water is due to the presence of soluble.
1. chloride of calcium
2. bicarbonate of calcium
3. sulphate of magnesium
4. bicarbonate of magnesium
Permanent hardness of water is due to the presence of soluble.
1. chloride of calcium
2. bicarbonate of calcium
3. sulphate of magnesium
4. bicarbonate of magnesium
Q. Select the correct answer using the codes given below.
a)
1 only
b)
1 and 3
c)
2 and 4
d)
1, 2 and 3

|
Gargi Saha answered |
Chlorides and sulphates of calcium and magnesium are responsible for permanent hardness of water. Note: Bicarbonates are responsible for temporary hardness of water.
Following statements are made in connection with carbon dioxide (CO2)
1. CO2 is a poisonous gas.
2. CO2 is an acidic oxide.
3. CO2 turns limewater milky.Which of the statements given above is/are correct?- a)1 and 2
- b)2 and 3
- c)3 only
- d)1 and 3
Correct answer is option 'B'. Can you explain this answer?
Following statements are made in connection with carbon dioxide (CO2)
1. CO2 is a poisonous gas.
2. CO2 is an acidic oxide.
3. CO2 turns limewater milky.
1. CO2 is a poisonous gas.
2. CO2 is an acidic oxide.
3. CO2 turns limewater milky.
Which of the statements given above is/are correct?
a)
1 and 2
b)
2 and 3
c)
3 only
d)
1 and 3

|
Palak Nambiar answered |
(i) CO2 is an acidic oxide. It dissolve in water formic unstable carbonic acid.
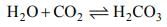
(ii) Limewater Ca(OH)2 is turned milky on passing CO2
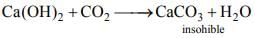
Consider the following parts of spectra:
1. Visible
2. Infrared
3. Ultraviolet
4. Microwave Q. Which of the following is the correct sequence in which the wavelengths increase?- a)4, 3, 1, 2
- b)4, 1, 2, 3
- c)3, 2, 1, 4
- d)3, 1, 2, 4
Correct answer is option 'D'. Can you explain this answer?
Consider the following parts of spectra:
1. Visible
2. Infrared
3. Ultraviolet
4. Microwave
1. Visible
2. Infrared
3. Ultraviolet
4. Microwave
Q. Which of the following is the correct sequence in which the wavelengths increase?
a)
4, 3, 1, 2
b)
4, 1, 2, 3
c)
3, 2, 1, 4
d)
3, 1, 2, 4

|
Poulomi Nair answered |
Ultra-violet rays < visible light < infrared radiation < microwaves (0.1 micrometres) (0.7 micrometres ) (0.01 mm) (less than 10 cm)
Q.Which of the following substances is/are ozone depleting?
1. Chlorofluorocarbons
2. Halons
3. Carbon tetrachloride- a)1 only
- b)1 and 2 only
- c)2 and 3 only
- d)1, 2 and 3
Correct answer is option 'D'. Can you explain this answer?
Q.Which of the following substances is/are ozone depleting?
1. Chlorofluorocarbons
2. Halons
3. Carbon tetrachloride
1. Chlorofluorocarbons
2. Halons
3. Carbon tetrachloride
a)
1 only
b)
1 and 2 only
c)
2 and 3 only
d)
1, 2 and 3

|
Sagar Chakraborty answered |
All the three substances are responsible for ozone layer depletion in different ways. Which are: CFC—mostly used in refrigeration, air conditioning and heat pump systems. Halons—used historically as fire suppression agents and fire fighting, but now only allowed in very limited situations. Carbon tetrachloride (tetrachloromethane)—limited solvent used in laboratories and chemical and pharmaceutical industries.
What is “ Kiss of death” ?- a)A flower whose smell was the basis of a discovery on smell that led to award of 2004 Nobel Prize in Medicine.
- b)A chemical whose discovery ultimately won the scientists the 2004 Nobel Prize in Chemistry.
- c)A good wine flavour working on which scientist won the 2004 Nobel Prize in Chemistry.
- d)A network in brain associated with smell whose discovery led the scientists win the 2004 Nobel Prize in Physiology and Medicine.
Correct answer is option 'B'. Can you explain this answer?
What is “ Kiss of death” ?
a)
A flower whose smell was the basis of a discovery on smell that led to award of 2004 Nobel Prize in Medicine.
b)
A chemical whose discovery ultimately won the scientists the 2004 Nobel Prize in Chemistry.
c)
A good wine flavour working on which scientist won the 2004 Nobel Prize in Chemistry.
d)
A network in brain associated with smell whose discovery led the scientists win the 2004 Nobel Prize in Physiology and Medicine.
|
|
Saptarshi Patel answered |
Your question?
While tinning of brass utensils, the ammonium chloride powder used to clean the hot utensil produces fumes of- a)ammonia
- b)carbon monoxide
- c)hydrochloric acid
- d)ammonia and hydrochloric acid
Correct answer is option 'D'. Can you explain this answer?
While tinning of brass utensils, the ammonium chloride powder used to clean the hot utensil produces fumes of
a)
ammonia
b)
carbon monoxide
c)
hydrochloric acid
d)
ammonia and hydrochloric acid

|
Saanvi Reddy answered |
It produces fumes of ammonia and hydrochloric acid.
Hydrofluoric acid is not kept in glass bottles because it reacts with- a)visible light
- b)sodium oxide of glass
- c)aluminium oxide of glass
- d)silicon dioxide of glass
Correct answer is option 'D'. Can you explain this answer?
Hydrofluoric acid is not kept in glass bottles because it reacts with
a)
visible light
b)
sodium oxide of glass
c)
aluminium oxide of glass
d)
silicon dioxide of glass

|
Aashna Nambiar answered |
Hydrofluoric acid is one of the most dangerous acids known. It needs to be treated differently then even strong acids like sulphuric and hydrochloric. HF reacts with many materials, therefore, avoid contact with glass, concrete, metals, water, other acids, oxidizers, reducers, alkalis, combustibles, organics and ceramics.
The inexhaustible source of energy of the stars is due to- a)conversion of hydrogen to helium
- b)conversion of helium to hydrogen
- c)decay of radioactive elements
- d)excess of oxygen that helps burning and release of energy
Correct answer is option 'A'. Can you explain this answer?
The inexhaustible source of energy of the stars is due to
a)
conversion of hydrogen to helium
b)
conversion of helium to hydrogen
c)
decay of radioactive elements
d)
excess of oxygen that helps burning and release of energy

|
Raksha Khanna answered |
The energy released from the collapse of the gas into a star causes the centre of the star to become extremely hot. When the core is hot enough, nuclear fusion commences. Fusion is the process where two hydrogen atoms combine to form a helium atom, releasing energy. The fusion reaction is a very efficient process, releasing a huge amount of energy. This is because a single helium atom contains less mass than two hydrogen atoms. The excess mass is released as energy.
In cold weather, aquatic animals survive even when water to the top layer of the lake freezes into ice because- a)they can breathe in ice
- b)they have enough of accumulated oxygen inside them
- c)their body structure is such that they can survive without oxygen.
- d)water has highest density of 4°C so underneath the top layer of ice there is layer of water
Correct answer is option 'D'. Can you explain this answer?
In cold weather, aquatic animals survive even when water to the top layer of the lake freezes into ice because
a)
they can breathe in ice
b)
they have enough of accumulated oxygen inside them
c)
their body structure is such that they can survive without oxygen.
d)
water has highest density of 4°C so underneath the top layer of ice there is layer of water

|
Aravind Chatterjee answered |
In cold countries, when the temperature of fresh water reaches 4°C, the layers of water near the top in contact with cold air continue to lose heat energy and their temperature falls below 4°C. On cooling below 4°C, these layers do not sink but may rise up as fresh water has a maximum density at 4°C. Due to this, the layer of water at 4°C remains at the bottom and above this layers of water 3°C, 2°C, 1°C and 0°C are formed. Because ice is a poor conductor of heat, it does not absorb heat energy from the water beneath the layer of ice which prevents the water freezing. Thus, aquatic creatures survive in such places.
Which one of the following is NOT correct?- a)Theory of evolution was propounded by Charles Darwin.
- b)The breaking apart of the nucleus of an atom is called fusion.
- c)Dry ice is nothing but solid carbon dioxide.
- d)Telephone was invented by Graham Bell.
Correct answer is option 'B'. Can you explain this answer?
Which one of the following is NOT correct?
a)
Theory of evolution was propounded by Charles Darwin.
b)
The breaking apart of the nucleus of an atom is called fusion.
c)
Dry ice is nothing but solid carbon dioxide.
d)
Telephone was invented by Graham Bell.
|
|
Mihir Mukherjee answered |
Theory of Evolution
- Charles Darwin is credited with the formulation of the theory of evolution through natural selection, which explains how species adapt and evolve over time.
Fusion vs. Fission
- The statement in option B incorrectly describes a nuclear process.
- Fission is the process where the nucleus of an atom splits into smaller parts, usually releasing a significant amount of energy.
- Fusion, on the other hand, is the process where two light atomic nuclei combine to form a heavier nucleus, which also releases energy.
Dry Ice
- Dry ice is indeed solid carbon dioxide (CO2), used primarily as a refrigerant or for creating fog effects.
Invention of the Telephone
- Alexander Graham Bell is recognized for inventing the first practical telephone, revolutionizing communication.
Conclusion
- Therefore, the incorrect statement is option B, as it confuses the terms fusion and fission, misrepresenting fundamental nuclear physics. Understanding these concepts is vital, especially in scientific fields, and highlights the importance of precise language in science.
- Charles Darwin is credited with the formulation of the theory of evolution through natural selection, which explains how species adapt and evolve over time.
Fusion vs. Fission
- The statement in option B incorrectly describes a nuclear process.
- Fission is the process where the nucleus of an atom splits into smaller parts, usually releasing a significant amount of energy.
- Fusion, on the other hand, is the process where two light atomic nuclei combine to form a heavier nucleus, which also releases energy.
Dry Ice
- Dry ice is indeed solid carbon dioxide (CO2), used primarily as a refrigerant or for creating fog effects.
Invention of the Telephone
- Alexander Graham Bell is recognized for inventing the first practical telephone, revolutionizing communication.
Conclusion
- Therefore, the incorrect statement is option B, as it confuses the terms fusion and fission, misrepresenting fundamental nuclear physics. Understanding these concepts is vital, especially in scientific fields, and highlights the importance of precise language in science.
Biogas mainly consists of- a)Carbon dioxide and hydrogen
- b)Hydrogen and methane
- c)Carbon dioxide and methane
- d)Hydrogen and oxygen
Correct answer is option 'C'. Can you explain this answer?
Biogas mainly consists of
a)
Carbon dioxide and hydrogen
b)
Hydrogen and methane
c)
Carbon dioxide and methane
d)
Hydrogen and oxygen

|
Kaavya Sarkar answered |
Bio gas is primarily methane (CH4) and carbon dioxide (CO2) and may have small amounts of hydrogen sulphide (H2S), moisture and siloxanes.
Consider the following statements:
Assertion (A): LPG is a pollution free vehicular fuel.
Reason (R): Plying of CNG fuelled-buses is recommended for metropolitan cities in India.- a)Both A and R are true, and R is the correct explanation of A.
- b)Both A and R are true, but R is not the correct explanation of A.
- c)A is true, but R is false.
- d)A is false, but R is true.
Correct answer is option 'D'. Can you explain this answer?
Consider the following statements:
Assertion (A): LPG is a pollution free vehicular fuel.
Reason (R): Plying of CNG fuelled-buses is recommended for metropolitan cities in India.
Assertion (A): LPG is a pollution free vehicular fuel.
Reason (R): Plying of CNG fuelled-buses is recommended for metropolitan cities in India.
a)
Both A and R are true, and R is the correct explanation of A.
b)
Both A and R are true, but R is not the correct explanation of A.
c)
A is true, but R is false.
d)
A is false, but R is true.

|
Gauri Bose answered |
Two recent studies have examined LPG-fueloil fuel mixes and found that smoke emissions and fuel consumption are reduced but hydrocarbon emissions are increased.
Match List–I with List–II and select the correct answer using the codes given below :

- a)A
- b)B
- c)C
- d)D
Correct answer is option 'A'. Can you explain this answer?
Match List–I with List–II and select the correct answer using the codes given below :


a)
A
b)
B
c)
C
d)
D

|
Dipika Gupta answered |
These compounds are used in the manufacture of the following products. Cellulose nitrate- Gun powder, Potassium Sulphate- Fertiliser, Potassium salts of fatty acids- Soft soap, Calcium oxide- Glass.
Consider the following statements: If there were no phenomenon of capillarity
1. It would be difficult to use a kerosene lamp.
2. One would not be able to use a straw to consume a soft drink.
3. the blotting paper would fail to function.
4. the big trees that we see around would not have grown on the earth.Q. Which of the statements given above is/are correct?- a)1, 2 and 3 only
- b)1, 3 and 4 only
- c)2 and 4 only
- d)1, 2, 3 and 4
Correct answer is option 'B'. Can you explain this answer?
Consider the following statements: If there were no phenomenon of capillarity
1. It would be difficult to use a kerosene lamp.
2. One would not be able to use a straw to consume a soft drink.
3. the blotting paper would fail to function.
4. the big trees that we see around would not have grown on the earth.
1. It would be difficult to use a kerosene lamp.
2. One would not be able to use a straw to consume a soft drink.
3. the blotting paper would fail to function.
4. the big trees that we see around would not have grown on the earth.
Q. Which of the statements given above is/are correct?
a)
1, 2 and 3 only
b)
1, 3 and 4 only
c)
2 and 4 only
d)
1, 2, 3 and 4

|
Aashna Nambiar answered |
Except option (2), all are applications of capillary action. One would not be able to consume soft drink, if there is no atmospheric pressure, i.e., in vacuum.
Consider the following statements and select the correct code.
Assertion (A): A chemical reaction becomes faster at higher temperature.
Reason (R): At higher temperature, molecular motion becomes more rapid.- a)Both A and R are true and R is the correct explanation of A.
- b)Both A and R are true, but R is not correct explanation of A.
- c)A is true, but R is false.
- d)A is false, but R is true.
Correct answer is option 'A'. Can you explain this answer?
Consider the following statements and select the correct code.
Assertion (A): A chemical reaction becomes faster at higher temperature.
Reason (R): At higher temperature, molecular motion becomes more rapid.
Assertion (A): A chemical reaction becomes faster at higher temperature.
Reason (R): At higher temperature, molecular motion becomes more rapid.
a)
Both A and R are true and R is the correct explanation of A.
b)
Both A and R are true, but R is not correct explanation of A.
c)
A is true, but R is false.
d)
A is false, but R is true.

|
Avi Sengupta answered |
The rates of most reactions increase with a rise in temperature. Raising the temperature increases the fraction of molecules having very high kinetic energies. These are the ones most likely to react when they collide. The higher the temperature, the larger the fraction of molecules that can provide the activation energy needed for reaction.
When soggy biscuits are kept inside the fridge for sometime they become crisp because- a)cooling releases extra moisture
- b)humidity inside the fridge is low and extra moisture is absorbed
- c)humidity inside the fridge is high and extra moisture is absorbed
- d)pressure inside the fridge is high and helps in releasing extra moisture
Correct answer is option 'B'. Can you explain this answer?
When soggy biscuits are kept inside the fridge for sometime they become crisp because
a)
cooling releases extra moisture
b)
humidity inside the fridge is low and extra moisture is absorbed
c)
humidity inside the fridge is high and extra moisture is absorbed
d)
pressure inside the fridge is high and helps in releasing extra moisture

|
Kalyan Verma answered |
Because the humidity inside the fridge is low and extra moisture is absorbed.
Consider the following statements and select the correct code.
Assertion (A): The main constituent of the liquefied petroleum gas is methane.
Reason (R): Methane can be used directly for burning in homes and factories where it can be supplied through pipelines.- a)Both A and R are true, and R is the correct explanation of A.
- b)Both A and R are true, but R is not the correct explanation of A.
- c)A is true, but R is false.
- d)A is false, but R is true.
Correct answer is option 'D'. Can you explain this answer?
Consider the following statements and select the correct code.
Assertion (A): The main constituent of the liquefied petroleum gas is methane.
Reason (R): Methane can be used directly for burning in homes and factories where it can be supplied through pipelines.
Assertion (A): The main constituent of the liquefied petroleum gas is methane.
Reason (R): Methane can be used directly for burning in homes and factories where it can be supplied through pipelines.
a)
Both A and R are true, and R is the correct explanation of A.
b)
Both A and R are true, but R is not the correct explanation of A.
c)
A is true, but R is false.
d)
A is false, but R is true.

|
Janhavi Bajaj answered |
The main components of LPG are butane and propane.
Consider the following statements and select the correct code.
Assertion (A) : In the periodic table of chemical elements, electron affinity is always found to increase from top to bottom in a group
Reason (R) : In a group, the atomic radii generally increase from top to bottom.- a)Both A and R are individually true and R is correct explanation of A
- b)Both A and R are individually true and R is not the correct explanation of A
- c)A is true but R is false
- d)A is false but R is true
Correct answer is option 'D'. Can you explain this answer?
Consider the following statements and select the correct code.
Assertion (A) : In the periodic table of chemical elements, electron affinity is always found to increase from top to bottom in a group
Reason (R) : In a group, the atomic radii generally increase from top to bottom.
Assertion (A) : In the periodic table of chemical elements, electron affinity is always found to increase from top to bottom in a group
Reason (R) : In a group, the atomic radii generally increase from top to bottom.
a)
Both A and R are individually true and R is correct explanation of A
b)
Both A and R are individually true and R is not the correct explanation of A
c)
A is true but R is false
d)
A is false but R is true

|
Aarya Choudhary answered |
Electron affinity generally decreases from top to bottom in a group. Atomic radii increases from top to bottom as energy levels increases because as we move down a group number of electrons increases.
Which of the following statements about diamond are correct?
1. It is used as a gem in jewellery because of its ability to reflect light.
2. It is good conductor of electricity.
3. It is used for cutting glass, marble stones and other hard materials.
4. It is used for drilling of rocks.Q. Select the correct answer using the codes given below :- a)1, 3 and 4
- b)2, 3 and 4
- c)1, 2 and 3
- d)2 and 4
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements about diamond are correct?
1. It is used as a gem in jewellery because of its ability to reflect light.
2. It is good conductor of electricity.
3. It is used for cutting glass, marble stones and other hard materials.
4. It is used for drilling of rocks.
1. It is used as a gem in jewellery because of its ability to reflect light.
2. It is good conductor of electricity.
3. It is used for cutting glass, marble stones and other hard materials.
4. It is used for drilling of rocks.
Q. Select the correct answer using the codes given below :
a)
1, 3 and 4
b)
2, 3 and 4
c)
1, 2 and 3
d)
2 and 4

|
Shanaya Bajaj answered |
Diamond, an allotrope of carbon, has very high refractive because of which it is used as a gem in jewellery. It is used for cutting glass, marble stones and other hard materials and for drilling of rocks. It is a bad conductor of electricity. It is the hardest material known.
Match List-I with List-II and select the correct answer from the codes given below
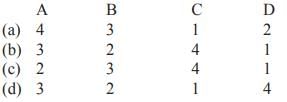
- a)A
- b)B
- c)C
- d)D
Correct answer is option 'A'. Can you explain this answer?
Match List-I with List-II and select the correct answer from the codes given below


a)
A
b)
B
c)
C
d)
D

|
Meghana Shah answered |
Quarks have fractional electric charge values– either 1/2 or 2/3 times the elementary charge. The positron has an electric charge of +1e, a spin of 1/2, and has the same mass as an electron. A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with halfinteger spin. All evidence suggest that neutrinos have mass but that their mass is tiny even by the standards of subatomic particles. Their mass has never been measured accurately.
Match List-I with List-II and select the correct answer from the codes given below:
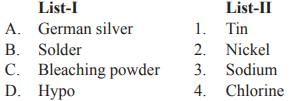

- a)A
- b)B
- c)C
- d)D
Correct answer is option 'D'. Can you explain this answer?
Match List-I with List-II and select the correct answer from the codes given below:
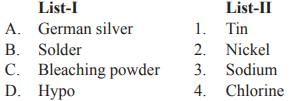
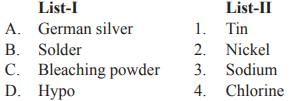

a)
A
b)
B
c)
C
d)
D

|
Nilanjan Banerjee answered |
Nickel silver, also known as German silver, is a copper alloy with nickel and often zinc. Solders are typically made from tin or lead or a combination of both in the ratio of 63:37 respectively. Calcium hypochlorite, also known as bleaching powder, is a chemical compound with formula Ca(ClO)2. It is widely used for water treatment and as a bleaching agent. This chemical is considered to be relatively stable and has greater available chlorine than sodium hypochlorite (liquid bleach). Hypo solution is the abbreviation for sodium thiosulphate or sodium hyposulphite, a chemical used to fix the image on photographic film after it has been developed.
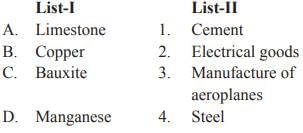
Match List-I with List-II and select the correct answer from the codes given below:

- a)A
- b)B
- c)C
- d)D
Correct answer is option 'A'. Can you explain this answer?
Match List-I with List-II and select the correct answer from the codes given below:



a)
A
b)
B
c)
C
d)
D

|
Shreya Desai answered |
Manganese is essential to iron and steel production. At present, steel making accounts 85 to 90% of the total demand, most of the total demand. Manganese is a key component of low-cost stainless steel formulations and certain widely used aluminium alloys. Limestone can be used in constructing buildings. It can be used for making cement and mortar. Limestone is used to make glass and even used to make roads. Bauxite is the mineral ore of aluminium which is used in the manufacture of cans, airplanes, sporting and electronic equipment and home appliances. The Wright Brother's first airplane to fly in 1903 only was able to get off the ground because they modified its engine with aluminium in order to reduce its weight. Without the ability of the strong aluminium, alloys to withstand the huge pressures and stresses involved, high altitude flying would not be conceivable. In fact, aluminium comprises about 80% of an aircraft’s unladen weight. The element copper is used extensively as an electrical conductor, for the making of electrical wire.
. Match List-I with List-II and select the correct answer from the codes given below:


- a)A
- b)B
- c)C
- d)D
Correct answer is option 'C'. Can you explain this answer?
. Match List-I with List-II and select the correct answer from the codes given below:




a)
A
b)
B
c)
C
d)
D

|
Kaavya Sarkar answered |
Sour milk – Lactic acid Vinegar and pickel – Acetic acid Soda water – Carbonic acid Apple – Malic acid
Chapter doubts & questions for Chemistry - Science & Technology for State PSC Exams 2025 is part of BPSC (Bihar) exam preparation. The chapters have been prepared according to the BPSC (Bihar) exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for BPSC (Bihar) 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemistry - Science & Technology for State PSC Exams in English & Hindi are available as part of BPSC (Bihar) exam.
Download more important topics, notes, lectures and mock test series for BPSC (Bihar) Exam by signing up for free.
Science & Technology for State PSC Exams
113 videos|527 docs|217 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









