All Exams >
ACT >
Science for ACT >
All Questions
All questions of Conflicting Viewpoints for ACT Exam
Magnets and electric charges show certain similarities. For example, both magnets and electric charges can exert a force on their surroundings. This force, when produced by a magnet, is called a magnetic field. When it is produced by an electric charge, the force is called an electric field. It has been observed that the strength of both magnetic fields and electric fields is inversely proportional to the square of the distance between a magnet or an electric charge and the objects that they affect.Below, three scientists debate the relationship between electricity and magnetism.Scientist 1:Electricity and magnetism are two different phenomena. Materials such as iron, cobalt, and nickel contain magnetic domains: tiny regions of magnetism, each with two poles. Normally, the domains have a random orientation and are not aligned, so the magnetism of some domains cancels out that of other domains; however, in magnets, domains line up in the same direction, creating the two poles of the magnet and causing magnetic behavior.In contrast, electricity is a moving electric charge which is caused by the flow of electrons through a material. Electrons flow through a material from a region of higher potential (more negative charge) to a region of lower potential (more positive charge). We can measure this flow of electrons as current, which refers to the amount of charge transferred over a period of time.Scientist 2:Electricity and magnetism are similar phenomena; however, one cannot be reduced to the other. Electricity involves two types of charges: positive and negative charge. Though electricity can occur in a moving form (in the form of current, or an electric charge moving through a wire), it can also occur in a static form. Static electricity involves no moving charge. Instead, objects can have a net excess of positive charge or a net excess of negative charge—because of having lost or gained electrons, respectively. When two static positive electric charges or two static negative electric charges are brought close together, they repel each other. However, when a positive and a negative static charge are brought together, they attract each other.Similarly, all magnets have two poles. Magnetic poles that are alike repel each other, while dissimilar magnetic poles attract each other. Magnets and static electric charges are alike in that they both show attraction and repulsion in similar circumstances. However, while isolated static electric charges occur in nature, there are no single, isolated magnetic poles. All magnets have two poles, which cannot be dissociated from each other.Scientist 3:Electricity and magnetism are two aspects of the same phenomenon. A moving flow of electrons creates a magnetic field around it. Thus, wherever an electric current exists, a magnetic field will also exist. The magnetic field created by an electric current is perpendicular to the electric current's direction of flow.Additionally, a magnetic field can induce an electric current. This can happen when a wire is moved across a magnetic field, or when a magnetic field is moved near a conductive wire. Because magnetic fields can produce electric fields and electric fields can produce magnetic fields, we can understand electricity and magnetism as parts of one phenomenon: electromagnetism.Q. Which of the following would be an example of electricity according to Scientist 2, but not according to Scientist 1?- a)A wire conducts electrons from the negative terminal of a battery to the positive terminal.
- b)Current flows along a wire between a negatively-charged object and an positively charged-object.
- c)Two negatively-charged objects repel each other.
- d)A positively-charged object is attracted to a negatively-charged object and receives excess electrons from it.
Correct answer is option 'C'. Can you explain this answer?
Magnets and electric charges show certain similarities. For example, both magnets and electric charges can exert a force on their surroundings. This force, when produced by a magnet, is called a magnetic field. When it is produced by an electric charge, the force is called an electric field. It has been observed that the strength of both magnetic fields and electric fields is inversely proportional to the square of the distance between a magnet or an electric charge and the objects that they affect.
Below, three scientists debate the relationship between electricity and magnetism.
Scientist 1:
Electricity and magnetism are two different phenomena. Materials such as iron, cobalt, and nickel contain magnetic domains: tiny regions of magnetism, each with two poles. Normally, the domains have a random orientation and are not aligned, so the magnetism of some domains cancels out that of other domains; however, in magnets, domains line up in the same direction, creating the two poles of the magnet and causing magnetic behavior.
In contrast, electricity is a moving electric charge which is caused by the flow of electrons through a material. Electrons flow through a material from a region of higher potential (more negative charge) to a region of lower potential (more positive charge). We can measure this flow of electrons as current, which refers to the amount of charge transferred over a period of time.
Scientist 2:
Electricity and magnetism are similar phenomena; however, one cannot be reduced to the other. Electricity involves two types of charges: positive and negative charge. Though electricity can occur in a moving form (in the form of current, or an electric charge moving through a wire), it can also occur in a static form. Static electricity involves no moving charge. Instead, objects can have a net excess of positive charge or a net excess of negative charge—because of having lost or gained electrons, respectively. When two static positive electric charges or two static negative electric charges are brought close together, they repel each other. However, when a positive and a negative static charge are brought together, they attract each other.
Similarly, all magnets have two poles. Magnetic poles that are alike repel each other, while dissimilar magnetic poles attract each other. Magnets and static electric charges are alike in that they both show attraction and repulsion in similar circumstances. However, while isolated static electric charges occur in nature, there are no single, isolated magnetic poles. All magnets have two poles, which cannot be dissociated from each other.
Scientist 3:
Electricity and magnetism are two aspects of the same phenomenon. A moving flow of electrons creates a magnetic field around it. Thus, wherever an electric current exists, a magnetic field will also exist. The magnetic field created by an electric current is perpendicular to the electric current's direction of flow.
Additionally, a magnetic field can induce an electric current. This can happen when a wire is moved across a magnetic field, or when a magnetic field is moved near a conductive wire. Because magnetic fields can produce electric fields and electric fields can produce magnetic fields, we can understand electricity and magnetism as parts of one phenomenon: electromagnetism.
Q. Which of the following would be an example of electricity according to Scientist 2, but not according to Scientist 1?
a)
A wire conducts electrons from the negative terminal of a battery to the positive terminal.
b)
Current flows along a wire between a negatively-charged object and an positively charged-object.
c)
Two negatively-charged objects repel each other.
d)
A positively-charged object is attracted to a negatively-charged object and receives excess electrons from it.

|
Orion Classes answered |
According to Scientist 2, electricity can take on two forms: static electricity and current electricity. Scientist 2 states that while current electricity consists of a moving electric charge, static electricity involves no moving charge. Scientist 2 also states that static electricity can cause two objects to repel or attract each other. In contrast, Scientist 1 defines electricity as a moving charge—he states that electricity must involve the flow of electrons.
So, a situation where there is no flow of electrons—where two objects repel each other due to static electricity—would be seen as an example of electricity by Scientist 2, but not by Scientist 1.
Magnets and electric charges show certain similarities. For example, both magnets and electric charges can exert a force on their surroundings. This force, when produced by a magnet, is called a magnetic field. When it is produced by an electric charge, the force is called an electric field. It has been observed that the strength of both magnetic fields and electric fields is inversely proportional to the square of the distance between a magnet or an electric charge and the objects that they affect.Below, three scientists debate the relationship between electricity and magnetism.Scientist 1:Electricity and magnetism are two different phenomena. Materials such as iron, cobalt, and nickel contain magnetic domains: tiny regions of magnetism, each with two poles. Normally, the domains have a random orientation and are not aligned, so the magnetism of some domains cancels out that of other domains; however, in magnets, domains line up in the same direction, creating the two poles of the magnet and causing magnetic behavior.In contrast, electricity is a moving electric charge which is caused by the flow of electrons through a material. Electrons flow through a material from a region of higher potential (more negative charge) to a region of lower potential (more positive charge). We can measure this flow of electrons as current, which refers to the amount of charge transferred over a period of time.Scientist 2:Electricity and magnetism are similar phenomena; however, one cannot be reduced to the other. Electricity involves two types of charges: positive and negative charge. Though electricity can occur in a moving form (in the form of current, or an electric charge moving through a wire), it can also occur in a static form. Static electricity involves no moving charge. Instead, objects can have a net excess of positive charge or a net excess of negative charge—because of having lost or gained electrons, respectively. When two static positive electric charges or two static negative electric charges are brought close together, they repel each other. However, when a positive and a negative static charge are brought together, they attract each other.Similarly, all magnets have two poles. Magnetic poles that are alike repel each other, while dissimilar magnetic poles attract each other. Magnets and static electric charges are alike in that they both show attraction and repulsion in similar circumstances. However, while isolated static electric charges occur in nature, there are no single, isolated magnetic poles. All magnets have two poles, which cannot be dissociated from each other.Scientist 3:Electricity and magnetism are two aspects of the same phenomenon. A moving flow of electrons creates a magnetic field around it. Thus, wherever an electric current exists, a magnetic field will also exist. The magnetic field created by an electric current is perpendicular to the electric current's direction of flow.Additionally, a magnetic field can induce an electric current. This can happen when a wire is moved across a magnetic field, or when a magnetic field is moved near a conductive wire. Because magnetic fields can produce electric fields and electric fields can produce magnetic fields, we can understand electricity and magnetism as parts of one phenomenon: electromagnetism.Q. In a compass, a needle spins to align North-South, following the Earth's magnetic field. Suppose that a compass is placed near wire through which an electric current flows, and it is observed that the needle of the compass no longer aligns to North-South. How would this affect the arguments of Scientist 2 and Scientist 3?- a)It would strengthen Scientist 2's argument, and it would strengthen Scientist 3's argument.
- b)It would strengthen Scientist 2's argument, and it would weaken Scientist 3's argument.
- c)It would have no effect on Scientist 2's argument, and it would strengthen Scientist 3's argument.
- d)It would weaken Scientist 2's argument, and it would weaken Scientist 3's argument.
Correct answer is option 'C'. Can you explain this answer?
Magnets and electric charges show certain similarities. For example, both magnets and electric charges can exert a force on their surroundings. This force, when produced by a magnet, is called a magnetic field. When it is produced by an electric charge, the force is called an electric field. It has been observed that the strength of both magnetic fields and electric fields is inversely proportional to the square of the distance between a magnet or an electric charge and the objects that they affect.
Below, three scientists debate the relationship between electricity and magnetism.
Scientist 1:
Electricity and magnetism are two different phenomena. Materials such as iron, cobalt, and nickel contain magnetic domains: tiny regions of magnetism, each with two poles. Normally, the domains have a random orientation and are not aligned, so the magnetism of some domains cancels out that of other domains; however, in magnets, domains line up in the same direction, creating the two poles of the magnet and causing magnetic behavior.
In contrast, electricity is a moving electric charge which is caused by the flow of electrons through a material. Electrons flow through a material from a region of higher potential (more negative charge) to a region of lower potential (more positive charge). We can measure this flow of electrons as current, which refers to the amount of charge transferred over a period of time.
Scientist 2:
Electricity and magnetism are similar phenomena; however, one cannot be reduced to the other. Electricity involves two types of charges: positive and negative charge. Though electricity can occur in a moving form (in the form of current, or an electric charge moving through a wire), it can also occur in a static form. Static electricity involves no moving charge. Instead, objects can have a net excess of positive charge or a net excess of negative charge—because of having lost or gained electrons, respectively. When two static positive electric charges or two static negative electric charges are brought close together, they repel each other. However, when a positive and a negative static charge are brought together, they attract each other.
Similarly, all magnets have two poles. Magnetic poles that are alike repel each other, while dissimilar magnetic poles attract each other. Magnets and static electric charges are alike in that they both show attraction and repulsion in similar circumstances. However, while isolated static electric charges occur in nature, there are no single, isolated magnetic poles. All magnets have two poles, which cannot be dissociated from each other.
Scientist 3:
Electricity and magnetism are two aspects of the same phenomenon. A moving flow of electrons creates a magnetic field around it. Thus, wherever an electric current exists, a magnetic field will also exist. The magnetic field created by an electric current is perpendicular to the electric current's direction of flow.
Additionally, a magnetic field can induce an electric current. This can happen when a wire is moved across a magnetic field, or when a magnetic field is moved near a conductive wire. Because magnetic fields can produce electric fields and electric fields can produce magnetic fields, we can understand electricity and magnetism as parts of one phenomenon: electromagnetism.
Q. In a compass, a needle spins to align North-South, following the Earth's magnetic field. Suppose that a compass is placed near wire through which an electric current flows, and it is observed that the needle of the compass no longer aligns to North-South. How would this affect the arguments of Scientist 2 and Scientist 3?
a)
It would strengthen Scientist 2's argument, and it would strengthen Scientist 3's argument.
b)
It would strengthen Scientist 2's argument, and it would weaken Scientist 3's argument.
c)
It would have no effect on Scientist 2's argument, and it would strengthen Scientist 3's argument.
d)
It would weaken Scientist 2's argument, and it would weaken Scientist 3's argument.
|
|
Ayesha Joshi answered |
Here, since the (magnetic) compass no longer aligns to North-South when it is near the wire, this implies that there is some kind of magnetic field near the wire which is interfering with the compass. This supports what Scientist 3 says in the first paragraph of her explanation: that an electric current can induce a magnetic field around it.
Scientist 2, however, makes no mention of this kind of electromagnetic induction in his explanation; however, he also does not say that it is not possible. His explanation is mostly about how magnetic poles are similar to and different from static electric charges. So, his argument is not affected by the observation that an electric current induces a magnetic field.
In a physics class, students conducted a series of experiments by placing different objects into a beaker of water. They conducted twenty trials for each object. For each trial, they recorded whether or not the object floated.First, they placed a steel paper clip into the water. They observed that the paper clip usually sank; however, they also saw that occasionally, the paper clip stayed afloat if it was placed very gently on top of the water. Next, they repeated the the same procedure using a cork, a toy boat made of aluminum, and a glass marble. They observed that both the cork and the toy boat always stayed afloat in the water, but that the glass marble always sank.Below, three students give their explanations for these observations.Student 1:Objects float when they are less dense than the liquid in which they are immersed. For example, when immiscible liquids of varying densities are mixed together in a container, the most dense liquid will sink to the bottom of the container, while the least dense liquid will rise to the top. This same principle applies to solid objects. Because the cork and the aluminum toy boat always float, cork and the aluminum of the boat must be less dense than water. Because the glass marble always sinks, the glass of the marble must be more dense than water.Objects that are more dense than water can also float due to surface tension. Surface tension occurs because molecules of a liquid are more attracted to each other more than they are to other objects. Molecules on the surface of water are attracted to the molecules around them and below them. This attraction causes a liquid's surface to behave if it were covered by a thin film, which resists penetration by other objects. Therefore, small objects such as paper clips can sometimes float on water when the upward force of water's surface tension exceeds the force of gravity pulling such objects down. Because the paper clips often sink and only float sometimes, we can conclude that they are indeed more dense than water, and that their floating is due to surface tension.Student 2:Objects float in two different cases: when they are buoyed by a liquid's surface tension or when their average density is less than that of the liquid in which they are immersed. The average density of cork is less than that of water. This is why the cork floats. In contrast, the density of glass is more than that of water. This is why the glass marble sinks.However, the densities of aluminum and of steel are greater than that of water. Thus, density cannot be used to explain why the aluminum toy boat and the paper clip float. Both of these objects float because of surface tension. Because the paper clip does not have much mass, the normal upward force created by water's surface tension can be enough to allow it to float. Other objects with greater mass, like the toy boat, employ a particular shape to magnify the force of surface tension. The curved shape of the boat's bottom both stabilizes the boat and increases the amount of the boat's surface area that touches the water, maximizing the force due to surface tension that the boat receives.Student 3:In this experiment, the paper clip floats because of surface tension; however, the cork, toy boat, and marble float or sink because of their relationship to a buoyant force. All objects immersed in a liquid experience a buoyant force, which pushes them upward. The strength of this force is equal to the weight of the liquid displaced, or pushed aside, by an object. Every object also experiences a downward force due to gravity, which is measured as the object's weight, and which is directly proportional to the object's mass. When the buoyant force acting on an object is greater than the downward force due to gravity, the object floats. However, when the buoyant force is less than the force due to gravity, the object sinks. Both the cork and the aluminum toy boat are able to displace enough water to create a buoyant force that exceeds the force due to gravity, so they float. However, the glass marble does not displace enough water to create a sufficient buoyant force, so it sinks.Q. The density of fresh, newly cut wood is less than water, and fresh wood always floats; however, over time, floating pieces of wood may sink. Which of the following explanations would Student 1 most likely give for this observation?- a)As time passes, water saturates pieces of wood, increasing their density until it exceeds the density of water.
- b)Pieces of wood float due to water's surface tension; however, when this surface tension breaks, they sink.
- c)As wood is broken down by decomposition, gases accumulate within the wood, decreasing its density.
- d)In cold weather, the density of water increases, which causes wood to sink.
Correct answer is option 'A'. Can you explain this answer?
In a physics class, students conducted a series of experiments by placing different objects into a beaker of water. They conducted twenty trials for each object. For each trial, they recorded whether or not the object floated.
First, they placed a steel paper clip into the water. They observed that the paper clip usually sank; however, they also saw that occasionally, the paper clip stayed afloat if it was placed very gently on top of the water. Next, they repeated the the same procedure using a cork, a toy boat made of aluminum, and a glass marble. They observed that both the cork and the toy boat always stayed afloat in the water, but that the glass marble always sank.
Below, three students give their explanations for these observations.
Student 1:
Objects float when they are less dense than the liquid in which they are immersed. For example, when immiscible liquids of varying densities are mixed together in a container, the most dense liquid will sink to the bottom of the container, while the least dense liquid will rise to the top. This same principle applies to solid objects. Because the cork and the aluminum toy boat always float, cork and the aluminum of the boat must be less dense than water. Because the glass marble always sinks, the glass of the marble must be more dense than water.
Objects that are more dense than water can also float due to surface tension. Surface tension occurs because molecules of a liquid are more attracted to each other more than they are to other objects. Molecules on the surface of water are attracted to the molecules around them and below them. This attraction causes a liquid's surface to behave if it were covered by a thin film, which resists penetration by other objects. Therefore, small objects such as paper clips can sometimes float on water when the upward force of water's surface tension exceeds the force of gravity pulling such objects down. Because the paper clips often sink and only float sometimes, we can conclude that they are indeed more dense than water, and that their floating is due to surface tension.
Student 2:
Objects float in two different cases: when they are buoyed by a liquid's surface tension or when their average density is less than that of the liquid in which they are immersed. The average density of cork is less than that of water. This is why the cork floats. In contrast, the density of glass is more than that of water. This is why the glass marble sinks.
However, the densities of aluminum and of steel are greater than that of water. Thus, density cannot be used to explain why the aluminum toy boat and the paper clip float. Both of these objects float because of surface tension. Because the paper clip does not have much mass, the normal upward force created by water's surface tension can be enough to allow it to float. Other objects with greater mass, like the toy boat, employ a particular shape to magnify the force of surface tension. The curved shape of the boat's bottom both stabilizes the boat and increases the amount of the boat's surface area that touches the water, maximizing the force due to surface tension that the boat receives.
Student 3:
In this experiment, the paper clip floats because of surface tension; however, the cork, toy boat, and marble float or sink because of their relationship to a buoyant force. All objects immersed in a liquid experience a buoyant force, which pushes them upward. The strength of this force is equal to the weight of the liquid displaced, or pushed aside, by an object. Every object also experiences a downward force due to gravity, which is measured as the object's weight, and which is directly proportional to the object's mass. When the buoyant force acting on an object is greater than the downward force due to gravity, the object floats. However, when the buoyant force is less than the force due to gravity, the object sinks. Both the cork and the aluminum toy boat are able to displace enough water to create a buoyant force that exceeds the force due to gravity, so they float. However, the glass marble does not displace enough water to create a sufficient buoyant force, so it sinks.
Q. The density of fresh, newly cut wood is less than water, and fresh wood always floats; however, over time, floating pieces of wood may sink. Which of the following explanations would Student 1 most likely give for this observation?
a)
As time passes, water saturates pieces of wood, increasing their density until it exceeds the density of water.
b)
Pieces of wood float due to water's surface tension; however, when this surface tension breaks, they sink.
c)
As wood is broken down by decomposition, gases accumulate within the wood, decreasing its density.
d)
In cold weather, the density of water increases, which causes wood to sink.
|
|
Ayesha Joshi answered |
Student 1 says that objects can float due to surface tension, or because they are less dense than water; however, Student 1 also says that floating due to surface tension happens in small objects that are more dense than water. Since this question tells us that fresh wood is less dense than water and always floats, the reason why it floats must be because of the low density of the wood. If a piece of wood then sinks, the density of the wood must have changed to become greater than the density of water.
In a physics class, students conducted a series of experiments by placing different objects into a beaker of water. They conducted twenty trials for each object. For each trial, they recorded whether or not the object floated.First, they placed a steel paper clip into the water. They observed that the paper clip usually sank; however, they also saw that occasionally, the paper clip stayed afloat if it was placed very gently on top of the water. Next, they repeated the the same procedure using a cork, a toy boat made of aluminum, and a glass marble. They observed that both the cork and the toy boat always stayed afloat in the water, but that the glass marble always sank.Below, three students give their explanations for these observations.Student 1:Objects float when they are less dense than the liquid in which they are immersed. For example, when immiscible liquids of varying densities are mixed together in a container, the most dense liquid will sink to the bottom of the container, while the least dense liquid will rise to the top. This same principle applies to solid objects. Because the cork and the aluminum toy boat always float, cork and the aluminum of the boat must be less dense than water. Because the glass marble always sinks, the glass of the marble must be more dense than water.Objects that are more dense than water can also float due to surface tension. Surface tension occurs because molecules of a liquid are more attracted to each other more than they are to other objects. Molecules on the surface of water are attracted to the molecules around them and below them. This attraction causes a liquid's surface to behave if it were covered by a thin film, which resists penetration by other objects. Therefore, small objects such as paper clips can sometimes float on water when the upward force of water's surface tension exceeds the force of gravity pulling such objects down. Because the paper clips often sink and only float sometimes, we can conclude that they are indeed more dense than water, and that their floating is due to surface tension.Student 2:Objects float in two different cases: when they are buoyed by a liquid's surface tension or when their average density is less than that of the liquid in which they are immersed. The average density of cork is less than that of water. This is why the cork floats. In contrast, the density of glass is more than that of water. This is why the glass marble sinks.However, the densities of aluminum and of steel are greater than that of water. Thus, density cannot be used to explain why the aluminum toy boat and the paper clip float. Both of these objects float because of surface tension. Because the paper clip does not have much mass, the normal upward force created by water's surface tension can be enough to allow it to float. Other objects with greater mass, like the toy boat, employ a particular shape to magnify the force of surface tension. The curved shape of the boat's bottom both stabilizes the boat and increases the amount of the boat's surface area that touches the water, maximizing the force due to surface tension that the boat receives.Student 3:In this experiment, the paper clip floats because of surface tension; however, the cork, toy boat, and marble float or sink because of their relationship to a buoyant force. All objects immersed in a liquid experience a buoyant force, which pushes them upward. The strength of this force is equal to the weight of the liquid displaced, or pushed aside, by an object. Every object also experiences a downward force due to gravity, which is measured as the object's weight, and which is directly proportional to the object's mass. When the buoyant force acting on an object is greater than the downward force due to gravity, the object floats. However, when the buoyant force is less than the force due to gravity, the object sinks. Both the cork and the aluminum toy boat are able to displace enough water to create a buoyant force that exceeds the force due to gravity, so they float. However, the glass marble does not displace enough water to create a sufficient buoyant force, so it sinks.Q. Paint is more dense than cooking oil; however, when a drop of paint is dripped into a container of cooking oil, it floats on top of the oil. If Student 1's explanation is correct, which of the following is most likely the reason for this observation?- a)The buoyant force on the drop of paint is less than the force due to gravity.
- b)The force on the drop of paint due to surface tension is greater than the force due to gravity.
- c)The force on the drop of paint due to surface tension is less than the buoyant force.
- d)The force on the drop of paint due to surface tension is less than the force due to gravity.
Correct answer is option 'B'. Can you explain this answer?
In a physics class, students conducted a series of experiments by placing different objects into a beaker of water. They conducted twenty trials for each object. For each trial, they recorded whether or not the object floated.
First, they placed a steel paper clip into the water. They observed that the paper clip usually sank; however, they also saw that occasionally, the paper clip stayed afloat if it was placed very gently on top of the water. Next, they repeated the the same procedure using a cork, a toy boat made of aluminum, and a glass marble. They observed that both the cork and the toy boat always stayed afloat in the water, but that the glass marble always sank.
Below, three students give their explanations for these observations.
Student 1:
Objects float when they are less dense than the liquid in which they are immersed. For example, when immiscible liquids of varying densities are mixed together in a container, the most dense liquid will sink to the bottom of the container, while the least dense liquid will rise to the top. This same principle applies to solid objects. Because the cork and the aluminum toy boat always float, cork and the aluminum of the boat must be less dense than water. Because the glass marble always sinks, the glass of the marble must be more dense than water.
Objects that are more dense than water can also float due to surface tension. Surface tension occurs because molecules of a liquid are more attracted to each other more than they are to other objects. Molecules on the surface of water are attracted to the molecules around them and below them. This attraction causes a liquid's surface to behave if it were covered by a thin film, which resists penetration by other objects. Therefore, small objects such as paper clips can sometimes float on water when the upward force of water's surface tension exceeds the force of gravity pulling such objects down. Because the paper clips often sink and only float sometimes, we can conclude that they are indeed more dense than water, and that their floating is due to surface tension.
Student 2:
Objects float in two different cases: when they are buoyed by a liquid's surface tension or when their average density is less than that of the liquid in which they are immersed. The average density of cork is less than that of water. This is why the cork floats. In contrast, the density of glass is more than that of water. This is why the glass marble sinks.
However, the densities of aluminum and of steel are greater than that of water. Thus, density cannot be used to explain why the aluminum toy boat and the paper clip float. Both of these objects float because of surface tension. Because the paper clip does not have much mass, the normal upward force created by water's surface tension can be enough to allow it to float. Other objects with greater mass, like the toy boat, employ a particular shape to magnify the force of surface tension. The curved shape of the boat's bottom both stabilizes the boat and increases the amount of the boat's surface area that touches the water, maximizing the force due to surface tension that the boat receives.
Student 3:
In this experiment, the paper clip floats because of surface tension; however, the cork, toy boat, and marble float or sink because of their relationship to a buoyant force. All objects immersed in a liquid experience a buoyant force, which pushes them upward. The strength of this force is equal to the weight of the liquid displaced, or pushed aside, by an object. Every object also experiences a downward force due to gravity, which is measured as the object's weight, and which is directly proportional to the object's mass. When the buoyant force acting on an object is greater than the downward force due to gravity, the object floats. However, when the buoyant force is less than the force due to gravity, the object sinks. Both the cork and the aluminum toy boat are able to displace enough water to create a buoyant force that exceeds the force due to gravity, so they float. However, the glass marble does not displace enough water to create a sufficient buoyant force, so it sinks.
Q. Paint is more dense than cooking oil; however, when a drop of paint is dripped into a container of cooking oil, it floats on top of the oil. If Student 1's explanation is correct, which of the following is most likely the reason for this observation?
a)
The buoyant force on the drop of paint is less than the force due to gravity.
b)
The force on the drop of paint due to surface tension is greater than the force due to gravity.
c)
The force on the drop of paint due to surface tension is less than the buoyant force.
d)
The force on the drop of paint due to surface tension is less than the force due to gravity.
|
|
Ayesha Joshi answered |
Student 1 says that objects may either float because they are less dense than water or because they rest on top of water due to the water's surface tension. Since we know that the drop of paint is more dense than water, it must float because of surface tension. According to Student 1, when something floats due to surface tension, the upward force from surface tension exceeds the downward force that gravity exerts on the drop of paint.
Two students are studying hydrocarbon combustion, or the burning of compounds containing carbon and hydrogen in the presence of oxygen gas. Both students express their views on this phenomenon. Student 1: Hydrocarbons are high in energy and therefore naturally burn in order to release that energy. That energy is released in the form of light and heat. If water is thrown onto a fire, it will extinguish it because it cuts the combustion from the oxygen gas required for it to burn.Student 2: Hydrocarbons are compounds at a greater energy state than the compounds produced when they burn. This excess energy changes to heat when hydrocarbons burn. Lastly, hydrocarbons require a spark to initiate the combustion. Q. Which of the following statements would both students be most likely to agree?- a)Hydrocarbons produce light when they burn.
- b)The products of hydrocarbons do not burn.
- c)Hydrocarbons are high energy compounds.
- d)The products of combustion are low in energy.
Correct answer is option 'C'. Can you explain this answer?
Two students are studying hydrocarbon combustion, or the burning of compounds containing carbon and hydrogen in the presence of oxygen gas. Both students express their views on this phenomenon.
Student 1: Hydrocarbons are high in energy and therefore naturally burn in order to release that energy. That energy is released in the form of light and heat. If water is thrown onto a fire, it will extinguish it because it cuts the combustion from the oxygen gas required for it to burn.
Student 2: Hydrocarbons are compounds at a greater energy state than the compounds produced when they burn. This excess energy changes to heat when hydrocarbons burn. Lastly, hydrocarbons require a spark to initiate the combustion.
Q. Which of the following statements would both students be most likely to agree?
a)
Hydrocarbons produce light when they burn.
b)
The products of hydrocarbons do not burn.
c)
Hydrocarbons are high energy compounds.
d)
The products of combustion are low in energy.
|
|
Ayesha Joshi answered |
The correct answer is that hydrocarbons are high energy compounds. The other answers are not statements explicitly expressed by both students.
In the 17th century, scientists were just beginning to understand the circulatory system of the heart. The two following viewpoints are the two most popular theories of the day.Scientist I The heart pumps blood through arteries and veins but the two systems are separate. They are similar, just as the senses of smell and taste are when observing food, but ultimately they are two separate systems which perform separate functions. Hot blood flows from the heart, through the arteries, and to the organs which consume the blood much as a human would consume nourishment to survive. Venous blood originates in the liver and follows the veins to the organs where it is similarly consumed.Scientist II The arteries and veins are two parts of one system. Blood flows from the heart, around the body, and back into the heart through the veins like two sets of one way streets. The idea of two systems, each pumping blood to the organs is unreasonable. If the heart can pump 6 oz of blood per minute, then the liver would have to produce 540 pounds of blood per day. A simple measurement of a human’s weight shows how unlikely that solution is. The single circulatory system is far superior as it explains the function of the heart, the arteries, and the veins clearly.Q. Why does Scientist I compare the arteries and veins to smell and taste?- a)The senses of taste and smell are the strongest.
- b)A person with liver issues will produce blood that tastes and smells different.
- c)To illustrate another example of systems that are linked but are not the same.
- d)Because blood has a very distinct odor and taste.
Correct answer is option 'C'. Can you explain this answer?
In the 17th century, scientists were just beginning to understand the circulatory system of the heart. The two following viewpoints are the two most popular theories of the day.
Scientist I The heart pumps blood through arteries and veins but the two systems are separate. They are similar, just as the senses of smell and taste are when observing food, but ultimately they are two separate systems which perform separate functions. Hot blood flows from the heart, through the arteries, and to the organs which consume the blood much as a human would consume nourishment to survive. Venous blood originates in the liver and follows the veins to the organs where it is similarly consumed.
Scientist II The arteries and veins are two parts of one system. Blood flows from the heart, around the body, and back into the heart through the veins like two sets of one way streets. The idea of two systems, each pumping blood to the organs is unreasonable. If the heart can pump 6 oz of blood per minute, then the liver would have to produce 540 pounds of blood per day. A simple measurement of a human’s weight shows how unlikely that solution is. The single circulatory system is far superior as it explains the function of the heart, the arteries, and the veins clearly.
Q. Why does Scientist I compare the arteries and veins to smell and taste?
a)
The senses of taste and smell are the strongest.
b)
A person with liver issues will produce blood that tastes and smells different.
c)
To illustrate another example of systems that are linked but are not the same.
d)
Because blood has a very distinct odor and taste.

|
Orion Classes answered |
The scientist is trying to make a comparison that the reader will already have experience with, as he is suggesting that the two systems are similar and yet different.
Genes are hereditary units that are responsible for the phenotypes of an organism. Genes are the directions for the body. Genetic change exists when genes are altered from their previous form. Genes are made up of DNA, or deoxyribonucleic acid. DNA is made up of four bases- adenine, guanine, cytosine, and thymine. Genetic change can result from a variety of factors. Both scientists mentioned below agree on this basic information about genes. However, the scientists do not agree on the primary driving force behind genetic change.Scientist 1A mutation is a permanent change in the sequence of the DNA of a gene. There are several types of mutations—point mutations, silent mutations, frame mutations, and nonsense mutations. Mutations are very important because proteins are synthesized by reading the DNA sequence. If the DNA sequence is changed, the proteins transcribed from the DNA will be different proteins. Mutations directly and substantially change the genes by changing the sequence of the four bases. Therefore, mutations are the main factor when looking at genetic change.Scientist 2 Sexual reproduction is the biggest contributor to genetic change. New combinations of genes are created with every random union of a sperm and egg. During division of the sex cells, or meiosis, crossing over can occur. Crossing over describes the situation when the genes from one parent’s chromosome are traded with genes from the other parent’s chromosome. This results in new combinations of genes. Lastly, a phenomenon called independent assortment results from sexual reproduction. Independent assortment is the random assortment of chromosomes during reproduction. Therefore, by its random nature, sexual reproduction is the largest contributor to genetic change.Q. The viewpoints of Scientist 1 and Scientist 2 both support what theory? - a)Sexual reproduction results in genetic change.
- b)Mutations are the biggest contributor to genetic change.
- c)Mutations during crossing over are the biggest contributor to genetic change.
- d)Genetic change is the variation of genes from one generation to another.
Correct answer is option 'D'. Can you explain this answer?
Genes are hereditary units that are responsible for the phenotypes of an organism. Genes are the directions for the body. Genetic change exists when genes are altered from their previous form. Genes are made up of DNA, or deoxyribonucleic acid. DNA is made up of four bases- adenine, guanine, cytosine, and thymine. Genetic change can result from a variety of factors. Both scientists mentioned below agree on this basic information about genes. However, the scientists do not agree on the primary driving force behind genetic change.
Scientist 1
A mutation is a permanent change in the sequence of the DNA of a gene. There are several types of mutations—point mutations, silent mutations, frame mutations, and nonsense mutations. Mutations are very important because proteins are synthesized by reading the DNA sequence. If the DNA sequence is changed, the proteins transcribed from the DNA will be different proteins. Mutations directly and substantially change the genes by changing the sequence of the four bases. Therefore, mutations are the main factor when looking at genetic change.
Scientist 2
Sexual reproduction is the biggest contributor to genetic change. New combinations of genes are created with every random union of a sperm and egg. During division of the sex cells, or meiosis, crossing over can occur. Crossing over describes the situation when the genes from one parent’s chromosome are traded with genes from the other parent’s chromosome. This results in new combinations of genes. Lastly, a phenomenon called independent assortment results from sexual reproduction. Independent assortment is the random assortment of chromosomes during reproduction. Therefore, by its random nature, sexual reproduction is the largest contributor to genetic change.
Q. The viewpoints of Scientist 1 and Scientist 2 both support what theory?
a)
Sexual reproduction results in genetic change.
b)
Mutations are the biggest contributor to genetic change.
c)
Mutations during crossing over are the biggest contributor to genetic change.
d)
Genetic change is the variation of genes from one generation to another.

|
Orion Classes answered |
The first paragraph discusses genetic change and describes it as the variation of genes from one generation to another. It goes on to say both scientists "agree on this basic information about genes."
Before modern technologies and experiments allowed scientists to understand different organisms' mechanisms of reproduction, numerous theories existed about how populations came to exist. Two scientists from the 1800s describe their theories. Here are their arguments.Scientist IJust like some plants come from seeds and others are capable of vegetative (asexual) reproduction, some animal organisms come from non-sexual reproduction as well. Maggots, for example, appear on rotting carcasses. It is clearly illogical to suggest that the dead animal created or gave birth to the maggots, as it is no longer alive and is therefore incapable of sexual reproduction. The only rational conclusion for the appearance of maggots is a spontaneous generation. This is similar to how, if one were to leave a bowl of broth in the open air for a week, it would turn cloudy from bacteria appearing in the liquid.Scientist IIAnimate objects cannot arise from inanimate objects. Even when plants perform asexual reproduction, daughter plants are still coming from parent plants. There is no other example in nature of a living organism spontaneously coming into being. It is true that we observe maggots on rotting carcasses, but that does not necessarily mean that the maggots came from the rotting carcass. Similarly, bacteria growing in broth do not necessarily come directly from the broth. If a living organism appears, then it must have come from another animate object, even if we did not witness it. It is more likely that these invisible organisms have come in through the air and we simply do not see them until they have had a chance to replicate in these locations.Q. An experiment is performed in which a rotting carcass is vacuum sealed. After several weeks, no maggots are observed. The rotting carcass is removed from the vacuum seal, and several days later maggots are observed. Which theory does this best support?- a)Scientist II
- b)Neither theory is supported
- c)Both theories are supported
- d)Scientist I
Correct answer is option 'A'. Can you explain this answer?
Before modern technologies and experiments allowed scientists to understand different organisms' mechanisms of reproduction, numerous theories existed about how populations came to exist. Two scientists from the 1800s describe their theories. Here are their arguments.
Scientist I
Just like some plants come from seeds and others are capable of vegetative (asexual) reproduction, some animal organisms come from non-sexual reproduction as well. Maggots, for example, appear on rotting carcasses. It is clearly illogical to suggest that the dead animal created or gave birth to the maggots, as it is no longer alive and is therefore incapable of sexual reproduction. The only rational conclusion for the appearance of maggots is a spontaneous generation. This is similar to how, if one were to leave a bowl of broth in the open air for a week, it would turn cloudy from bacteria appearing in the liquid.
Scientist II
Animate objects cannot arise from inanimate objects. Even when plants perform asexual reproduction, daughter plants are still coming from parent plants. There is no other example in nature of a living organism spontaneously coming into being. It is true that we observe maggots on rotting carcasses, but that does not necessarily mean that the maggots came from the rotting carcass. Similarly, bacteria growing in broth do not necessarily come directly from the broth. If a living organism appears, then it must have come from another animate object, even if we did not witness it. It is more likely that these invisible organisms have come in through the air and we simply do not see them until they have had a chance to replicate in these locations.
Q. An experiment is performed in which a rotting carcass is vacuum sealed. After several weeks, no maggots are observed. The rotting carcass is removed from the vacuum seal, and several days later maggots are observed. Which theory does this best support?
a)
Scientist II
b)
Neither theory is supported
c)
Both theories are supported
d)
Scientist I

|
Orion Classes answered |
Scientist II would use this as proof that spontaneous generation cannot exist; otherwise maggots would have developed inside of the vacuum seal. Scientist I's theory would not work because the maggots did not appear until after the carcass was removed from the seal.
There are two types of forces that occur with all substances on Earth. Intramolecular forces occur between atoms in a molecule, while intermolecular forces occur between neighboring molecules. Intermolecular forces can be dipole-dipole forces, hydrogen bonding, or London dispersion forces.Professor 1:Water molecules represent an example of hydrogen bonding due to the attraction between the hydrogen atoms and the oxygen atoms in the molecule. This strong dipole-dipole occurs due to lone pairs present on such atoms as Fluorine, Nitrogen, and Oxygen, which are able to pair more closely to the hydrogen atom in another nearby molecule. Water can be present in a solid, liquid, or gaseous state on Earth depending on the competition between the strength of intermolecular bonds and the thermal energy of the system. In 1873, a Dutch scientist, Van der Waals derived an equation that included both the force of attraction between the particles of a gas and the volume of the particles at high pressures. This equation led to a better fit for experimental data than the Ideal Gas Law.Professor 2: Water is the only substance on Earth that we routinely encounter as a solid, liquid, and gas. At low temperatures, the water molecules lock into a rigid structure, but as the temperature increases, the average kinetic energy of the water molecules increases and the molecules are able to move more creating its other natural states of matter. The higher the temperature, the more likely water is to be a gas. Water is proof of the kinetic theory, which assumes that there is no force of attraction between the particles of the gas state. The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law, since there is no interaction between the gaseous molecules. This law accounts for all of the forces that occur with gases on Earth.Q. Which of these statements made by professor 2 is not contradicted by professor 1?- a)The best fit for experimental data involving water in a gaseous state is found by using the Ideal Gas Law.
- b)As temperature increases, the average kinetic energy of the water molecules increases.
- c)The Ideal Gas Law accounts for all of the forces that occur with gases.
- d)There is no force of attraction between water's molecules in the gaseous state.
Correct answer is option 'B'. Can you explain this answer?
There are two types of forces that occur with all substances on Earth. Intramolecular forces occur between atoms in a molecule, while intermolecular forces occur between neighboring molecules. Intermolecular forces can be dipole-dipole forces, hydrogen bonding, or London dispersion forces.
Professor 1:
Water molecules represent an example of hydrogen bonding due to the attraction between the hydrogen atoms and the oxygen atoms in the molecule. This strong dipole-dipole occurs due to lone pairs present on such atoms as Fluorine, Nitrogen, and Oxygen, which are able to pair more closely to the hydrogen atom in another nearby molecule. Water can be present in a solid, liquid, or gaseous state on Earth depending on the competition between the strength of intermolecular bonds and the thermal energy of the system. In 1873, a Dutch scientist, Van der Waals derived an equation that included both the force of attraction between the particles of a gas and the volume of the particles at high pressures. This equation led to a better fit for experimental data than the Ideal Gas Law.
Professor 2:
Water is the only substance on Earth that we routinely encounter as a solid, liquid, and gas. At low temperatures, the water molecules lock into a rigid structure, but as the temperature increases, the average kinetic energy of the water molecules increases and the molecules are able to move more creating its other natural states of matter. The higher the temperature, the more likely water is to be a gas. Water is proof of the kinetic theory, which assumes that there is no force of attraction between the particles of the gas state. The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law, since there is no interaction between the gaseous molecules. This law accounts for all of the forces that occur with gases on Earth.
Q. Which of these statements made by professor 2 is not contradicted by professor 1?
a)
The best fit for experimental data involving water in a gaseous state is found by using the Ideal Gas Law.
b)
As temperature increases, the average kinetic energy of the water molecules increases.
c)
The Ideal Gas Law accounts for all of the forces that occur with gases.
d)
There is no force of attraction between water's molecules in the gaseous state.
|
|
Ayesha Joshi answered |
All of the other answer choices are proven wrong with the first professor's statements. The only choice that involves a statement only dicussed by professor 2 is "As temperature increases, the average kinetic energy of the water molecules increases."
In its refined form, iron is a shiny, silver-gray metal; however, when refined iron is exposed to atmospheric conditions for an extended period of time, its surface becomes flaky, pitted, and red- or orange-colored. This process is known as "rusting," and the new flaky, orange or red substance is called "rust."Below, two scientists discuss how rust forms and the composition of rust.Scientist 1:Both water and oxygen are needed for rust to form. Water is an electrolyte, meaning that it allows ions to move within it. When iron comes into contact with water, some iron naturally dissociates into iron ions (Fe2+) and free electrons. Additionally, when atmospheric oxygen (O2) dissolves in water, some oxygen reacts with water to form hydroxide ions (OH-). Because water allows ions to move freely, iron ions and hydroxide ions combine to form a new compound: iron hydroxide. However, iron hydroxide is not a stable compound. Over time, as water evaporates, it changes into a hydrated form of iron oxide. This is rust.Salts can act as catalysts for rust formation, meaning that they speed up the rate at which rust forms. However, rust can form in pure water, in the absence of added salts.Increasing the ambient temperature increases the rate of rust formation. Additionally, increasing the amount of iron's surface area that is exposed to water also increases the rate at which rust forms. However, because a layer of rust is porous to water and oxygen, water and oxygen will continue to cause the interior of a piece of iron to rust even after the iron's surface has been rusted.Scientist 2:Attack by acids causes rust to form. In water, acids ionize to create positively-charged hydronium (H+) ions and negatively-charged anions. Hydronium ions are electron-deficient; because of this, they attract electrons from iron. This creates iron ions (Fe2+), which are soluble in water. Once dissolved in water, iron ions react with dissolved atmospheric oxygen (O2) to create iron oxide, or rust.Acids can come from a variety of sources. For example, when carbon dioxide in the atmosphere dissolves in water, carbonic acid (H2CO3) is created. Carbonic acid is the most common cause of rusting. However, other environmental sources of acids exist. Rainwater is normally slightly acidic because it has come into contact with molecules in the atmosphere, like sulfur dioxide and nitrogen oxides. These molecules also dissolve in water to form acids. Additionally, iron itself may contain impurities such as phosphorous and sulfur, which react with water to produce acids. Both acidic environments and impurities within iron itself create the conditions under which iron rusts.Rusting can be prevented by painting the surface of iron, thus preventing it from coming into contact with water, oxygen, and acids. Iron can also be protected in a process called "galvanizing," which involves coating iron in a thin layer of zinc. Because zinc is more reactive than iron, it is corroded while the iron is protected.Q. Given that all of the following are true, which of the following, if found, provides the strongest evidence against Scientist 1's hypothesis?- a)When the concentration of hydroxide ions in a solution is increased, rust forms less quickly on iron in the solution.
- b)When table salt is dissolved in water, the water is better able to conduct an electrical current.
- c)When table salt is dissolved in water, the rate at which Fe2+ ions are produced increases.
- d)When the concentration of dissolved oxygen in a solution is increased, rust forms more quickly on iron in the solution.
Correct answer is option 'D'. Can you explain this answer?
In its refined form, iron is a shiny, silver-gray metal; however, when refined iron is exposed to atmospheric conditions for an extended period of time, its surface becomes flaky, pitted, and red- or orange-colored. This process is known as "rusting," and the new flaky, orange or red substance is called "rust."
Below, two scientists discuss how rust forms and the composition of rust.
Scientist 1:
Both water and oxygen are needed for rust to form. Water is an electrolyte, meaning that it allows ions to move within it. When iron comes into contact with water, some iron naturally dissociates into iron ions (Fe2+) and free electrons. Additionally, when atmospheric oxygen (O2) dissolves in water, some oxygen reacts with water to form hydroxide ions (OH-). Because water allows ions to move freely, iron ions and hydroxide ions combine to form a new compound: iron hydroxide. However, iron hydroxide is not a stable compound. Over time, as water evaporates, it changes into a hydrated form of iron oxide. This is rust.
Salts can act as catalysts for rust formation, meaning that they speed up the rate at which rust forms. However, rust can form in pure water, in the absence of added salts.
Increasing the ambient temperature increases the rate of rust formation. Additionally, increasing the amount of iron's surface area that is exposed to water also increases the rate at which rust forms. However, because a layer of rust is porous to water and oxygen, water and oxygen will continue to cause the interior of a piece of iron to rust even after the iron's surface has been rusted.
Scientist 2:
Attack by acids causes rust to form. In water, acids ionize to create positively-charged hydronium (H+) ions and negatively-charged anions. Hydronium ions are electron-deficient; because of this, they attract electrons from iron. This creates iron ions (Fe2+), which are soluble in water. Once dissolved in water, iron ions react with dissolved atmospheric oxygen (O2) to create iron oxide, or rust.
Acids can come from a variety of sources. For example, when carbon dioxide in the atmosphere dissolves in water, carbonic acid (H2CO3) is created. Carbonic acid is the most common cause of rusting. However, other environmental sources of acids exist. Rainwater is normally slightly acidic because it has come into contact with molecules in the atmosphere, like sulfur dioxide and nitrogen oxides. These molecules also dissolve in water to form acids. Additionally, iron itself may contain impurities such as phosphorous and sulfur, which react with water to produce acids. Both acidic environments and impurities within iron itself create the conditions under which iron rusts.
Rusting can be prevented by painting the surface of iron, thus preventing it from coming into contact with water, oxygen, and acids. Iron can also be protected in a process called "galvanizing," which involves coating iron in a thin layer of zinc. Because zinc is more reactive than iron, it is corroded while the iron is protected.
Q. Given that all of the following are true, which of the following, if found, provides the strongest evidence against Scientist 1's hypothesis?
a)
When the concentration of hydroxide ions in a solution is increased, rust forms less quickly on iron in the solution.
b)
When table salt is dissolved in water, the water is better able to conduct an electrical current.
c)
When table salt is dissolved in water, the rate at which Fe2+ ions are produced increases.
d)
When the concentration of dissolved oxygen in a solution is increased, rust forms more quickly on iron in the solution.
|
|
Ayesha Joshi answered |
According to Scientist 1, the production of hydroxide ions is needed in order for rust to form. Scientist 1 states that hydroxide ions combine with soluble iron ions to form iron hydroxide, which then changes into hydrated iron oxide, or rust. Since hydroxide ions are one of the reactants used to produce rust, increasing the concentration of hydroxide ions in a solution should speed up the formation of rust. However, if increasing the hydroxide concentration actually slows down the formation of rust, this would suggest that Scientist 1's explanation is incorrect.
 The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below:
The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below: 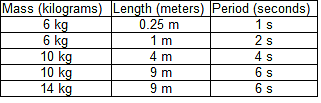 Q. During an earthquake, several chandeliers in a mansion begin to swing. Some of the chandeliers are quite small while others, such as one found in the dining room, are very large. However, all of the chandeliers hang the same exact distance from the ceiling. What would the two students predict would happen?
Q. During an earthquake, several chandeliers in a mansion begin to swing. Some of the chandeliers are quite small while others, such as one found in the dining room, are very large. However, all of the chandeliers hang the same exact distance from the ceiling. What would the two students predict would happen?- a)More information is necessary to make a prediction.
- b)Both Student 1 and Student 2: Every chandelier would display a different period of swinging
- c)Both Student 1 and Student 2: Every chandelier would have the same exact period of swinging.
- d)Student 1: Every chandelier would display a different period of swinging; Student 2: Every chandelier would have the same exact period of swinging.
Correct answer is option 'D'. Can you explain this answer?

The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.
Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.
Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.
The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below:

Q. During an earthquake, several chandeliers in a mansion begin to swing. Some of the chandeliers are quite small while others, such as one found in the dining room, are very large. However, all of the chandeliers hang the same exact distance from the ceiling. What would the two students predict would happen?
a)
More information is necessary to make a prediction.
b)
Both Student 1 and Student 2: Every chandelier would display a different period of swinging
c)
Both Student 1 and Student 2: Every chandelier would have the same exact period of swinging.
d)
Student 1: Every chandelier would display a different period of swinging; Student 2: Every chandelier would have the same exact period of swinging.
|
|
Ayesha Joshi answered |
What is important here is to recognize the analogy. The chandeliers are essentially pendulums—masses hanging from a certain point which are allowed to swing. The correct answer is the one in which Student 1 predicts variations in period of swinging and Student 2 predicts no variations. This comes down to their fundamental disagreement, which is that Student 1 believes the mass of a pendulum affects the pendulum's period
 The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below:
The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below:  Q. Before analyzing the data collected, the two students go out into a local playground and use the swing set to test their hypotheses in an approximate manner. Student 1 and Student 2 are almost exactly the same mass, so Student 2 swings wearing his backpack full of books. Both students begin swinging from the same height and swing exactly three times each in exactly twelve seconds. Whose hypothesis has been supported in this brief trial?
Q. Before analyzing the data collected, the two students go out into a local playground and use the swing set to test their hypotheses in an approximate manner. Student 1 and Student 2 are almost exactly the same mass, so Student 2 swings wearing his backpack full of books. Both students begin swinging from the same height and swing exactly three times each in exactly twelve seconds. Whose hypothesis has been supported in this brief trial?- a)Student 2's
- b)Student 1's
- c)Both Student 1's and Student 2's
- d)More information is needed.
Correct answer is option 'A'. Can you explain this answer?

The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.
Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.
Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.
The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below:

Q. Before analyzing the data collected, the two students go out into a local playground and use the swing set to test their hypotheses in an approximate manner. Student 1 and Student 2 are almost exactly the same mass, so Student 2 swings wearing his backpack full of books. Both students begin swinging from the same height and swing exactly three times each in exactly twelve seconds. Whose hypothesis has been supported in this brief trial?
a)
Student 2's
b)
Student 1's
c)
Both Student 1's and Student 2's
d)
More information is needed.
|
|
Ayesha Joshi answered |
The answer is Student 2's hypothesis. Since the students swung the same amount of times over the same period of time, we can extrapolate that the "period" involved in this pendulum was the same. To derive this, we need an understanding of the definition of a pendulum's period as defined by the passage. Since the students varied in mass because of Student 2's backpack, we can see that this supports the hypothesis that mass does not affect a pendulum's period.
 The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below:
The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below:  Q. On which of the following points would the scientists most likely disagree?
Q. On which of the following points would the scientists most likely disagree?- a)Two children of identical masses swinging on swings of different length would show different swinging periods.
- b)Length of a pendulum is not important to consider when measuring period T.
- c)A child swinging at a height of one meter would show the same period of swinging as a child swinging at a height of two meters.
- d)Two children of different masses swinging on identical swings would show the exact same swinging period.
Correct answer is option 'D'. Can you explain this answer?

The period of a simple pendulum T is defined as the amount of time that it takes for a pendulum to swing from one end to the other and back. In studying the period of a simple pendulum, two students express their opinions.
Student 1: The period of a pendulum depends on two factors: the mass of the pendulum's bob (the object swinging at the end of the pendulum) and the length of the pendulum. The height at which the pendulum is originally dropped does not affect the period T.
Student 2: The period of a pendulum T only depends on the length of the pendulum. Varying the mass and the height at which the pendulum is originally dropped does not affect how long the pendulum takes to swing across.
The two students ran a series of trials to measure the period of a simple pendulum using varying weights and lengths. The students did not measure height as a factor. The results of the trials can be seen in the table below:

Q. On which of the following points would the scientists most likely disagree?
a)
Two children of identical masses swinging on swings of different length would show different swinging periods.
b)
Length of a pendulum is not important to consider when measuring period T.
c)
A child swinging at a height of one meter would show the same period of swinging as a child swinging at a height of two meters.
d)
Two children of different masses swinging on identical swings would show the exact same swinging period.
|
|
Ayesha Joshi answered |
The correct answer is "Two children of different masses swinging on identical swings would show the exact same swinging period." According to the passage, only Student 2 would agree that the children would show the same period of swinging, while Student 1 would argue that they would differ. Gravitational force was not mentioned in the passage (although it is a true statement) and both students agree that the length is important to consider.
In its refined form, iron is a shiny, silver-gray metal; however, when refined iron is exposed to atmospheric conditions for an extended period of time, its surface becomes flaky, pitted, and red- or orange-colored. This process is known as "rusting," and the new flaky, orange or red substance is called "rust."Below, two scientists discuss how rust forms and the composition of rust.Scientist 1:Both water and oxygen are needed for rust to form. Water is an electrolyte, meaning that it allows ions to move within it. When iron comes into contact with water, some iron naturally dissociates into iron ions (Fe2+) and free electrons. Additionally, when atmospheric oxygen (O2) dissolves in water, some oxygen reacts with water to form hydroxide ions (OH-). Because water allows ions to move freely, iron ions and hydroxide ions combine to form a new compound: iron hydroxide. However, iron hydroxide is not a stable compound. Over time, as water evaporates, it changes into a hydrated form of iron oxide. This is rust.Salts can act as catalysts for rust formation, meaning that they speed up the rate at which rust forms. However, rust can form in pure water, in the absence of added salts.Increasing the ambient temperature increases the rate of rust formation. Additionally, increasing the amount of iron's surface area that is exposed to water also increases the rate at which rust forms. However, because a layer of rust is porous to water and oxygen, water and oxygen will continue to cause the interior of a piece of iron to rust even after the iron's surface has been rusted.Scientist 2:Attack by acids causes rust to form. In water, acids ionize to create positively-charged hydronium (H+) ions and negatively-charged anions. Hydronium ions are electron-deficient; because of this, they attract electrons from iron. This creates iron ions (Fe2+), which are soluble in water. Once dissolved in water, iron ions react with dissolved atmospheric oxygen (O2) to create iron oxide, or rust.Acids can come from a variety of sources. For example, when carbon dioxide in the atmosphere dissolves in water, carbonic acid (H2CO3) is created. Carbonic acid is the most common cause of rusting. However, other environmental sources of acids exist. Rainwater is normally slightly acidic because it has come into contact with molecules in the atmosphere, like sulfur dioxide and nitrogen oxides. These molecules also dissolve in water to form acids. Additionally, iron itself may contain impurities such as phosphorous and sulfur, which react with water to produce acids. Both acidic environments and impurities within iron itself create the conditions under which iron rusts.Rusting can be prevented by painting the surface of iron, thus preventing it from coming into contact with water, oxygen, and acids. Iron can also be protected in a process called "galvanizing," which involves coating iron in a thin layer of zinc. Because zinc is more reactive than iron, it is corroded while the iron is protected.Q. Lye (sodium hydroxide) is a base that neutralizes acids. Suppose that lye is added to water in which an iron pipe has been immersed. According to Scientist 2, the pipe's rate of rusting will most likely __________.- a)increase, because more Fe2+ ions will be produced
- b)increase, because rust requires hydroxide ions to form
- c)increase, because the concentration of H+ ions in the solution will increase
- d)decrease, because the solution will become less acidic
Correct answer is option 'D'. Can you explain this answer?
In its refined form, iron is a shiny, silver-gray metal; however, when refined iron is exposed to atmospheric conditions for an extended period of time, its surface becomes flaky, pitted, and red- or orange-colored. This process is known as "rusting," and the new flaky, orange or red substance is called "rust."
Below, two scientists discuss how rust forms and the composition of rust.
Scientist 1:
Both water and oxygen are needed for rust to form. Water is an electrolyte, meaning that it allows ions to move within it. When iron comes into contact with water, some iron naturally dissociates into iron ions (Fe2+) and free electrons. Additionally, when atmospheric oxygen (O2) dissolves in water, some oxygen reacts with water to form hydroxide ions (OH-). Because water allows ions to move freely, iron ions and hydroxide ions combine to form a new compound: iron hydroxide. However, iron hydroxide is not a stable compound. Over time, as water evaporates, it changes into a hydrated form of iron oxide. This is rust.
Salts can act as catalysts for rust formation, meaning that they speed up the rate at which rust forms. However, rust can form in pure water, in the absence of added salts.
Increasing the ambient temperature increases the rate of rust formation. Additionally, increasing the amount of iron's surface area that is exposed to water also increases the rate at which rust forms. However, because a layer of rust is porous to water and oxygen, water and oxygen will continue to cause the interior of a piece of iron to rust even after the iron's surface has been rusted.
Scientist 2:
Attack by acids causes rust to form. In water, acids ionize to create positively-charged hydronium (H+) ions and negatively-charged anions. Hydronium ions are electron-deficient; because of this, they attract electrons from iron. This creates iron ions (Fe2+), which are soluble in water. Once dissolved in water, iron ions react with dissolved atmospheric oxygen (O2) to create iron oxide, or rust.
Acids can come from a variety of sources. For example, when carbon dioxide in the atmosphere dissolves in water, carbonic acid (H2CO3) is created. Carbonic acid is the most common cause of rusting. However, other environmental sources of acids exist. Rainwater is normally slightly acidic because it has come into contact with molecules in the atmosphere, like sulfur dioxide and nitrogen oxides. These molecules also dissolve in water to form acids. Additionally, iron itself may contain impurities such as phosphorous and sulfur, which react with water to produce acids. Both acidic environments and impurities within iron itself create the conditions under which iron rusts.
Rusting can be prevented by painting the surface of iron, thus preventing it from coming into contact with water, oxygen, and acids. Iron can also be protected in a process called "galvanizing," which involves coating iron in a thin layer of zinc. Because zinc is more reactive than iron, it is corroded while the iron is protected.
Q. Lye (sodium hydroxide) is a base that neutralizes acids. Suppose that lye is added to water in which an iron pipe has been immersed. According to Scientist 2, the pipe's rate of rusting will most likely __________.
a)
increase, because more Fe2+ ions will be produced
b)
increase, because rust requires hydroxide ions to form
c)
increase, because the concentration of H+ ions in the solution will increase
d)
decrease, because the solution will become less acidic
|
|
Ayesha Joshi answered |
According to Scientist 2, acid is needed for rust to form. However, the question tells us that lye neutralizes acids. So, if lye is added to the solution, the solution will become less acidic, and rust will not form, or form at a slower rate.
In its refined form, iron is a shiny, silver-gray metal; however, when refined iron is exposed to atmospheric conditions for an extended period of time, its surface becomes flaky, pitted, and red- or orange-colored. This process is known as "rusting," and the new flaky, orange or red substance is called "rust."Below, two scientists discuss how rust forms and the composition of rust.Scientist 1:Both water and oxygen are needed for rust to form. Water is an electrolyte, meaning that it allows ions to move within it. When iron comes into contact with water, some iron naturally dissociates into iron ions (Fe2+) and free electrons. Additionally, when atmospheric oxygen (O2) dissolves in water, some oxygen reacts with water to form hydroxide ions (OH-). Because water allows ions to move freely, iron ions and hydroxide ions combine to form a new compound: iron hydroxide. However, iron hydroxide is not a stable compound. Over time, as water evaporates, it changes into a hydrated form of iron oxide. This is rust.Salts can act as catalysts for rust formation, meaning that they speed up the rate at which rust forms. However, rust can form in pure water, in the absence of added salts.Increasing the ambient temperature increases the rate of rust formation. Additionally, increasing the amount of iron's surface area that is exposed to water also increases the rate at which rust forms. However, because a layer of rust is porous to water and oxygen, water and oxygen will continue to cause the interior of a piece of iron to rust even after the iron's surface has been rusted.Scientist 2:Attack by acids causes rust to form. In water, acids ionize to create positively-charged hydronium (H+) ions and negatively-charged anions. Hydronium ions are electron-deficient; because of this, they attract electrons from iron. This creates iron ions (Fe2+), which are soluble in water. Once dissolved in water, iron ions react with dissolved atmospheric oxygen (O2) to create iron oxide, or rust.Acids can come from a variety of sources. For example, when carbon dioxide in the atmosphere dissolves in water, carbonic acid (H2CO3) is created. Carbonic acid is the most common cause of rusting. However, other environmental sources of acids exist. Rainwater is normally slightly acidic because it has come into contact with molecules in the atmosphere, like sulfur dioxide and nitrogen oxides. These molecules also dissolve in water to form acids. Additionally, iron itself may contain impurities such as phosphorous and sulfur, which react with water to produce acids. Both acidic environments and impurities within iron itself create the conditions under which iron rusts.Rusting can be prevented by painting the surface of iron, thus preventing it from coming into contact with water, oxygen, and acids. Iron can also be protected in a process called "galvanizing," which involves coating iron in a thin layer of zinc. Because zinc is more reactive than iron, it is corroded while the iron is protected.Boiling water does not contain dissolved oxygen. Suppose that a new piece of iron is immersed in boiling water for an extended period of time. Afterwards, scientists observe that the iron has rusted. How would this affect the arguments of Scientist 1 and Scientist 2?- a)It would strengthen Scientist 1's argument, and it would strengthen Scientist 2's argument.
- b)It would strengthen Scientist 1's argument, and it would weaken Scientist 2's argument.
- c)It would weaken Scientist 1's argument, and it would strengthen Scientist 2's argument.
- d)It would weaken Scientist 1's argument, and it would weaken Scientist 2's argument.
Correct answer is option 'D'. Can you explain this answer?
In its refined form, iron is a shiny, silver-gray metal; however, when refined iron is exposed to atmospheric conditions for an extended period of time, its surface becomes flaky, pitted, and red- or orange-colored. This process is known as "rusting," and the new flaky, orange or red substance is called "rust."
Below, two scientists discuss how rust forms and the composition of rust.
Scientist 1:
Both water and oxygen are needed for rust to form. Water is an electrolyte, meaning that it allows ions to move within it. When iron comes into contact with water, some iron naturally dissociates into iron ions (Fe2+) and free electrons. Additionally, when atmospheric oxygen (O2) dissolves in water, some oxygen reacts with water to form hydroxide ions (OH-). Because water allows ions to move freely, iron ions and hydroxide ions combine to form a new compound: iron hydroxide. However, iron hydroxide is not a stable compound. Over time, as water evaporates, it changes into a hydrated form of iron oxide. This is rust.
Salts can act as catalysts for rust formation, meaning that they speed up the rate at which rust forms. However, rust can form in pure water, in the absence of added salts.
Increasing the ambient temperature increases the rate of rust formation. Additionally, increasing the amount of iron's surface area that is exposed to water also increases the rate at which rust forms. However, because a layer of rust is porous to water and oxygen, water and oxygen will continue to cause the interior of a piece of iron to rust even after the iron's surface has been rusted.
Scientist 2:
Attack by acids causes rust to form. In water, acids ionize to create positively-charged hydronium (H+) ions and negatively-charged anions. Hydronium ions are electron-deficient; because of this, they attract electrons from iron. This creates iron ions (Fe2+), which are soluble in water. Once dissolved in water, iron ions react with dissolved atmospheric oxygen (O2) to create iron oxide, or rust.
Acids can come from a variety of sources. For example, when carbon dioxide in the atmosphere dissolves in water, carbonic acid (H2CO3) is created. Carbonic acid is the most common cause of rusting. However, other environmental sources of acids exist. Rainwater is normally slightly acidic because it has come into contact with molecules in the atmosphere, like sulfur dioxide and nitrogen oxides. These molecules also dissolve in water to form acids. Additionally, iron itself may contain impurities such as phosphorous and sulfur, which react with water to produce acids. Both acidic environments and impurities within iron itself create the conditions under which iron rusts.
Rusting can be prevented by painting the surface of iron, thus preventing it from coming into contact with water, oxygen, and acids. Iron can also be protected in a process called "galvanizing," which involves coating iron in a thin layer of zinc. Because zinc is more reactive than iron, it is corroded while the iron is protected.
Boiling water does not contain dissolved oxygen. Suppose that a new piece of iron is immersed in boiling water for an extended period of time. Afterwards, scientists observe that the iron has rusted. How would this affect the arguments of Scientist 1 and Scientist 2?
a)
It would strengthen Scientist 1's argument, and it would strengthen Scientist 2's argument.
b)
It would strengthen Scientist 1's argument, and it would weaken Scientist 2's argument.
c)
It would weaken Scientist 1's argument, and it would strengthen Scientist 2's argument.
d)
It would weaken Scientist 1's argument, and it would weaken Scientist 2's argument.
|
|
Ayesha Joshi answered |
The arguments of both Scientist 1 and Scientist 2 involve atmospheric oxygen diffusing into water. For both scientists, this is one of the necessary events leading up to the formation of rust. According to the question, however, boiling water does not contain dissolved oxygen. So, if iron still rusted in water that did not contain oxygen, this would imply that the explanations of both Scientist 1 and Scientist 2 are wrong or incomplete.
Magnets and electric charges show certain similarities. For example, both magnets and electric charges can exert a force on their surroundings. This force, when produced by a magnet, is called a magnetic field. When it is produced by an electric charge, the force is called an electric field. It has been observed that the strength of both magnetic fields and electric fields is inversely proportional to the square of the distance between a magnet or an electric charge and the objects that they affect.Below, three scientists debate the relationship between electricity and magnetism.Scientist 1:Electricity and magnetism are two different phenomena. Materials such as iron, cobalt, and nickel contain magnetic domains: tiny regions of magnetism, each with two poles. Normally, the domains have a random orientation and are not aligned, so the magnetism of some domains cancels out that of other domains; however, in magnets, domains line up in the same direction, creating the two poles of the magnet and causing magnetic behavior.In contrast, electricity is a moving electric charge which is caused by the flow of electrons through a material. Electrons flow through a material from a region of higher potential (more negative charge) to a region of lower potential (more positive charge). We can measure this flow of electrons as current, which refers to the amount of charge transferred over a period of time.Scientist 2:Electricity and magnetism are similar phenomena; however, one cannot be reduced to the other. Electricity involves two types of charges: positive and negative charge. Though electricity can occur in a moving form (in the form of current, or an electric charge moving through a wire), it can also occur in a static form. Static electricity involves no moving charge. Instead, objects can have a net excess of positive charge or a net excess of negative charge—because of having lost or gained electrons, respectively. When two static positive electric charges or two static negative electric charges are brought close together, they repel each other. However, when a positive and a negative static charge are brought together, they attract each other.Similarly, all magnets have two poles. Magnetic poles that are alike repel each other, while dissimilar magnetic poles attract each other. Magnets and static electric charges are alike in that they both show attraction and repulsion in similar circumstances. However, while isolated static electric charges occur in nature, there are no single, isolated magnetic poles. All magnets have two poles, which cannot be dissociated from each other.Scientist 3:Electricity and magnetism are two aspects of the same phenomenon. A moving flow of electrons creates a magnetic field around it. Thus, wherever an electric current exists, a magnetic field will also exist. The magnetic field created by an electric current is perpendicular to the electric current's direction of flow.Additionally, a magnetic field can induce an electric current. This can happen when a wire is moved across a magnetic field, or when a magnetic field is moved near a conductive wire. Because magnetic fields can produce electric fields and electric fields can produce magnetic fields, we can understand electricity and magnetism as parts of one phenomenon: electromagnetism.Q. In an experiment, an iron bar that showed no magnetism was heated and allowed to cool while aligned North-South with the Earth's magnetic field. After it cooled, the iron bar was found to be magnetic. Scientist 1 would most likely explain this result by saying which of the following?- a)The experiment caused the two magnetic poles of the bar to move so that they were aligned with the Earth's magnetic field.
- b)The experiment allowed the magnetic domains of the bar to line up, causing the bar to become magnetic.
- c)Interference occurred between the electric field of the bar and the magnetic field of the Earth, causing the bar to become magnetic.
- d)The experiment caused the magnetic domains of the bar to move out of alignment with each other.
Correct answer is option 'B'. Can you explain this answer?
Magnets and electric charges show certain similarities. For example, both magnets and electric charges can exert a force on their surroundings. This force, when produced by a magnet, is called a magnetic field. When it is produced by an electric charge, the force is called an electric field. It has been observed that the strength of both magnetic fields and electric fields is inversely proportional to the square of the distance between a magnet or an electric charge and the objects that they affect.
Below, three scientists debate the relationship between electricity and magnetism.
Scientist 1:
Electricity and magnetism are two different phenomena. Materials such as iron, cobalt, and nickel contain magnetic domains: tiny regions of magnetism, each with two poles. Normally, the domains have a random orientation and are not aligned, so the magnetism of some domains cancels out that of other domains; however, in magnets, domains line up in the same direction, creating the two poles of the magnet and causing magnetic behavior.
In contrast, electricity is a moving electric charge which is caused by the flow of electrons through a material. Electrons flow through a material from a region of higher potential (more negative charge) to a region of lower potential (more positive charge). We can measure this flow of electrons as current, which refers to the amount of charge transferred over a period of time.
Scientist 2:
Electricity and magnetism are similar phenomena; however, one cannot be reduced to the other. Electricity involves two types of charges: positive and negative charge. Though electricity can occur in a moving form (in the form of current, or an electric charge moving through a wire), it can also occur in a static form. Static electricity involves no moving charge. Instead, objects can have a net excess of positive charge or a net excess of negative charge—because of having lost or gained electrons, respectively. When two static positive electric charges or two static negative electric charges are brought close together, they repel each other. However, when a positive and a negative static charge are brought together, they attract each other.
Similarly, all magnets have two poles. Magnetic poles that are alike repel each other, while dissimilar magnetic poles attract each other. Magnets and static electric charges are alike in that they both show attraction and repulsion in similar circumstances. However, while isolated static electric charges occur in nature, there are no single, isolated magnetic poles. All magnets have two poles, which cannot be dissociated from each other.
Scientist 3:
Electricity and magnetism are two aspects of the same phenomenon. A moving flow of electrons creates a magnetic field around it. Thus, wherever an electric current exists, a magnetic field will also exist. The magnetic field created by an electric current is perpendicular to the electric current's direction of flow.
Additionally, a magnetic field can induce an electric current. This can happen when a wire is moved across a magnetic field, or when a magnetic field is moved near a conductive wire. Because magnetic fields can produce electric fields and electric fields can produce magnetic fields, we can understand electricity and magnetism as parts of one phenomenon: electromagnetism.
Q. In an experiment, an iron bar that showed no magnetism was heated and allowed to cool while aligned North-South with the Earth's magnetic field. After it cooled, the iron bar was found to be magnetic. Scientist 1 would most likely explain this result by saying which of the following?
a)
The experiment caused the two magnetic poles of the bar to move so that they were aligned with the Earth's magnetic field.
b)
The experiment allowed the magnetic domains of the bar to line up, causing the bar to become magnetic.
c)
Interference occurred between the electric field of the bar and the magnetic field of the Earth, causing the bar to become magnetic.
d)
The experiment caused the magnetic domains of the bar to move out of alignment with each other.
|
|
Ayesha Joshi answered |
Scientist 1 states that magnetism occurs when the magnetic domains in a material align. Since the iron bar initially showed no magnetism, we can assume that its magnetic domains were initially oriented randomly, and that it had no magnetic poles. Since the iron bar became magnetic after it was heated and cooled, the heating and cooling process likely reoriented the magnetic domains in the iron so that they became more aligned, creating two magnetic poles.
Three doctors are discussing the most optimal way to approach cancer treatment. While they all acknowledge that cancer is uncontrolled cell proliferation, they have different opinions on whether chemotherapy is the best treatment method. Chemotherapy is the treatment of cancer with cytotoxic antienoplastic drugs. These drugs are used to kill fast-growing cancerous cells. All three doctors agree that chemotherapy has many associated side effects.Doctor 1While the drugs used for chemotherapy can be very strong, they need to be. Cancer, by its very definition, is made up of cells growing at a faster than normal rate. This means the treatment needs to be aggressive. The slower the effects of treatment, the more time the cancer has to spread; therefore, while the chemotherapy can also kill some healthy noncancerous cells in the process, it is still the best option. Doctor 2 Chemotherapy does much more harm than good. Chemotherapy might temporarily destroy the cancer, but it does not cure the cancer. In addition, killing the cancerous cells means poisoning the body with chemicals and toxins. Instead, we should be addressing the reasons cancer exists in the first place, treating it at that step. Cancer is due to toxins in the body, industrial pollutions, and drugs. Avoiding sugar, exercising, and maintaining a healthy lifestyle—free of toxins, processed food, and other containments—is the best approach.Doctor 3Chemotherapy is effective in the sense that it kills the cancer cells. The downfall is that chemotherapy also kills the healthy cells in the process; therefore, we should be looking at way to decrease the amount of chemotherapy needed, so that we are only introducing the minimum amount of toxins into the body. Insulin Potentiating Therapy is a type of chemotherapy in which lower doses of chemotherapy are used because they are combined with insulin. Cancer cells have more insulin receptors than non-cancerous cells; therefore, cancer cells will have a biased absorption of such insulin-based chemotherapy when compared with noncancerous cells. In other words, piggybacking chemotherapy onto insulin allows cancer cells to absorb more of the chemotherapy, meaning less chemotherapy is needed and fewer noncancerous cells absorb the chemotherapy.Q. What fact do all three doctors seem to agree upon?- a)Chemotherapy has many harmful side effects that might outweigh the good effects of chemotherapy.
- b)Chemotherapy should be used as a last option.
- c)Chemotherapy should not be used.
- d)Chemotherapy has side effects.
Correct answer is option 'D'. Can you explain this answer?
Three doctors are discussing the most optimal way to approach cancer treatment. While they all acknowledge that cancer is uncontrolled cell proliferation, they have different opinions on whether chemotherapy is the best treatment method. Chemotherapy is the treatment of cancer with cytotoxic antienoplastic drugs. These drugs are used to kill fast-growing cancerous cells. All three doctors agree that chemotherapy has many associated side effects.
Doctor 1
While the drugs used for chemotherapy can be very strong, they need to be. Cancer, by its very definition, is made up of cells growing at a faster than normal rate. This means the treatment needs to be aggressive. The slower the effects of treatment, the more time the cancer has to spread; therefore, while the chemotherapy can also kill some healthy noncancerous cells in the process, it is still the best option.
Doctor 2
Chemotherapy does much more harm than good. Chemotherapy might temporarily destroy the cancer, but it does not cure the cancer. In addition, killing the cancerous cells means poisoning the body with chemicals and toxins. Instead, we should be addressing the reasons cancer exists in the first place, treating it at that step. Cancer is due to toxins in the body, industrial pollutions, and drugs. Avoiding sugar, exercising, and maintaining a healthy lifestyle—free of toxins, processed food, and other containments—is the best approach.
Doctor 3
Chemotherapy is effective in the sense that it kills the cancer cells. The downfall is that chemotherapy also kills the healthy cells in the process; therefore, we should be looking at way to decrease the amount of chemotherapy needed, so that we are only introducing the minimum amount of toxins into the body. Insulin Potentiating Therapy is a type of chemotherapy in which lower doses of chemotherapy are used because they are combined with insulin. Cancer cells have more insulin receptors than non-cancerous cells; therefore, cancer cells will have a biased absorption of such insulin-based chemotherapy when compared with noncancerous cells. In other words, piggybacking chemotherapy onto insulin allows cancer cells to absorb more of the chemotherapy, meaning less chemotherapy is needed and fewer noncancerous cells absorb the chemotherapy.
Q. What fact do all three doctors seem to agree upon?
a)
Chemotherapy has many harmful side effects that might outweigh the good effects of chemotherapy.
b)
Chemotherapy should be used as a last option.
c)
Chemotherapy should not be used.
d)
Chemotherapy has side effects.

|
Orion Classes answered |
The answer is clearly stated in the first paragraph, last sentence—"All three doctors agree that chemotherapy has many associated side effects."
Three doctors are discussing the most optimal way to approach cancer treatment. While they all acknowledge that cancer is uncontrolled cell proliferation, they have different opinions on whether chemotherapy is the best treatment method. Chemotherapy is the treatment of cancer with cytotoxic antienoplastic drugs. These drugs are used to kill fast-growing cancerous cells. All three doctors agree that chemotherapy has many associated side effects.Doctor 1While the drugs used for chemotherapy can be very strong, they need to be. Cancer, by its very definition, is made up of cells growing at a faster than normal rate. This means the treatment needs to be aggressive. The slower the effects of treatment, the more time the cancer has to spread; therefore, while the chemotherapy can also kill some healthy noncancerous cells in the process, it is still the best option. Doctor 2 Chemotherapy does much more harm than good. Chemotherapy might temporarily destroy the cancer, but it does not cure the cancer. In addition, killing the cancerous cells means poisoning the body with chemicals and toxins. Instead, we should be addressing the reasons cancer exists in the first place, treating it at that step. Cancer is due to toxins in the body, industrial pollutions, and drugs. Avoiding sugar, exercising, and maintaining a healthy lifestyle—free of toxins, processed food, and other containments—is the best approach.Doctor 3Chemotherapy is effective in the sense that it kills the cancer cells. The downfall is that chemotherapy also kills the healthy cells in the process; therefore, we should be looking at way to decrease the amount of chemotherapy needed, so that we are only introducing the minimum amount of toxins into the body. Insulin Potentiating Therapy is a type of chemotherapy in which lower doses of chemotherapy are used because they are combined with insulin. Cancer cells have more insulin receptors than non-cancerous cells; therefore, cancer cells will have a biased absorption of such insulin-based chemotherapy when compared with noncancerous cells. In other words, piggybacking chemotherapy onto insulin allows cancer cells to absorb more of the chemotherapy, meaning less chemotherapy is needed and fewer noncancerous cells absorb the chemotherapy.Q. Which of the following best states the opinion of Doctor 1?- a)None of the choices listed.
- b)Chemotherapy does more harm than good.
- c)Chemotherapy is the best treatment because cancer needs an aggressive treatment method.
- d)Chemotherapy is one of the most effective methods to treat cancer.
Correct answer is option 'C'. Can you explain this answer?
Three doctors are discussing the most optimal way to approach cancer treatment. While they all acknowledge that cancer is uncontrolled cell proliferation, they have different opinions on whether chemotherapy is the best treatment method. Chemotherapy is the treatment of cancer with cytotoxic antienoplastic drugs. These drugs are used to kill fast-growing cancerous cells. All three doctors agree that chemotherapy has many associated side effects.
Doctor 1
While the drugs used for chemotherapy can be very strong, they need to be. Cancer, by its very definition, is made up of cells growing at a faster than normal rate. This means the treatment needs to be aggressive. The slower the effects of treatment, the more time the cancer has to spread; therefore, while the chemotherapy can also kill some healthy noncancerous cells in the process, it is still the best option.
Doctor 2
Chemotherapy does much more harm than good. Chemotherapy might temporarily destroy the cancer, but it does not cure the cancer. In addition, killing the cancerous cells means poisoning the body with chemicals and toxins. Instead, we should be addressing the reasons cancer exists in the first place, treating it at that step. Cancer is due to toxins in the body, industrial pollutions, and drugs. Avoiding sugar, exercising, and maintaining a healthy lifestyle—free of toxins, processed food, and other containments—is the best approach.
Doctor 3
Chemotherapy is effective in the sense that it kills the cancer cells. The downfall is that chemotherapy also kills the healthy cells in the process; therefore, we should be looking at way to decrease the amount of chemotherapy needed, so that we are only introducing the minimum amount of toxins into the body. Insulin Potentiating Therapy is a type of chemotherapy in which lower doses of chemotherapy are used because they are combined with insulin. Cancer cells have more insulin receptors than non-cancerous cells; therefore, cancer cells will have a biased absorption of such insulin-based chemotherapy when compared with noncancerous cells. In other words, piggybacking chemotherapy onto insulin allows cancer cells to absorb more of the chemotherapy, meaning less chemotherapy is needed and fewer noncancerous cells absorb the chemotherapy.
Q. Which of the following best states the opinion of Doctor 1?
a)
None of the choices listed.
b)
Chemotherapy does more harm than good.
c)
Chemotherapy is the best treatment because cancer needs an aggressive treatment method.
d)
Chemotherapy is one of the most effective methods to treat cancer.

|
Orion Classes answered |
Doctor 1 states in his last sentence that chemotherapy " is still the best option" because "Cancer . . . is cells growing at a faster than normal rate . . . treatment needs to be aggressive." Don't be fooled to answer, "Chemotherapy is one of the most effective methods to treat cancer." Doctor 1 never makes this statement nor does he explicitly compare its effectiveness to other methods.
Doctor 1 states in his last sentence that chemotherapy " is still the best option" because "Cancer...is cells growing at a faster than normal rate . . . treatment needs to be aggressive." Dont be fooled to answer "Chemotherapy is one of the most effective methods to treat cancer." Doctor 1 never makes this statement nor does he explicitly compare its effectivness to other methods.
Genes are hereditary units that are responsible for the phenotypes of an organism. Genes are the directions for the body. Genetic change exists when genes are altered from their previous form. Genes are made up of DNA, or deoxyribonucleic acid. DNA is made up of four bases- adenine, guanine, cytosine, and thymine. Genetic change can result from a variety of factors. Both scientists mentioned below agree on this basic information about genes. However, the scientists do not agree on the primary driving force behind genetic change.Scientist 1A mutation is a permanent change in the sequence of the DNA of a gene. There are several types of mutations—point mutations, silent mutations, frame mutations, and nonsense mutations. Mutations are very important because proteins are synthesized by reading the DNA sequence. If the DNA sequence is changed, the proteins transcribed from the DNA will be different proteins. Mutations directly and substantially change the genes by changing the sequence of the four bases. Therefore, mutations are the main factor when looking at genetic change.Scientist 2 Sexual reproduction is the biggest contributor to genetic change. New combinations of genes are created with every random union of a sperm and egg. During division of the sex cells, or meiosis, crossing over can occur. Crossing over describes the situation when the genes from one parent’s chromosome are traded with genes from the other parent’s chromosome. This results in new combinations of genes. Lastly, a phenomenon called independent assortment results from sexual reproduction. Independent assortment is the random assortment of chromosomes during reproduction. Therefore, by its random nature, sexual reproduction is the largest contributor to genetic change.What information would weaken the viewpoint of Scientist 1?- a)Most mutations are not detectable.
- b)Genes are not easily changed by outside factors, and variation only results from mutations.
- c)Mutations are extremely common.
- d)The majority of mutations are silent mutations, which result in a nucleotide change but not a change in the protein.
Correct answer is option 'D'. Can you explain this answer?
Genes are hereditary units that are responsible for the phenotypes of an organism. Genes are the directions for the body. Genetic change exists when genes are altered from their previous form. Genes are made up of DNA, or deoxyribonucleic acid. DNA is made up of four bases- adenine, guanine, cytosine, and thymine. Genetic change can result from a variety of factors. Both scientists mentioned below agree on this basic information about genes. However, the scientists do not agree on the primary driving force behind genetic change.
Scientist 1
A mutation is a permanent change in the sequence of the DNA of a gene. There are several types of mutations—point mutations, silent mutations, frame mutations, and nonsense mutations. Mutations are very important because proteins are synthesized by reading the DNA sequence. If the DNA sequence is changed, the proteins transcribed from the DNA will be different proteins. Mutations directly and substantially change the genes by changing the sequence of the four bases. Therefore, mutations are the main factor when looking at genetic change.
Scientist 2
Sexual reproduction is the biggest contributor to genetic change. New combinations of genes are created with every random union of a sperm and egg. During division of the sex cells, or meiosis, crossing over can occur. Crossing over describes the situation when the genes from one parent’s chromosome are traded with genes from the other parent’s chromosome. This results in new combinations of genes. Lastly, a phenomenon called independent assortment results from sexual reproduction. Independent assortment is the random assortment of chromosomes during reproduction. Therefore, by its random nature, sexual reproduction is the largest contributor to genetic change.
What information would weaken the viewpoint of Scientist 1?
a)
Most mutations are not detectable.
b)
Genes are not easily changed by outside factors, and variation only results from mutations.
c)
Mutations are extremely common.
d)
The majority of mutations are silent mutations, which result in a nucleotide change but not a change in the protein.

|
Orion Classes answered |
If the majority of mutations were silent and did not affect the protein read from the DNA, one could argue the impact of the mutation would be greatly reduced.
In the 17th century, scientists were just beginning to understand the circulatory system of the heart. The two following viewpoints are the two most popular theories of the day.Scientist I The heart pumps blood through arteries and veins but the two systems are separate. They are similar, just as the senses of smell and taste are when observing food, but ultimately they are two separate systems which perform separate functions. Hot blood flows from the heart, through the arteries, and to the organs which consume the blood much as a human would consume nourishment to survive. Venous blood originates in the liver and follows the veins to the organs where it is similarly consumed.Scientist II The arteries and veins are two parts of one system. Blood flows from the heart, around the body, and back into the heart through the veins like two sets of one way streets. The idea of two systems, each pumping blood to the organs is unreasonable. If the heart can pump 6 oz of blood per minute, then the liver would have to produce 540 pounds of blood per day. A simple measurement of a human’s weight shows how unlikely that solution is. The single circulatory system is far superior as it explains the function of the heart, the arteries, and the veins clearly.How would Scientist I respond to Scientist II's claims that a human's weight disproves the theory that a liver pumps blood?- a)When the liver pumps blood, it is tiring, so there would be a comparable amount of sweat released at the same time.
- b)The temperature of the blood would make it feel lighter in the human body.
- c)The weight of blood cannot be accurately measured by modern tools.
- d)The organs consume the blood at the same rate as which it is being produced, therefore no change in weight would be noticed.
Correct answer is option 'D'. Can you explain this answer?
In the 17th century, scientists were just beginning to understand the circulatory system of the heart. The two following viewpoints are the two most popular theories of the day.
Scientist I The heart pumps blood through arteries and veins but the two systems are separate. They are similar, just as the senses of smell and taste are when observing food, but ultimately they are two separate systems which perform separate functions. Hot blood flows from the heart, through the arteries, and to the organs which consume the blood much as a human would consume nourishment to survive. Venous blood originates in the liver and follows the veins to the organs where it is similarly consumed.
Scientist II The arteries and veins are two parts of one system. Blood flows from the heart, around the body, and back into the heart through the veins like two sets of one way streets. The idea of two systems, each pumping blood to the organs is unreasonable. If the heart can pump 6 oz of blood per minute, then the liver would have to produce 540 pounds of blood per day. A simple measurement of a human’s weight shows how unlikely that solution is. The single circulatory system is far superior as it explains the function of the heart, the arteries, and the veins clearly.
How would Scientist I respond to Scientist II's claims that a human's weight disproves the theory that a liver pumps blood?
a)
When the liver pumps blood, it is tiring, so there would be a comparable amount of sweat released at the same time.
b)
The temperature of the blood would make it feel lighter in the human body.
c)
The weight of blood cannot be accurately measured by modern tools.
d)
The organs consume the blood at the same rate as which it is being produced, therefore no change in weight would be noticed.

|
Orion Classes answered |
Scientist I believes that blood is consumed by the organs. If the blood is consumed at the same rate as which it is produced, there would be no noticeable change.
There are two types of forces that occur with all substances on Earth. Intramolecular forces occur between atoms in a molecule, while intermolecular forces occur between neighboring molecules. Intermolecular forces can be dipole-dipole forces, hydrogen bonding, or London dispersion forces. Professor 1:Water molecules represent an example of hydrogen bonding due to the attraction between the hydrogen atoms and the oxygen atoms in the molecule. This strong dipole-dipole occurs due to lone pairs present on such atoms as Fluorine, Nitrogen, and Oxygen, which are able to pair more closely to the hydrogen atom in another nearby molecule. Water can be present in a solid, liquid, or gaseous state on Earth depending on the competition between the strength of intermolecular bonds and the thermal energy of the system. In 1873, a Dutch scientist, Van der Waals derived an equation that included both the force of attraction between the particles of a gas and the volume of the particles at high pressures. This equation led to a better fit for experimental data than the Ideal Gas Law.Professor 2: Water is the only substance on Earth that we routinely encounter as a solid, liquid, and gas. At low temperatures, the water molecules lock into a rigid structure, but as the temperature increases, the average kinetic energy of the water molecules increases and the molecules are able to move more creating its other natural states of matter. The higher the temperature, the more likely water is to be a gas. Water is proof of the kinetic theory, which assumes that there is no force of attraction between the particles of the gas state. The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law, since there is no interaction between the gaseous molecules. This law accounts for all of the forces that occur with gases on Earth.Q. With which of the following statements would both professors agree?- a)The Ideal Gas Law is used to simulate experimental data involving gases.
- b)Van der Waals' equation is used to simulate experimental data invloving gases.
- c)The state of water is dependent upon the thermal energy of the system.
- d)Water is proof of the Kinetic Theory.
Correct answer is option 'A'. Can you explain this answer?
There are two types of forces that occur with all substances on Earth. Intramolecular forces occur between atoms in a molecule, while intermolecular forces occur between neighboring molecules. Intermolecular forces can be dipole-dipole forces, hydrogen bonding, or London dispersion forces.
Professor 1:
Water molecules represent an example of hydrogen bonding due to the attraction between the hydrogen atoms and the oxygen atoms in the molecule. This strong dipole-dipole occurs due to lone pairs present on such atoms as Fluorine, Nitrogen, and Oxygen, which are able to pair more closely to the hydrogen atom in another nearby molecule. Water can be present in a solid, liquid, or gaseous state on Earth depending on the competition between the strength of intermolecular bonds and the thermal energy of the system. In 1873, a Dutch scientist, Van der Waals derived an equation that included both the force of attraction between the particles of a gas and the volume of the particles at high pressures. This equation led to a better fit for experimental data than the Ideal Gas Law.
Professor 2:
Water is the only substance on Earth that we routinely encounter as a solid, liquid, and gas. At low temperatures, the water molecules lock into a rigid structure, but as the temperature increases, the average kinetic energy of the water molecules increases and the molecules are able to move more creating its other natural states of matter. The higher the temperature, the more likely water is to be a gas. Water is proof of the kinetic theory, which assumes that there is no force of attraction between the particles of the gas state. The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law, since there is no interaction between the gaseous molecules. This law accounts for all of the forces that occur with gases on Earth.
Q. With which of the following statements would both professors agree?
a)
The Ideal Gas Law is used to simulate experimental data involving gases.
b)
Van der Waals' equation is used to simulate experimental data invloving gases.
c)
The state of water is dependent upon the thermal energy of the system.
d)
Water is proof of the Kinetic Theory.
|
|
Ayesha Joshi answered |
Both professors mention the Ideal Gas Law as a method used to mirror experimental data using a math equation. Though professor 1 prefers using the Van der Waals' equation, he still mentions the Ideal Gas Law as the traditional option used.
There are two types of forces that occur with all substances on Earth. Intramolecular forces occur between atoms in a molecule, while intermolecular forces occur between neighboring molecules. Intermolecular forces can be dipole-dipole forces, hydrogen bonding, or London dispersion forces.Professor 1:Water molecules represent an example of hydrogen bonding due to the attraction between the hydrogen atoms and the oxygen atoms in the molecule. This strong dipole-dipole occurs due to lone pairs present on such atoms as Fluorine, Nitrogen, and Oxygen, which are able to pair more closely to the hydrogen atom in another nearby molecule. Water can be present in a solid, liquid, or gaseous state on Earth depending on the competition between the strength of intermolecular bonds and the thermal energy of the system. In 1873, a Dutch scientist, Van der Waals derived an equation that included both the force of attraction between the particles of a gas and the volume of the particles at high pressures. This equation led to a better fit for experimental data than the Ideal Gas Law.Professor 2: Water is the only substance on Earth that we routinely encounter as a solid, liquid, and gas. At low temperatures, the water molecules lock into a rigid structure, but as the temperature increases, the average kinetic energy of the water molecules increases and the molecules are able to move more creating its other natural states of matter. The higher the temperature, the more likely water is to be a gas. Water is proof of the kinetic theory, which assumes that there is no force of attraction between the particles of the gas state. The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law, since there is no interaction between the gaseous molecules. This law accounts for all of the forces that occur with gases on Earth.Q. Which of the following statements is professor 1 most likely to agree with?- a)The Ideal Gas Law is the best way to simulate experimental data involving gases on Earth.
- b)Van der Waals is responsible for finding a better method to simulate experimental data involving gases on Earth.
- c)Water is the only example of hydrogen bonding that exists on Earth.
- d)The higher the temperature, the more likely water is to be a gas.
Correct answer is option 'B'. Can you explain this answer?
There are two types of forces that occur with all substances on Earth. Intramolecular forces occur between atoms in a molecule, while intermolecular forces occur between neighboring molecules. Intermolecular forces can be dipole-dipole forces, hydrogen bonding, or London dispersion forces.
Professor 1:
Water molecules represent an example of hydrogen bonding due to the attraction between the hydrogen atoms and the oxygen atoms in the molecule. This strong dipole-dipole occurs due to lone pairs present on such atoms as Fluorine, Nitrogen, and Oxygen, which are able to pair more closely to the hydrogen atom in another nearby molecule. Water can be present in a solid, liquid, or gaseous state on Earth depending on the competition between the strength of intermolecular bonds and the thermal energy of the system. In 1873, a Dutch scientist, Van der Waals derived an equation that included both the force of attraction between the particles of a gas and the volume of the particles at high pressures. This equation led to a better fit for experimental data than the Ideal Gas Law.
Professor 2:
Water is the only substance on Earth that we routinely encounter as a solid, liquid, and gas. At low temperatures, the water molecules lock into a rigid structure, but as the temperature increases, the average kinetic energy of the water molecules increases and the molecules are able to move more creating its other natural states of matter. The higher the temperature, the more likely water is to be a gas. Water is proof of the kinetic theory, which assumes that there is no force of attraction between the particles of the gas state. The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law, since there is no interaction between the gaseous molecules. This law accounts for all of the forces that occur with gases on Earth.
Q. Which of the following statements is professor 1 most likely to agree with?
a)
The Ideal Gas Law is the best way to simulate experimental data involving gases on Earth.
b)
Van der Waals is responsible for finding a better method to simulate experimental data involving gases on Earth.
c)
Water is the only example of hydrogen bonding that exists on Earth.
d)
The higher the temperature, the more likely water is to be a gas.
|
|
Ayesha Joshi answered |
Professor 1 states that "In 1873, a Dutch scientist, Van der Waals derived an equation that... led to a better fit for experimental data than the Ideal Gas Law." This shows that the correct answer is "Van der Waals is responsible for finding a better method to simulate experimental data involving gases on Earth."
Additionally, "The higher the temperature, the more likely water is to be a gas." and "The Ideal Gas Law is the best way to simulate experimental data involving gases on Earth." are statements that match up with what professor 2 said in his statement. Finally, professor 1 states that "Water molecules represent AN example of hydrogen bonding" implying that water is one of many examples present.
In a physics class, students conducted a series of experiments by placing different objects into a beaker of water. They conducted twenty trials for each object. For each trial, they recorded whether or not the object floated.First, they placed a steel paper clip into the water. They observed that the paper clip usually sank; however, they also saw that occasionally, the paper clip stayed afloat if it was placed very gently on top of the water. Next, they repeated the the same procedure using a cork, a toy boat made of aluminum, and a glass marble. They observed that both the cork and the toy boat always stayed afloat in the water, but that the glass marble always sank.Below, three students give their explanations for these observations.Student 1:Objects float when they are less dense than the liquid in which they are immersed. For example, when immiscible liquids of varying densities are mixed together in a container, the most dense liquid will sink to the bottom of the container, while the least dense liquid will rise to the top. This same principle applies to solid objects. Because the cork and the aluminum toy boat always float, cork and the aluminum of the boat must be less dense than water. Because the glass marble always sinks, the glass of the marble must be more dense than water.Objects that are more dense than water can also float due to surface tension. Surface tension occurs because molecules of a liquid are more attracted to each other more than they are to other objects. Molecules on the surface of water are attracted to the molecules around them and below them. This attraction causes a liquid's surface to behave if it were covered by a thin film, which resists penetration by other objects. Therefore, small objects such as paper clips can sometimes float on water when the upward force of water's surface tension exceeds the force of gravity pulling such objects down. Because the paper clips often sink and only float sometimes, we can conclude that they are indeed more dense than water, and that their floating is due to surface tension.Student 2:Objects float in two different cases: when they are buoyed by a liquid's surface tension or when their average density is less than that of the liquid in which they are immersed. The average density of cork is less than that of water. This is why the cork floats. In contrast, the density of glass is more than that of water. This is why the glass marble sinks.However, the densities of aluminum and of steel are greater than that of water. Thus, density cannot be used to explain why the aluminum toy boat and the paper clip float. Both of these objects float because of surface tension. Because the paper clip does not have much mass, the normal upward force created by water's surface tension can be enough to allow it to float. Other objects with greater mass, like the toy boat, employ a particular shape to magnify the force of surface tension. The curved shape of the boat's bottom both stabilizes the boat and increases the amount of the boat's surface area that touches the water, maximizing the force due to surface tension that the boat receives.Student 3:In this experiment, the paper clip floats because of surface tension; however, the cork, toy boat, and marble float or sink because of their relationship to a buoyant force. All objects immersed in a liquid experience a buoyant force, which pushes them upward. The strength of this force is equal to the weight of the liquid displaced, or pushed aside, by an object. Every object also experiences a downward force due to gravity, which is measured as the object's weight, and which is directly proportional to the object's mass. When the buoyant force acting on an object is greater than the downward force due to gravity, the object floats. However, when the buoyant force is less than the force due to gravity, the object sinks. Both the cork and the aluminum toy boat are able to displace enough water to create a buoyant force that exceeds the force due to gravity, so they float. However, the glass marble does not displace enough water to create a sufficient buoyant force, so it sinks.Q. Given that Student 3's explanation is correct, how does the buoyant force on an object held down completely under water compare to the buoyant force on the same object when it is held down partially under water? Compared to the force on the completely-submerged object, the force on the partially-submerged object is __________.- a)the same
- b)less
- c)more
- d)The force may be more, less, or the same, depending on the composition of the object.
Correct answer is option 'B'. Can you explain this answer?
In a physics class, students conducted a series of experiments by placing different objects into a beaker of water. They conducted twenty trials for each object. For each trial, they recorded whether or not the object floated.
First, they placed a steel paper clip into the water. They observed that the paper clip usually sank; however, they also saw that occasionally, the paper clip stayed afloat if it was placed very gently on top of the water. Next, they repeated the the same procedure using a cork, a toy boat made of aluminum, and a glass marble. They observed that both the cork and the toy boat always stayed afloat in the water, but that the glass marble always sank.
Below, three students give their explanations for these observations.
Student 1:
Objects float when they are less dense than the liquid in which they are immersed. For example, when immiscible liquids of varying densities are mixed together in a container, the most dense liquid will sink to the bottom of the container, while the least dense liquid will rise to the top. This same principle applies to solid objects. Because the cork and the aluminum toy boat always float, cork and the aluminum of the boat must be less dense than water. Because the glass marble always sinks, the glass of the marble must be more dense than water.
Objects that are more dense than water can also float due to surface tension. Surface tension occurs because molecules of a liquid are more attracted to each other more than they are to other objects. Molecules on the surface of water are attracted to the molecules around them and below them. This attraction causes a liquid's surface to behave if it were covered by a thin film, which resists penetration by other objects. Therefore, small objects such as paper clips can sometimes float on water when the upward force of water's surface tension exceeds the force of gravity pulling such objects down. Because the paper clips often sink and only float sometimes, we can conclude that they are indeed more dense than water, and that their floating is due to surface tension.
Student 2:
Objects float in two different cases: when they are buoyed by a liquid's surface tension or when their average density is less than that of the liquid in which they are immersed. The average density of cork is less than that of water. This is why the cork floats. In contrast, the density of glass is more than that of water. This is why the glass marble sinks.
However, the densities of aluminum and of steel are greater than that of water. Thus, density cannot be used to explain why the aluminum toy boat and the paper clip float. Both of these objects float because of surface tension. Because the paper clip does not have much mass, the normal upward force created by water's surface tension can be enough to allow it to float. Other objects with greater mass, like the toy boat, employ a particular shape to magnify the force of surface tension. The curved shape of the boat's bottom both stabilizes the boat and increases the amount of the boat's surface area that touches the water, maximizing the force due to surface tension that the boat receives.
Student 3:
In this experiment, the paper clip floats because of surface tension; however, the cork, toy boat, and marble float or sink because of their relationship to a buoyant force. All objects immersed in a liquid experience a buoyant force, which pushes them upward. The strength of this force is equal to the weight of the liquid displaced, or pushed aside, by an object. Every object also experiences a downward force due to gravity, which is measured as the object's weight, and which is directly proportional to the object's mass. When the buoyant force acting on an object is greater than the downward force due to gravity, the object floats. However, when the buoyant force is less than the force due to gravity, the object sinks. Both the cork and the aluminum toy boat are able to displace enough water to create a buoyant force that exceeds the force due to gravity, so they float. However, the glass marble does not displace enough water to create a sufficient buoyant force, so it sinks.
Q. Given that Student 3's explanation is correct, how does the buoyant force on an object held down completely under water compare to the buoyant force on the same object when it is held down partially under water? Compared to the force on the completely-submerged object, the force on the partially-submerged object is __________.
a)
the same
b)
less
c)
more
d)
The force may be more, less, or the same, depending on the composition of the object.
|
|
Ayesha Joshi answered |
Student 3 says that the buoyant force that an object experiences is equal to the weight of the water that the object displaces. Since an object that's held down completely under water displaces more water than an object that's held down only partially under water, the object that is completely under water experiences a greater buoyant force. This is why it's hard to push an inner tube completely under the water in a pool!
Before modern technologies and experiments allowed scientists to understand different organisms' mechanisms of reproduction, numerous theories existed about how populations came to exist. Two scientists from the 1800s describe their theories. Here are their arguments.Scientist IJust like some plants come from seeds and others are capable of vegetative (asexual) reproduction, some animal organisms come from non-sexual reproduction as well. Maggots, for example, appear on rotting carcasses. It is clearly illogical to suggest that the dead animal created or gave birth to the maggots, as it is no longer alive and is therefore incapable of sexual reproduction. The only rational conclusion for the appearance of maggots is a spontaneous generation. This is similar to how, if one were to leave a bowl of broth in the open air for a week, it would turn cloudy from bacteria appearing in the liquid.Scientist IIAnimate objects cannot arise from inanimate objects. Even when plants perform asexual reproduction, daughter plants are still coming from parent plants. There is no other example in nature of a living organism spontaneously coming into being. It is true that we observe maggots on rotting carcasses, but that does not necessarily mean that the maggots came from the rotting carcass. Similarly, bacteria growing in broth do not necessarily come directly from the broth. If a living organism appears, then it must have come from another animate object, even if we did not witness it. It is more likely that these invisible organisms have come in through the air and we simply do not see them until they have had a chance to replicate in these locations.An experiment is performed in which a bowl of broth containing bacteria is boiled and then left in the open air. After a day, the broth is observed to be cloudy. How might Scientist I explain this result?- a)The broth was insufficiently boiled and the original bacteria in it were not killed
- b)The bacteria did not necessarily come from the broth itself
- c)The bacteria spontaneously generated in the broth, given the proper combination of nutrients and air
- d)The cloudiness is part of a natural cycle of the clarity of liquids
Correct answer is option 'C'. Can you explain this answer?
Before modern technologies and experiments allowed scientists to understand different organisms' mechanisms of reproduction, numerous theories existed about how populations came to exist. Two scientists from the 1800s describe their theories. Here are their arguments.
Scientist I
Just like some plants come from seeds and others are capable of vegetative (asexual) reproduction, some animal organisms come from non-sexual reproduction as well. Maggots, for example, appear on rotting carcasses. It is clearly illogical to suggest that the dead animal created or gave birth to the maggots, as it is no longer alive and is therefore incapable of sexual reproduction. The only rational conclusion for the appearance of maggots is a spontaneous generation. This is similar to how, if one were to leave a bowl of broth in the open air for a week, it would turn cloudy from bacteria appearing in the liquid.
Scientist II
Animate objects cannot arise from inanimate objects. Even when plants perform asexual reproduction, daughter plants are still coming from parent plants. There is no other example in nature of a living organism spontaneously coming into being. It is true that we observe maggots on rotting carcasses, but that does not necessarily mean that the maggots came from the rotting carcass. Similarly, bacteria growing in broth do not necessarily come directly from the broth. If a living organism appears, then it must have come from another animate object, even if we did not witness it. It is more likely that these invisible organisms have come in through the air and we simply do not see them until they have had a chance to replicate in these locations.
An experiment is performed in which a bowl of broth containing bacteria is boiled and then left in the open air. After a day, the broth is observed to be cloudy. How might Scientist I explain this result?
a)
The broth was insufficiently boiled and the original bacteria in it were not killed
b)
The bacteria did not necessarily come from the broth itself
c)
The bacteria spontaneously generated in the broth, given the proper combination of nutrients and air
d)
The cloudiness is part of a natural cycle of the clarity of liquids

|
Orion Classes answered |
Scientist I argues that organisms can spontaneously appear. He would likely suggest that the live bacteria spontaneously generated in the broth after it was left to sit.
The argument that the bacteria did not come from the broth is the viewpoint of Scientist II. Neither scientist discussed the clarity of the liquid or the idea of boiling liquids to remove bacteria.
Magnets and electric charges show certain similarities. For example, both magnets and electric charges can exert a force on their surroundings. This force, when produced by a magnet, is called a magnetic field. When it is produced by an electric charge, the force is called an electric field. It has been observed that the strength of both magnetic fields and electric fields is inversely proportional to the square of the distance between a magnet or an electric charge and the objects that they affect.Below, three scientists debate the relationship between electricity and magnetism.Scientist 1:Electricity and magnetism are two different phenomena. Materials such as iron, cobalt, and nickel contain magnetic domains: tiny regions of magnetism, each with two poles. Normally, the domains have a random orientation and are not aligned, so the magnetism of some domains cancels out that of other domains; however, in magnets, domains line up in the same direction, creating the two poles of the magnet and causing magnetic behavior.In contrast, electricity is a moving electric charge which is caused by the flow of electrons through a material. Electrons flow through a material from a region of higher potential (more negative charge) to a region of lower potential (more positive charge). We can measure this flow of electrons as current, which refers to the amount of charge transferred over a period of time.Scientist 2:Electricity and magnetism are similar phenomena; however, one cannot be reduced to the other. Electricity involves two types of charges: positive and negative charge. Though electricity can occur in a moving form (in the form of current, or an electric charge moving through a wire), it can also occur in a static form. Static electricity involves no moving charge. Instead, objects can have a net excess of positive charge or a net excess of negative charge—because of having lost or gained electrons, respectively. When two static positive electric charges or two static negative electric charges are brought close together, they repel each other. However, when a positive and a negative static charge are brought together, they attract each other.Similarly, all magnets have two poles. Magnetic poles that are alike repel each other, while dissimilar magnetic poles attract each other. Magnets and static electric charges are alike in that they both show attraction and repulsion in similar circumstances. However, while isolated static electric charges occur in nature, there are no single, isolated magnetic poles. All magnets have two poles, which cannot be dissociated from each other.Scientist 3:Electricity and magnetism are two aspects of the same phenomenon. A moving flow of electrons creates a magnetic field around it. Thus, wherever an electric current exists, a magnetic field will also exist. The magnetic field created by an electric current is perpendicular to the electric current's direction of flow.Additionally, a magnetic field can induce an electric current. This can happen when a wire is moved across a magnetic field, or when a magnetic field is moved near a conductive wire. Because magnetic fields can produce electric fields and electric fields can produce magnetic fields, we can understand electricity and magnetism as parts of one phenomenon: electromagnetism.Q. According to Scientist 2, which of the following would be an example of a static electric charge?- a)A wire that carries charge from the negative to the positive terminal of a battery
- b)A ring of a conductive material, such copper, that has not lost or gained electrons
- c)A conductive material, such as copper, that is placed in an magnetic field
- d)A balloon that has been rubbed against hair so that it has picked up excess electrons
Correct answer is option 'D'. Can you explain this answer?
Magnets and electric charges show certain similarities. For example, both magnets and electric charges can exert a force on their surroundings. This force, when produced by a magnet, is called a magnetic field. When it is produced by an electric charge, the force is called an electric field. It has been observed that the strength of both magnetic fields and electric fields is inversely proportional to the square of the distance between a magnet or an electric charge and the objects that they affect.
Below, three scientists debate the relationship between electricity and magnetism.
Scientist 1:
Electricity and magnetism are two different phenomena. Materials such as iron, cobalt, and nickel contain magnetic domains: tiny regions of magnetism, each with two poles. Normally, the domains have a random orientation and are not aligned, so the magnetism of some domains cancels out that of other domains; however, in magnets, domains line up in the same direction, creating the two poles of the magnet and causing magnetic behavior.
In contrast, electricity is a moving electric charge which is caused by the flow of electrons through a material. Electrons flow through a material from a region of higher potential (more negative charge) to a region of lower potential (more positive charge). We can measure this flow of electrons as current, which refers to the amount of charge transferred over a period of time.
Scientist 2:
Electricity and magnetism are similar phenomena; however, one cannot be reduced to the other. Electricity involves two types of charges: positive and negative charge. Though electricity can occur in a moving form (in the form of current, or an electric charge moving through a wire), it can also occur in a static form. Static electricity involves no moving charge. Instead, objects can have a net excess of positive charge or a net excess of negative charge—because of having lost or gained electrons, respectively. When two static positive electric charges or two static negative electric charges are brought close together, they repel each other. However, when a positive and a negative static charge are brought together, they attract each other.
Similarly, all magnets have two poles. Magnetic poles that are alike repel each other, while dissimilar magnetic poles attract each other. Magnets and static electric charges are alike in that they both show attraction and repulsion in similar circumstances. However, while isolated static electric charges occur in nature, there are no single, isolated magnetic poles. All magnets have two poles, which cannot be dissociated from each other.
Scientist 3:
Electricity and magnetism are two aspects of the same phenomenon. A moving flow of electrons creates a magnetic field around it. Thus, wherever an electric current exists, a magnetic field will also exist. The magnetic field created by an electric current is perpendicular to the electric current's direction of flow.
Additionally, a magnetic field can induce an electric current. This can happen when a wire is moved across a magnetic field, or when a magnetic field is moved near a conductive wire. Because magnetic fields can produce electric fields and electric fields can produce magnetic fields, we can understand electricity and magnetism as parts of one phenomenon: electromagnetism.
Q. According to Scientist 2, which of the following would be an example of a static electric charge?
a)
A wire that carries charge from the negative to the positive terminal of a battery
b)
A ring of a conductive material, such copper, that has not lost or gained electrons
c)
A conductive material, such as copper, that is placed in an magnetic field
d)
A balloon that has been rubbed against hair so that it has picked up excess electrons
|
|
Ayesha Joshi answered |
Scientist 2 states that static electric charges occur when an object has a net excess of positive or negative charge. According to Scientist 2, static electric charges also don't involve a moving charge. So, one example of a static electric charge is a balloon that has picked up excess electrons: it has an excess of negative charge, but the charge is not moving.
Eukaryotic cells, cells that contain membrane-bound organelles and generally reside within multicellular organisms, contain DNA, or deoxyribonucleic acid, which is organized into chromosomes. DNA is a double-stranded nucleic acid that forms a double helix. The bases found within a DNA molecule are adenine (A), thymine (T), guanine (G), and cytosine (C). DNA is organized into functional units, called genes, that encode the basic traits and characteristics of living organisms. DNA can be replicated within the nucleus prior to cell division to ensure each daughter cell receives an identical copy of DNA. The central dogma of molecular biology states that DNA is transcribed to RNA which is then translated into protein. RNA, or ribonucleic acid, is a nucleic acid found in all cells that serves a messenger to carry the genetic code from DNA to produce a functional molecule, the protein. RNA is a single-stranded nucleic acid and consists of the bases adenine (A), uracil (U), guanine (G), and cytosine (C). RNA is translated into amino acids on the ribosome to produce a polypeptide chain, or a protein. There are two general hypotheses for the original evolutionary molecule. The “RNA world” hypothesis states that the original genetic molecule is RNA, and RNA was able to be translated into protein and reverse transcribed to produce DNA. Alternatively, the “DNA, RNA, and Protein World” suggests that DNA was the original genetic molecule and was responsible for subsequent production of RNA and protein.Q. A new organism is identified and the only nucleic acid contained within its cells is RNA. Which hypothesis would be supported by such a finding?- a)RNA World
- b)DNA, RNA, Protein World
- c)Transcription
- d)The Central Dogma
Correct answer is option 'A'. Can you explain this answer?
Eukaryotic cells, cells that contain membrane-bound organelles and generally reside within multicellular organisms, contain DNA, or deoxyribonucleic acid, which is organized into chromosomes. DNA is a double-stranded nucleic acid that forms a double helix. The bases found within a DNA molecule are adenine (A), thymine (T), guanine (G), and cytosine (C). DNA is organized into functional units, called genes, that encode the basic traits and characteristics of living organisms. DNA can be replicated within the nucleus prior to cell division to ensure each daughter cell receives an identical copy of DNA. The central dogma of molecular biology states that DNA is transcribed to RNA which is then translated into protein. RNA, or ribonucleic acid, is a nucleic acid found in all cells that serves a messenger to carry the genetic code from DNA to produce a functional molecule, the protein. RNA is a single-stranded nucleic acid and consists of the bases adenine (A), uracil (U), guanine (G), and cytosine (C). RNA is translated into amino acids on the ribosome to produce a polypeptide chain, or a protein. There are two general hypotheses for the original evolutionary molecule. The “RNA world” hypothesis states that the original genetic molecule is RNA, and RNA was able to be translated into protein and reverse transcribed to produce DNA. Alternatively, the “DNA, RNA, and Protein World” suggests that DNA was the original genetic molecule and was responsible for subsequent production of RNA and protein.
Q. A new organism is identified and the only nucleic acid contained within its cells is RNA. Which hypothesis would be supported by such a finding?
a)
RNA World
b)
DNA, RNA, Protein World
c)
Transcription
d)
The Central Dogma

|
Orion Classes answered |
The RNA World hypothesis states that RNA was the first genetic material. Idenitifying and organism that contains RNA but not DNA suggests that organisms can survive without DNA, supporting the idea that RNA may have been the first sole genetic information.
Before modern technologies and experiments allowed scientists to understand different organisms' mechanisms of reproduction, numerous theories existed about how populations came to exist. Two scientists from the 1800s describe their theories. Here are their arguments.Scientist IJust like some plants come from seeds and others are capable of vegetative (asexual) reproduction, some animal organisms come from non-sexual reproduction as well. Maggots, for example, appear on rotting carcasses. It is clearly illogical to suggest that the dead animal created or gave birth to the maggots, as it is no longer alive and is therefore incapable of sexual reproduction. The only rational conclusion for the appearance of maggots is a spontaneous generation. This is similar to how, if one were to leave a bowl of broth in the open air for a week, it would turn cloudy from bacteria appearing in the liquid.Scientist IIAnimate objects cannot arise from inanimate objects. Even when plants perform asexual reproduction, daughter plants are still coming from parent plants. There is no other example in nature of a living organism spontaneously coming into being. It is true that we observe maggots on rotting carcasses, but that does not necessarily mean that the maggots came from the rotting carcass. Similarly, bacteria growing in broth do not necessarily come directly from the broth. If a living organism appears, then it must have come from another animate object, even if we did not witness it. It is more likely that these invisible organisms have come in through the air and we simply do not see them until they have had a chance to replicate in these locations.Which of the following is a valid summary of Scientist II's argument against Scientist I?- a)Not all plants come from seeds
- b)Just because we did not see an event occur does not mean it did not happen
- c)There is insufficient experimental evidence to prove spontaneous generation
- d)Maggots do not appear on rotting carcasses
Correct answer is option 'B'. Can you explain this answer?
Before modern technologies and experiments allowed scientists to understand different organisms' mechanisms of reproduction, numerous theories existed about how populations came to exist. Two scientists from the 1800s describe their theories. Here are their arguments.
Scientist I
Just like some plants come from seeds and others are capable of vegetative (asexual) reproduction, some animal organisms come from non-sexual reproduction as well. Maggots, for example, appear on rotting carcasses. It is clearly illogical to suggest that the dead animal created or gave birth to the maggots, as it is no longer alive and is therefore incapable of sexual reproduction. The only rational conclusion for the appearance of maggots is a spontaneous generation. This is similar to how, if one were to leave a bowl of broth in the open air for a week, it would turn cloudy from bacteria appearing in the liquid.
Scientist II
Animate objects cannot arise from inanimate objects. Even when plants perform asexual reproduction, daughter plants are still coming from parent plants. There is no other example in nature of a living organism spontaneously coming into being. It is true that we observe maggots on rotting carcasses, but that does not necessarily mean that the maggots came from the rotting carcass. Similarly, bacteria growing in broth do not necessarily come directly from the broth. If a living organism appears, then it must have come from another animate object, even if we did not witness it. It is more likely that these invisible organisms have come in through the air and we simply do not see them until they have had a chance to replicate in these locations.
Which of the following is a valid summary of Scientist II's argument against Scientist I?
a)
Not all plants come from seeds
b)
Just because we did not see an event occur does not mean it did not happen
c)
There is insufficient experimental evidence to prove spontaneous generation
d)
Maggots do not appear on rotting carcasses

|
Orion Classes answered |
Scientist II argues that just because we have not witnessed something does not mean it does not exist. He suggests that an unseen animate object is responsible for the growth and reproduction of organisms in an area that previously appeared to have no life.
Scientist II does not dispute that maggots appear on rotting carcasses or that some plants do not come from seeds. He also does not argue that the first scientist has insufficient experimental evidence. Rather, he gives an alternative explanation for the experimental results cited by Scientist I.
There are two types of forces that occur with all substances on Earth. Intramolecular forces occur between atoms in a molecule, while intermolecular forces occur between neighboring molecules. Intermolecular forces can be dipole-dipole forces, hydrogen bonding, or London dispersion forces.Professor 1:Water molecules represent an example of hydrogen bonding due to the attraction between the hydrogen atoms and the oxygen atoms in the molecule. This strong dipole-dipole occurs due to lone pairs present on such atoms as Fluorine, Nitrogen, and Oxygen, which are able to pair more closely to the hydrogen atom in another nearby molecule. Water can be present in a solid, liquid, or gaseous state on Earth depending on the competition between the strength of intermolecular bonds and the thermal energy of the system. In 1873, a Dutch scientist, Van der Waals derived an equation that included both the force of attraction between the particles of a gas and the volume of the particles at high pressures. This equation led to a better fit for experimental data than the Ideal Gas Law.Professor 2: Water is the only substance on Earth that we routinely encounter as a solid, liquid, and gas. At low temperatures, the water molecules lock into a rigid structure, but as the temperature increases, the average kinetic energy of the water molecules increases and the molecules are able to move more creating its other natural states of matter. The higher the temperature, the more likely water is to be a gas. Water is proof of the kinetic theory, which assumes that there is no force of attraction between the particles of the gas state. The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law, since there is no interaction between the gaseous molecules. This law accounts for all of the forces that occur with gases on Earth.Q. Which of the following statements would professor 2 agree with?- a)Van der Waals' equation most closely mirrors the gas interactions that occur in nature.
- b)The Ideal Gas Law most closely mirrors the gas interactions that occur in nature.
- c)At low temperatures, water is present as a gas.
- d)The state of water is dependent upon the strength of intramolecular forces and the thermal energy present in the system.
Correct answer is option 'B'. Can you explain this answer?
There are two types of forces that occur with all substances on Earth. Intramolecular forces occur between atoms in a molecule, while intermolecular forces occur between neighboring molecules. Intermolecular forces can be dipole-dipole forces, hydrogen bonding, or London dispersion forces.
Professor 1:
Water molecules represent an example of hydrogen bonding due to the attraction between the hydrogen atoms and the oxygen atoms in the molecule. This strong dipole-dipole occurs due to lone pairs present on such atoms as Fluorine, Nitrogen, and Oxygen, which are able to pair more closely to the hydrogen atom in another nearby molecule. Water can be present in a solid, liquid, or gaseous state on Earth depending on the competition between the strength of intermolecular bonds and the thermal energy of the system. In 1873, a Dutch scientist, Van der Waals derived an equation that included both the force of attraction between the particles of a gas and the volume of the particles at high pressures. This equation led to a better fit for experimental data than the Ideal Gas Law.
Professor 2:
Water is the only substance on Earth that we routinely encounter as a solid, liquid, and gas. At low temperatures, the water molecules lock into a rigid structure, but as the temperature increases, the average kinetic energy of the water molecules increases and the molecules are able to move more creating its other natural states of matter. The higher the temperature, the more likely water is to be a gas. Water is proof of the kinetic theory, which assumes that there is no force of attraction between the particles of the gas state. The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law, since there is no interaction between the gaseous molecules. This law accounts for all of the forces that occur with gases on Earth.
Q. Which of the following statements would professor 2 agree with?
a)
Van der Waals' equation most closely mirrors the gas interactions that occur in nature.
b)
The Ideal Gas Law most closely mirrors the gas interactions that occur in nature.
c)
At low temperatures, water is present as a gas.
d)
The state of water is dependent upon the strength of intramolecular forces and the thermal energy present in the system.
|
|
Ayesha Joshi answered |
Professor 2 states "The best fit for experimental data involving water in a gaseous form is found by using the Ideal Gas Law" so the correct answer is "The Ideal Gas Law most closely mirrors the gas interactions that occur in nature."
Additionally, "Van der Waals' equation most closely mirrors the gas interactions that occur in nature." and "The state of water is dependent upon the strength of intramolecular forces and the thermal energy present in the system." are both statements that match up with the first professor's statements. Finally, professor 2 states "The higher the temperature, the more likely water is to be a gas" , not "At low temperatures, water is present as a gas."
Chapter doubts & questions for Conflicting Viewpoints - Science for ACT 2025 is part of ACT exam preparation. The chapters have been prepared according to the ACT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for ACT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Conflicting Viewpoints - Science for ACT in English & Hindi are available as part of ACT exam.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Science for ACT
486 videos|517 docs|337 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup










