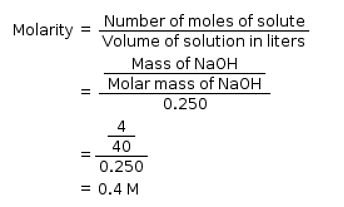All Exams >
ACT >
Science for ACT >
All Questions
All questions of Some Basic Concepts Of Chemistry for ACT Exam
Number of significant figures in 0.001520 is:a)4b)7c)6d)3Correct answer is 'A'. Can you explain this answer?

|
Shruti Mehta answered |
All non-zero digits are significant. Zeros before the decimal point are not significant. Zeros to the right of the decimal point are significant.
Can you explain the answer of this question below:Molecular mass of glucose molecule (C6H12O6) is:
- A:
180 g
- B:
172 g
- C:
182 g
- D:
192 g
The answer is a.
Molecular mass of glucose molecule (C6H12O6) is:
180 g
172 g
182 g
192 g

|
Samridhi Kaur answered |
Molecular mass : The sum of the atomic masses of all the atoms in a molecule of a substance is called the molecular mass of the molecule.
Generally we use relative atomic masses of atoms for calculating the molecular mass of 1 mole of any molecular or ionic substances.
Molecular formula of glucose is C6H12O6
Atomic mass of H = 1
Atomic mass pf C = 12
Atomic mass of O = 16
Molecular mass of C6H12O6 = 12(Atomic mass of Hydrogen) + 6(Atomic mass of carbon) + 6(Atomic mass of oxygen)
= 12 x 1 + 6X12 + 6X16
= 12 + 72 + 96 = 180 u.
If we add the numbers 6.68 and 0.8, the result will be:
- a)7.4
- b)7.5
- c)7.58
- d)7.48
Correct answer is option 'B'. Can you explain this answer?
If we add the numbers 6.68 and 0.8, the result will be:
a)
7.4
b)
7.5
c)
7.58
d)
7.48

|
Simran Mishra answered |
During addition, the result cannot have more digits to the right of the decimal point than either of the original numbers. Since there is only 1 digit after the decimal point in 0.8 so the result 7.48 should be rounded off to 1 decimal point i.e. 7.5.
The scientific notation for 0.00016 is:- a)1.6 Χ 10-4
- b)16 Χ 10-4
- c)1.6 Χ 10-2
- d)1.6 Χ 104
Correct answer is option 'A'. Can you explain this answer?
The scientific notation for 0.00016 is:
a)
1.6 Χ 10-4
b)
16 Χ 10-4
c)
1.6 Χ 10-2
d)
1.6 Χ 104
|
|
Arya Dasgupta answered |
Here, the decimal has to be moved four places to the right side and hence -4 is the exponent in the scientific notation.
Can you explain the answer of this question below:If the true value for a result is 2.00 g and a student takes 2 measurements and reports the results as 1.95 g and 1.93 g, then we can conclude that:
a) The values are both precise and accurateb) The values are accuratec) The value are neither precise not accurated) The values are precisee) The values are precise but not accurateCorrect answer option is E.
|
|
Anjana Sharma answered |
Precision indicates how closely repeated measurements match each other. Since both the values are close to each other, they are precise and since there is a difference between the mean value and true value, they are not accurate.
Which law is also known as Law of constant composition?- a)Law of multiple proportions
- b)Avogadro’s law
- c)Law of conservation of mass
- d)Law of definite proportions
Correct answer is option 'D'. Can you explain this answer?
Which law is also known as Law of constant composition?
a)
Law of multiple proportions
b)
Avogadro’s law
c)
Law of conservation of mass
d)
Law of definite proportions
|
|
Pooja Shah answered |
The law of definite proportions, also known as the law of constant composition states that all pure samples of the same chemical compound contain the same elements combined in the same proportions by mass.
What this law emphasizes is that, if pure samples of the same chemical substance, wherever they may be found, are analyzed, it will be found that they all consist of the same elements, as well as having these elements combine in the same proportions by mass.
For examples, pure sample of copper(II) oxide is composed of copper and oxygen, in the proportion of 1:1 by mole, or 64 g of copper to 16 g of oxygen or 1 g of copper to 0.25 g of oxygen.
What this law emphasizes is that, if pure samples of the same chemical substance, wherever they may be found, are analyzed, it will be found that they all consist of the same elements, as well as having these elements combine in the same proportions by mass.
For examples, pure sample of copper(II) oxide is composed of copper and oxygen, in the proportion of 1:1 by mole, or 64 g of copper to 16 g of oxygen or 1 g of copper to 0.25 g of oxygen.
Vapour density of a gas is 22. Its molecular mass will be:- a)33g
- b)44g
- c)22g
- d)11g
Correct answer is 'B'. Can you explain this answer?
Vapour density of a gas is 22. Its molecular mass will be:
a)
33g
b)
44g
c)
22g
d)
11g
|
|
Nandini Patel answered |
Molecular mass = 2 x vapour density= 2 x 22= 44Therefore, molecular mass of a gas will be 44.
Can you explain the answer of this question below:Number of nitrogen atoms present in 1.4 g of N2:- A:6.023 X 1022
- B:6.023 X 1023
- C:3.012 X 1023
- D:3.012 X 1022
The answer is a.
Number of nitrogen atoms present in 1.4 g of N2:
A:
6.023 X 1022
B:
6.023 X 1023
C:
3.012 X 1023
D:
3.012 X 1022
|
|
Raghav Bansal answered |
Given weight of N₂ gas = 1.4 g
Molar mass of N₂ gas = 28 g
So, mole = given mass/ molar mass
⇒ mole = 1.4/28 = 1/20 mole
Now, number of molecules = mole × avogadro number
⇒ number of molecules = 1/20 × 6.022 × 10²³
⇒ number of molecules = 3.011 × 10²²
Now, we are asked for number of atoms. In N₂, there are 2 atoms, so to obtain number of atoms we will multiply with 2 in number of molecules.
⇒ number of atoms = 2 × 3.011 × 10²²
⇒ number of atoms = 6.022 × 10²²
What is the mass of 0.20 mole of C2H5OH (ethanol)?- a)46 g
- b)23 g
- c)4.6 g
- d)none of these
Correct answer is option 'D'. Can you explain this answer?
What is the mass of 0.20 mole of C2H5OH (ethanol)?
a)
46 g
b)
23 g
c)
4.6 g
d)
none of these
|
|
Neha Joshi answered |
Molecular Mass of Ethanol
= 2(12) + 5 (1) + 16 + 1
= 24 + 5 + 17
= 46
Now,
Moles (n) = Given Mass (m)/Molecular Mass (M)
Thus, to = nM
= (0.2) (46)
= 9.2
= 2(12) + 5 (1) + 16 + 1
= 24 + 5 + 17
= 46
Now,
Moles (n) = Given Mass (m)/Molecular Mass (M)
Thus, to = nM
= (0.2) (46)
= 9.2
Can you explain the answer of this question below:Molar mass of F2 is 38 g. How many atoms are present in 0.147 mole of F2?
- A:
6.02 Χ 1023 atoms
- B:
8.78 Χ 10222 atoms
- C:
0.292 atoms
- D:
1.76 Χ 1023 atoms
The answer is d.
Molar mass of F2 is 38 g. How many atoms are present in 0.147 mole of F2?
6.02 Χ 1023 atoms
8.78 Χ 10222 atoms
0.292 atoms
1.76 Χ 1023 atoms

|
Puja Das answered |
One molecule of F2 contains 2 atoms of fluorine.
1 mol of fluorine contains 2 X 6.023 X 1023 atoms
So, 0.147 moles will contain 0.147 X 2 X 6.023 X 1023 = 1.76 X 1023 atoms of flourine.
The molar mass of ZnSO4 is- a)161.47 g
- b)136.4g
- c)166.4g
- d)156.4g
Correct answer is option 'A'. Can you explain this answer?
The molar mass of ZnSO4 is
a)
161.47 g
b)
136.4g
c)
166.4g
d)
156.4g
|
|
Lavanya Menon answered |
Chemical Formula: ZnSO4
Molar Mass: 161.47 g/mol (anhydrous)
Molar Mass: 161.47 g/mol (anhydrous)
Vapour density of a gas is 22. Its molecular mass will be:- a)33g
- b)44g
- c)22g
- d)11g
Correct answer is option 'B'. Can you explain this answer?
Vapour density of a gas is 22. Its molecular mass will be:
a)
33g
b)
44g
c)
22g
d)
11g

|
Ameya Mukherjee answered |
Molecular mass = 2 x vapour density
= 2 x 22
= 44
Therefore, molecular mass of a gas will be 44.
= 2 x 22
= 44
Therefore, molecular mass of a gas will be 44.
The coefficient in 6.65 x 104 is:- a)10-4
- b)6.65
- c)6.65 x 104
- d)104
Correct answer is 'B'. Can you explain this answer?
The coefficient in 6.65 x 104 is:
a)
10-4
b)
6.65
c)
6.65 x 104
d)
104
|
|
Om Desai answered |
Here 6.65 is the coefficient and 104 is the exponent.
The ‘Candela’ is the unit of:- a)Luminous intensity
- b)Amount of substance
- c)Thermodynamic temperature
- d)Electric curren
Correct answer is option 'A'. Can you explain this answer?
The ‘Candela’ is the unit of:
a)
Luminous intensity
b)
Amount of substance
c)
Thermodynamic temperature
d)
Electric curren
|
|
Anjana Sharma answered |
The SI unit of luminous intensity is the candela , which is a primary unit. The derived SI unit of luminous flux is the lumen (abbreviated lm). These are based on the sensitivity of the human eye to light. Light sources are often evaluated based on their luminous efficacy, which is defined as the luminous flux divided by the power consumed and is measured in lm W−1. In a physical or online store, find manufacturer’s specifications for representative incandescent, halogen, high-temperature-discharge, LED, and fluorescent lamps of similar luminous flux and compare their luminous efficacy.
Can you explain the answer of this question below:100mL of gaseous hydrogen combines with 50mL of gaseous oxygen to give 100mL of water vapours. This can be explained on the basis of:- A:Law of definite proportions
- B:Gay Lussac’s law
- C:Law of multiple proportions
- D:Avogadro law
The answer is b.
100mL of gaseous hydrogen combines with 50mL of gaseous oxygen to give 100mL of water vapours. This can be explained on the basis of:
A:
Law of definite proportions
B:
Gay Lussac’s law
C:
Law of multiple proportions
D:
Avogadro law
|
|
Nandini Patel answered |
Volume of 17g of NH3 at N.T.P. will be- a)22.4 L
- b)2.24 L
- c)4.48 L
- d)44.8 L
Correct answer is 'A'. Can you explain this answer?
Volume of 17g of NH3 at N.T.P. will be
a)
22.4 L
b)
2.24 L
c)
4.48 L
d)
44.8 L
|
|
Neha Sharma answered |
Molar mass of NH3 is 17g. So the volume of 17g of NH3 i.e 1 mole NH3 at N.T.P. will be 22.4L.
How many moles of NaCl should be dissolved in 100 mL of water to get 0.2 M solution?- a)0.2
- b)0.02
- c)0.01
- d)0.1
Correct answer is option 'B'. Can you explain this answer?
How many moles of NaCl should be dissolved in 100 mL of water to get 0.2 M solution?
a)
0.2
b)
0.02
c)
0.01
d)
0.1

|
Sai Mishra answered |
Molarity = no of moles /volume( in litre) 0.2 =no of moles / 0.1 No of moles =0.2 × 0.1 = 0.02
Which of the following has maximum number of moles?- a)1g of O2
- b)1g of H2
- c)1g of Cl2
- d)1g of N2
Correct answer is 'B'. Can you explain this answer?
Which of the following has maximum number of moles?
a)
1g of O2
b)
1g of H2
c)
1g of Cl2
d)
1g of N2

|
Priyanka Roy answered |
1g hydrogen has the maximum number of moles because atomic mass of hydrogen is smallest among all.
Number of significant figures in 0.001520 is:- a)0
- b)7
- c)6
- d)3
Correct answer is option 'A'. Can you explain this answer?
Number of significant figures in 0.001520 is:
a)
0
b)
7
c)
6
d)
3

|
Raghav Chakraborty answered |
All non-zero digits are significant. Zeros before the decimal point are not significant. Zeros to the right of the decimal point are significant.
The SI unit of time is:- a)minute
- b)metre
- c)second
- d)kilogram
Correct answer is option 'C'. Can you explain this answer?
The SI unit of time is:
a)
minute
b)
metre
c)
second
d)
kilogram
|
|
Krishna Iyer answered |
The SI unit of time, the second, symbols, is easy enough to define, but time itself can only be defined in reference to other quantities.
For the reaction 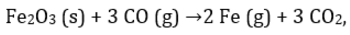 224 g of CO is available to react with 400 g Fe2O3, the yield of iron and CO2, are:
224 g of CO is available to react with 400 g Fe2O3, the yield of iron and CO2, are:- a)225 and 279
- b)280 and 330 g
- c)210 and 290
- d)210 and 279 g
Correct answer is 'B'. Can you explain this answer?
For the reaction
224 g of CO is available to react with 400 g Fe2O3, the yield of iron and CO2, are:
a)
225 and 279
b)
280 and 330 g
c)
210 and 290
d)
210 and 279 g
|
|
Neha Sharma answered |
Moles of CO =8 moles Moles of Fe2O3= 2.5 moles.
3 moles of CO is needed for 1 mole of Fe2O3 so 8 moles of CO will require 2.66 mole of Fe2O3 so Fe2O3 is limiting reagent.
1 mole of Fe2O3 produce 2 mole of Fe so 2.5 mole of Fe2O3will produce 5 mole of Fe = 280g of Fe.
Also 1 mole of Fe2O3 also produce 3 mole of CO2 so 2.5 mole of Fe2O3 will produce 7.5 mole of CO2=330g.
Can you explain the answer of this question below:The number of significant figures in 3.1205 are
- A:
3
- B:
7
- C:
6
- D:
5
The answer is d.
The number of significant figures in 3.1205 are
3
7
6
5

|
Arpita Nambiar answered |
The answer is 5 because all non zero digits and zero between the non zero digits are significant.
Atomic mass of bromine is 80 g. The mass of four moles of molecular bromine (Br2) is:- a)80 g
- b)40 g
- c)640 g
- d)320 g
Correct answer is option 'C'. Can you explain this answer?
Atomic mass of bromine is 80 g. The mass of four moles of molecular bromine (Br2) is:
a)
80 g
b)
40 g
c)
640 g
d)
320 g
|
|
Nandini Patel answered |
Mass of one mole of Br2 is 160 g.
Hence mass of four moles of molecular bromine is 4 X 160 = 640 g.
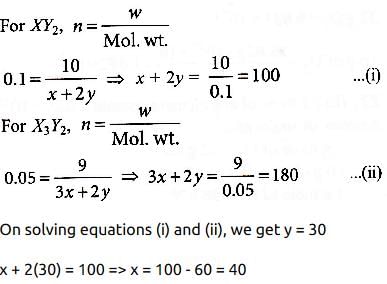
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are- a)40, 30
- b)60, 40
- c)20, 30
- d)30, 20
Correct answer is option 'A'. Can you explain this answer?
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are
a)
40, 30
b)
60, 40
c)
20, 30
d)
30, 20
|
|
Pranavi Chopra answered |
Let atomic weight of element X is x and that of element Y is y
What will be the molality of the solution containing 18.25 g of HCl gas in 500 g of water?- a)0.1 m
- b)1 M
- c)0.5 m
- d)1 m
Correct answer is option 'D'. Can you explain this answer?
What will be the molality of the solution containing 18.25 g of HCl gas in 500 g of water?
a)
0.1 m
b)
1 M
c)
0.5 m
d)
1 m

|
Saikat Sharma answered |
Given:
Mass of HCl = 18.25 gm
Molar mass of HCl = 36.5 g/mol
Mass of water = 500 gm
= 0.5 kg
No. of moles of HCl;

Molality is,

Molality of solution is 1 m.
Can you explain the answer of this question below:The number of base SI units is:- A:5
- B:7
- C:6
- D:2
The answer is b.
The number of base SI units is:
A:
5
B:
7
C:
6
D:
2
|
|
Om Desai answered |
Base system means fundamental physical quantities and there are 9 fundamental physical quantities but only 7 have units so number of base SI units is 7.
The molar mass of Al2O3 is- a)112
- b)82
- c)102
- d)92
Correct answer is option 'C'. Can you explain this answer?
The molar mass of Al2O3 is
a)
112
b)
82
c)
102
d)
92
|
|
Pooja Shah answered |
Molar mass = 2(27) +3 (16) = 102g.
There are ____ in 12.0 ml?- a)0.12 L
- b)120 L
- c)0.012 L
- d)12000 L
Correct answer is option 'C'. Can you explain this answer?
There are ____ in 12.0 ml?
a)
0.12 L
b)
120 L
c)
0.012 L
d)
12000 L
|
|
Riya Banerjee answered |
Since we know that 1litre -1000ml 1ml-1\1000litre So,12ml-1/1000×12=12/1000 =0.012
The correct relationship between picometer and nanometer is- a)1nm = 1000pm
- b)1nm = 10pm
- c)1pm = 10nm
- d)1pm = 100nm
Correct answer is option 'A'. Can you explain this answer?
The correct relationship between picometer and nanometer is
a)
1nm = 1000pm
b)
1nm = 10pm
c)
1pm = 10nm
d)
1pm = 100nm
|
|
Neha Joshi answered |
1 picometer = 10-12 m
1 nanometer=10-9 m
So, 1 picometer = 0.001 nanometer .
From this we get 1 nanometer= 1000 picometer.
1 nanometer=10-9 m
So, 1 picometer = 0.001 nanometer .
From this we get 1 nanometer= 1000 picometer.
Hence, the correct answer is Option A.
Laws of chemical combinations can be explained on the basis of:- a)Avogadro law
- b)Gay lussacs law
- c)Dalton’s atomic theory
- d)Mole concept
Correct answer is option 'C'. Can you explain this answer?
Laws of chemical combinations can be explained on the basis of:
a)
Avogadro law
b)
Gay lussacs law
c)
Dalton’s atomic theory
d)
Mole concept
|
|
Gaurav Kumar answered |
The old ideas were put on a scientific scale by John Dalton in the form of a theory, known as Dalton’s atomic theory,
Main postulates of which are as follows :
• All matters are made of atoms. Atoms are indivisible and indestructible.
• All atoms of a given element are identical in mass and properties.
• Atoms of different elements differ in properties and have different masses and sizes.
• Compounds are formed by a combination of two or more different kinds of atoms.
• A chemical reaction is a rearrangement of atoms.These are neither created nor destroyed in a chemical reaction
Main postulates of which are as follows :
• All matters are made of atoms. Atoms are indivisible and indestructible.
• All atoms of a given element are identical in mass and properties.
• Atoms of different elements differ in properties and have different masses and sizes.
• Compounds are formed by a combination of two or more different kinds of atoms.
• A chemical reaction is a rearrangement of atoms.These are neither created nor destroyed in a chemical reaction
Can you explain the answer of this question below:Out of the numbers: 6.26, 5.8, 0.00267, 0.03, the one with the least significant figures is:
- A:
0.03
- B:
5.8
- C:
0.00267
- D:
6.26
The answer is a.
Out of the numbers: 6.26, 5.8, 0.00267, 0.03, the one with the least significant figures is:
0.03
5.8
0.00267
6.26
|
|
Stuti Joshi answered |
6.26 & 0.00267 contains 3 significant figures while 5.8 has two significant figures and 0.03 contains least that is 1.
A statement which is not a part of Dalton’s atomic theory is:- a)Matter is made of atoms.
- b)All atoms of a given element are identical.
- c)Atoms are composed of sub-atomic particles called electron, proton and neutron.
- d)Atoms of different elements have different mass and different properties.
Correct answer is option 'C'. Can you explain this answer?
A statement which is not a part of Dalton’s atomic theory is:
a)
Matter is made of atoms.
b)
All atoms of a given element are identical.
c)
Atoms are composed of sub-atomic particles called electron, proton and neutron.
d)
Atoms of different elements have different mass and different properties.
|
|
Shreya Gupta answered |
According to the Dalton’s atomic theory, atoms are indivisible i.e. they are not composed of any sub-atomic particles
How many atoms of hydrogen are in 67.2 L of H2 at STP?
- a)5.612 × 1024
- b)2.612 × 1024
- c)4.612 × 1024
- d)3.6132 × 1024
Correct answer is option 'D'. Can you explain this answer?
How many atoms of hydrogen are in 67.2 L of H2 at STP?
a)
5.612 × 1024
b)
2.612 × 1024
c)
4.612 × 1024
d)
3.6132 × 1024
|
|
Anjana Sharma answered |
Standard temperature and pressure (STP) is defined as 0 degrees Celsius and 1 atmosphere of pressure. At STP, 1 mole of any gas occupies 22.4 liters.
First, calculate the number of moles of hydrogen gas (H2) in 67.2 liters:
67.2 L / 22.4 L/mole = 3 moles of H2
Each molecule of H2 contains 2 atoms of hydrogen. Therefore, 3 moles of H2 contains:
3 moles * (6.022 x 10^23 molecules/mole) * 2 atoms/molecule = 3.6132 x 10^24 atoms of hydrogen.
First, calculate the number of moles of hydrogen gas (H2) in 67.2 liters:
67.2 L / 22.4 L/mole = 3 moles of H2
Each molecule of H2 contains 2 atoms of hydrogen. Therefore, 3 moles of H2 contains:
3 moles * (6.022 x 10^23 molecules/mole) * 2 atoms/molecule = 3.6132 x 10^24 atoms of hydrogen.
Which of the following statements is/are correct?- a)Two or more atoms combine to give molecules
- b)The atoms of different elements are present in a compound in a fixed and definite ratio
- c)The constituents of a compound can be separated by physical and chemical methods
- d)Both (a) and (b)
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements is/are correct?
a)
Two or more atoms combine to give molecules
b)
The atoms of different elements are present in a compound in a fixed and definite ratio
c)
The constituents of a compound can be separated by physical and chemical methods
d)
Both (a) and (b)
|
|
Preeti Khanna answered |
- Two or more atoms combine to give molecules.
- The atoms of different elements are present in a compound in a fixed and definite ratio.
- These two are correct by concepts of chemistry.
The unit of amount of substance is:- a)ampere
- b)second
- c)mole
- d)kilogram
Correct answer is option 'C'. Can you explain this answer?
The unit of amount of substance is:
a)
ampere
b)
second
c)
mole
d)
kilogram
|
|
Pooja Shah answered |
- Amount of Substance is also called as material quantity. The amount of substance refers to a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles.
- The amount of substance appears in thermodynamic relations such as the ideal gas law, and in stoichiometric relations between reacting molecules as in the law of multiple proportions. The unit symbol of the amount of substance is mole.
Which of the following is an element?- a)Sugar solution
- b)Brass
- c)HCl
- d)Copper metal
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an element?
a)
Sugar solution
b)
Brass
c)
HCl
d)
Copper metal
|
|
Krishna Iyer answered |
Copper is a chemical element with symbol Cu and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity.
The distinction between atoms and molecules was made by:- a)Dalton’s atomic theory
- b)Avogadro law
- c)Laws of chemical combination
- d)Gay lussacs law
Correct answer is 'B'. Can you explain this answer?
The distinction between atoms and molecules was made by:
a)
Dalton’s atomic theory
b)
Avogadro law
c)
Laws of chemical combination
d)
Gay lussacs law
|
|
Preeti Iyer answered |
Avogadro made the distinction between atoms and molecules, which today seems clear. However, Dalton rejected Avogadro's hypothesis because Dalton believed that atoms of the same kind could not combine. Since it was believed that atoms were held together by an electrical force, only unlike atoms would be attracted together, and like atoms should repel. Therefore it seemed impossible for a molecule of oxygen, O2, to exist. Avogadro's work, even if it was read appears not to have been understood, and was pushed into the dark recesses of chemistry libraries and ignored. Avogadro continued to teach at the university of Turin, when it was not closed because of the political upheavals going on in Italy at the time, and died in 1854, an unknown figure.
Which one of the following is not a mixture?- a)Distilled water
- b)Sugar dissolved in water
- c)Liquefied Petroleum Gas (LPG)
- d)Gasoline
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is not a mixture?
a)
Distilled water
b)
Sugar dissolved in water
c)
Liquefied Petroleum Gas (LPG)
d)
Gasoline
|
|
Gaurav Kumar answered |
Distilled water is the pure form of water with no impurities present in it; others are the mixture of two or more components.
If the true value for a result is 2.00 g and a student takes 2 measurements and reports the results as 1.95 g and 1.93 g, then we can conclude that:- a)The values are both precise and accurate
- b)The value are neither precise nor accurate
- c)The values are accurate
- d)The values are precise
Correct answer is option 'D'. Can you explain this answer?
If the true value for a result is 2.00 g and a student takes 2 measurements and reports the results as 1.95 g and 1.93 g, then we can conclude that:
a)
The values are both precise and accurate
b)
The value are neither precise nor accurate
c)
The values are accurate
d)
The values are precise
|
|
Anjana Sharma answered |
Precision indicates how closely repeated measurements match each other. Since both the values are close to each other, they are precise and since their is a difference between the mean value and true value, they are not accurate.
The molar mass of AgNO3 is- a)169.87 g
- b)189.9
- c)179.9
- d)159.9
Correct answer is option 'A'. Can you explain this answer?
The molar mass of AgNO3 is
a)
169.87 g
b)
189.9
c)
179.9
d)
159.9
|
|
Geetika Shah answered |
Molar mass of AgNO3 = mass of Ag + N + O3 = 107.87 + 14 + 3* 16 = 107. 87 + 14 + 48 = 169.87 g
What is incorrect about the Law of conservation of mass?- a)A given compound always conatains exactly the same proportion of elements by weight
- b)Mass of reactants is equal to the mass of products
- c)Matter can neither be created nor destroyed
- d)It was given by Antoine Lavoiser
Correct answer is option 'A'. Can you explain this answer?
What is incorrect about the Law of conservation of mass?
a)
A given compound always conatains exactly the same proportion of elements by weight
b)
Mass of reactants is equal to the mass of products
c)
Matter can neither be created nor destroyed
d)
It was given by Antoine Lavoiser
|
|
Jyoti Kumar answered |
The law of definite proportions, also known law of definite composition, states that regardless of the amount, a pure compound always contains the same elements in the same proportions by mass. Law of multiple proportions, also known as Dalton s Law, states that when one element combines with another to form more than one compound, the mass rations of the elements in the compounds are simple whole numbers of each other.
Law of conservation of mass was given by:- a)Avogadro
- b)Antoine Lavoisier
- c)John Dalton
- d)Joseph Proust
Correct answer is option 'B'. Can you explain this answer?
Law of conservation of mass was given by:
a)
Avogadro
b)
Antoine Lavoisier
c)
John Dalton
d)
Joseph Proust
|
|
Lavanya Menon answered |
The Law of Conservation of Mass (or Matter) in a chemical reaction can be stated thus:
In a chemical reaction, matter is neither created nor destroyed.
It was discovered by Antoine Laurent Lavoisier (1743-94) about 1785. However, philosophical speculation and even some quantitative experimentation preceeded him. In addition, he was certainly not the first to accept this law as true or to teach it, but he is credited as its discoverer.
In a chemical reaction, matter is neither created nor destroyed.
It was discovered by Antoine Laurent Lavoisier (1743-94) about 1785. However, philosophical speculation and even some quantitative experimentation preceeded him. In addition, he was certainly not the first to accept this law as true or to teach it, but he is credited as its discoverer.
The number of significant figures in 3256 is:- a)4
- b)3
- c)5
- d)2
Correct answer is option 'A'. Can you explain this answer?
The number of significant figures in 3256 is:
a)
4
b)
3
c)
5
d)
2
|
|
Suresh Reddy answered |
3256, This number has four non zero numbers. That's why it has four significant figures.
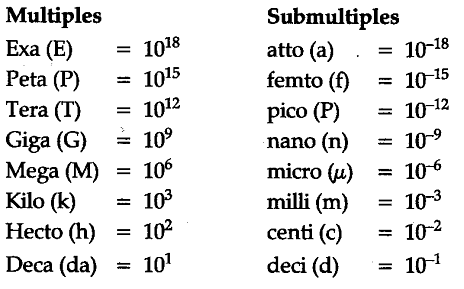
The prefix used for 10-18 is:- a)atto
- b)centi
- c)kilo
- d)deci
Correct answer is option 'A'. Can you explain this answer?
The prefix used for 10-18 is:
a)
atto
b)
centi
c)
kilo
d)
deci
|
|
Geetika Shah answered |
The prefix used for 10-18 is: atto-
Can you explain the answer of this question below:How many atoms of Oxygen are there in 18g of water? (Hint: Avagadro’s Number = 6.02 x 1023 atoms/mol)- a:8.02 x 1023
- b:6.02 x 1023
- c:5 .02 x 1023
- d:7.02 x 1023
Correct answer is 'b'.
How many atoms of Oxygen are there in 18g of water? (Hint: Avagadro’s Number = 6.02 x 1023 atoms/mol)
a:
8.02 x 1023
b:
6.02 x 1023
c:
5 .02 x 1023
d:
7.02 x 1023
|
|
Gaurav Kumar answered |
18g H2O = 1mol water = 6.02 x 1023 molecules of water = 6.02 x 1023 atoms of oxygen.
Can you explain the answer of this question below:Choose the most appropriate answer amongst the options given below for the statement. A solution of a desired concentration is prepared by diluting- a:solution of known higher concentration
- b:solution of known lower concentration
- c:from a serially diluted solution
- d:stock solution.
Correct answer is 'd'.
Choose the most appropriate answer amongst the options given below for the statement. A solution of a desired concentration is prepared by diluting
a:
solution of known higher concentration
b:
solution of known lower concentration
c:
from a serially diluted solution
d:
stock solution.
|
|
Geetika Shah answered |
Stock solution is diluted to prepare the solution of desired concentration.
A measured temperature is 100 0F on Fahrenheit scale, then what is this reading be on Celsius scale :- a)11.2 0C
- b)78 0C
- c)102.7 0C
- d)37.8 0C
Correct answer is option 'D'. Can you explain this answer?
A measured temperature is 100 0F on Fahrenheit scale, then what is this reading be on Celsius scale :
a)
11.2 0C
b)
78 0C
c)
102.7 0C
d)
37.8 0C
|
|
Krishna Iyer answered |
C-0/100-0 = F-32/180.
C/5= F-32/9.
C/5= 100-32/9.
C/5= 68/9.
C= 68×5/9.
C= 340/9.
C= 37.77.
C= 37.8
C/5= F-32/9.
C/5= 100-32/9.
C/5= 68/9.
C= 68×5/9.
C= 340/9.
C= 37.77.
C= 37.8
Can you explain the answer of this question below:Consider the reaction between hydrogen and oxygen gases to form water. Which of the following is/are not conserved in the reaction?
2H2(g) + O2(g) → 2H2O(l)- A:Atoms
- B:Mass
- C:Both moles of molecules and moles of atoms
- D:Moles of molecules
The answer is d.
Consider the reaction between hydrogen and oxygen gases to form water. Which of the following is/are not conserved in the reaction?
2H2(g) + O2(g) → 2H2O(l)
2H2(g) + O2(g) → 2H2O(l)
A:
Atoms
B:
Mass
C:
Both moles of molecules and moles of atoms
D:
Moles of molecules
|
|
Lavanya Menon answered |
In the equation both side of reaction moles of atoms is conserved ie, both reactant and product contain 4H and 2O but there is 3 (2+1) moles of reactant molecules turns into 2 moles of product molecules.
Chemistry does not play a major role in- a)Design and synthesis new materials having specific magnetic, electric and optical properties.
- b)Large scale production of a variety of fertilizers
- c)Explaining superconductivity
- d)Explaining ozone depletion
Correct answer is option 'C'. Can you explain this answer?
Chemistry does not play a major role in
a)
Design and synthesis new materials having specific magnetic, electric and optical properties.
b)
Large scale production of a variety of fertilizers
c)
Explaining superconductivity
d)
Explaining ozone depletion
|
|
Rajesh Gupta answered |
Chemistry does not deal in explaining superconductivity.
Chapter doubts & questions for Some Basic Concepts Of Chemistry - Science for ACT 2025 is part of ACT exam preparation. The chapters have been prepared according to the ACT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for ACT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Some Basic Concepts Of Chemistry - Science for ACT in English & Hindi are available as part of ACT exam.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Science for ACT
486 videos|517 docs|337 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup