All Exams >
ACT >
Biology for ACT >
All Questions
All questions of Biomolecules for ACT Exam
Which is true about enzymes?a)All enzymes are ... moreproteins.b)All proteins are enzymes.c)All enzymes are not proteins.d)All enzymes are vitamins.Correct answer is option 'C'. Can you explain this answer?
|
|
Gaurav Kumar answered |
Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. The latter are called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures.
The turnover of biomolecules is together called- a)Anabolism
- b)Bioenergetics
- c)Catabolism
- d)Metabolism
Correct answer is option 'D'. Can you explain this answer?
The turnover of biomolecules is together called
a)
Anabolism
b)
Bioenergetics
c)
Catabolism
d)
Metabolism
|
|
Hansa Sharma answered |
All the biomolecules are constantly being changed into some other biomolecules and also made from some other biomolecules. The turnover of biomolecules takes place continuously. All these reactions are together called metabolism.
Cellulose is made up of- a)Fructose
- b)Glucose
- c)Sucrose
- d)Ribose
Correct answer is option 'B'. Can you explain this answer?
Cellulose is made up of
a)
Fructose
b)
Glucose
c)
Sucrose
d)
Ribose
|
|
Ayush Chauhan answered |
Cellulose is a third polymer made from beta glucose molecules and the polymer molecules are straight cellulose serves a very different purpose in nature to starch and glycogen it make up the cell walls in plant cell.
The bacterial cell wall is formed of- a)Cellulose
- b)Hemicellulose
- c)Peptidoglycan
- d)Glycogen
Correct answer is option 'C'. Can you explain this answer?
The bacterial cell wall is formed of
a)
Cellulose
b)
Hemicellulose
c)
Peptidoglycan
d)
Glycogen
|
|
Pooja Shah answered |
Bacterial cell walls are made of peptidoglycan (also called murein), which is made from polysaccharide chains cross-linked by unusual peptides containing D-amino acids. Bacterial cell walls are different from the cell walls of plants and fungi which are made of cellulose and chitin, respectively.
The number of amino acids found in proteins are- a)20
- b)21
- c)18
- d)16
Correct answer is option 'A'. Can you explain this answer?
The number of amino acids found in proteins are
a)
20
b)
21
c)
18
d)
16
|
|
Rohan Singh answered |
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. ... Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.
The most abundant mineral of the animal body is- a)Potassium
- b)Sodium
- c)Calcium
- d)Iron
Correct answer is option 'C'. Can you explain this answer?
The most abundant mineral of the animal body is
a)
Potassium
b)
Sodium
c)
Calcium
d)
Iron
|
|
Hansa Sharma answered |
Calcium is the most abundant mineral in our body. It is essential for the development and maintenance of strong bones and teeth, where about 99% of the body's calcium is found. Calcium also helps the heart, nerves, muscles, and other body systems work properly.
Therefore, the correct answer is option C.
A homopolymer has only one type of building block called a monomer repeated ‘n’ number of times. A heteropolymer has more than one type of monomer. Proteins are heteropolymers made of amino acids. While a nucleic acid like DNA or RNA is made of only 4 types of nucleotide monomers, proteins are made of- a)20 types of monomers
- b)3 types of monomers
- c)40 types of monomers
- d)Only one type of monomer
Correct answer is option 'A'. Can you explain this answer?
A homopolymer has only one type of building block called a monomer repeated ‘n’ number of times. A heteropolymer has more than one type of monomer. Proteins are heteropolymers made of amino acids. While a nucleic acid like DNA or RNA is made of only 4 types of nucleotide monomers, proteins are made of
a)
20 types of monomers
b)
3 types of monomers
c)
40 types of monomers
d)
Only one type of monomer

|
Syed Hussain answered |
In living systems, like our own bodies, these larger molecules include carbohydrates, lipids, nucleic acids and proteins. The monomers of these organic groups are: Carbohydrates - monosaccharides. Lipids - glycerol and fatty acids
Which of the following is a catabolic pathway?- a)Photosynthesis
- b)Formation of proteins from amino acids
- c)Conversion of acetic acid to cholesterol
- d)Conversion of glucose to lactic acid
Correct answer is option 'D'. Can you explain this answer?
Which of the following is a catabolic pathway?
a)
Photosynthesis
b)
Formation of proteins from amino acids
c)
Conversion of acetic acid to cholesterol
d)
Conversion of glucose to lactic acid
|
|
Ankit Patel answered |
Catabolic pathways are metabolic pathways that involve the breakdown of complex molecules into simpler ones with the release of energy. The correct answer is option D, which states the conversion of glucose to lactic acid.
Explanation:
• Glucose is a simple sugar that is an important source of energy for living organisms.
• The breakdown of glucose into simpler molecules is called glycolysis, which is the first step in cellular respiration.
• During glycolysis, glucose is converted to pyruvate, which can be further metabolized to produce energy.
• In the absence of oxygen, pyruvate is converted to lactic acid through a process called fermentation.
• This process releases a small amount of energy and regenerates NAD+ for further glycolysis.
Therefore, the conversion of glucose to lactic acid is a catabolic pathway as it involves the breakdown of glucose into simpler molecules with the release of energy.
Explanation:
• Glucose is a simple sugar that is an important source of energy for living organisms.
• The breakdown of glucose into simpler molecules is called glycolysis, which is the first step in cellular respiration.
• During glycolysis, glucose is converted to pyruvate, which can be further metabolized to produce energy.
• In the absence of oxygen, pyruvate is converted to lactic acid through a process called fermentation.
• This process releases a small amount of energy and regenerates NAD+ for further glycolysis.
Therefore, the conversion of glucose to lactic acid is a catabolic pathway as it involves the breakdown of glucose into simpler molecules with the release of energy.
The most abundant organic molecule present on earth is- a)Protein
- b)Lipid
- c)Steroids
- d)Cellulose
Correct answer is option 'D'. Can you explain this answer?
The most abundant organic molecule present on earth is
a)
Protein
b)
Lipid
c)
Steroids
d)
Cellulose
|
|
Vijay Bansal answered |
The most abundant organic compound on Earth is cellulose, which is made up of many, many glucose molecules all linked together. Cellulose is found in the cell walls of every plant, which is why it is the most abundant organic molecule.
Can you explain the answer of this question below:The energy content in kcal/g of carbohydrate:protein:triglycerol is approximately in the ratio of
- A:
1:2:1
- B:
2:2:1
- C:
1:1:2
- D:
1:2:2
The answer is c.
The energy content in kcal/g of carbohydrate:protein:triglycerol is approximately in the ratio of
1:2:1
2:2:1
1:1:2
1:2:2
|
|
Shreya Gupta answered |
Carbohydrates supply energy at the physiological rate of 4 kcal/g (caloric value 4.1), fats are the source of energy with a physiological rate of 9 kcal/g (caloric value 9.45), while proteins may yield energy at 4.65 kcal/g caloric value or 4.0 kcal/g physiological fuel value.
When we homogenise any tissue in an acid, the acid-soluble pool represents- a)Mitochondria
- b)Nucleus
- c)Cell membrane
- d)Cytoplasm
Correct answer is option 'D'. Can you explain this answer?
When we homogenise any tissue in an acid, the acid-soluble pool represents
a)
Mitochondria
b)
Nucleus
c)
Cell membrane
d)
Cytoplasm
|
|
Rohit Shah answered |
When we homogenise any tissue in an acid, the acid soluble pool represents cytoplasm.
Which of the following is an anabolic pathway?- a)Conversion of glycogen to carbon dioxide and water
- b)Conversion of glucose to lactic acid
- c)Hydrolysis of sucrose
- d)Formation of proteins
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an anabolic pathway?
a)
Conversion of glycogen to carbon dioxide and water
b)
Conversion of glucose to lactic acid
c)
Hydrolysis of sucrose
d)
Formation of proteins
|
|
Preeti Iyer answered |
Anabolic pathways require an input of energy to synthesize complex molecules from simpler ones.
Example: Synthesis of large proteins from amino acid building blocks, and the synthesis of new DNA strands from nucleic acid building blocks.
Example: Synthesis of large proteins from amino acid building blocks, and the synthesis of new DNA strands from nucleic acid building blocks.
These biosynthetic processes are critical to the life of the cell, take place constantly, and demand energy provided by ATP and other high-energy molecules like NADH (nicotinamide adenine dinucleotide) and NADPH.
Chemical reactions require energy for- a)Entropy
- b)Activation
- c)Oxidation
- d)Enthalpy
Correct answer is option 'B'. Can you explain this answer?
Chemical reactions require energy for
a)
Entropy
b)
Activation
c)
Oxidation
d)
Enthalpy
|
|
Sounak Saini answered |
Chemical reactions require energy for Activation.
Explanation:
Activation energy is the minimum amount of energy required to start a chemical reaction. It is the energy required to break the bonds of the reactant molecules so that they can rearrange to form the product molecules.
During a chemical reaction, the reactant molecules collide with each other. However, not all collisions lead to a successful reaction. Only those collisions in which the reactant molecules possess sufficient energy to overcome the activation energy barrier lead to the formation of the product molecules.
Thus, energy is required for activation so that the reactant molecules can possess enough energy to overcome the activation energy barrier and form the product molecules.
Explanation:
Activation energy is the minimum amount of energy required to start a chemical reaction. It is the energy required to break the bonds of the reactant molecules so that they can rearrange to form the product molecules.
During a chemical reaction, the reactant molecules collide with each other. However, not all collisions lead to a successful reaction. Only those collisions in which the reactant molecules possess sufficient energy to overcome the activation energy barrier lead to the formation of the product molecules.
Thus, energy is required for activation so that the reactant molecules can possess enough energy to overcome the activation energy barrier and form the product molecules.
Ester linkages occur in- a)Proteins
- b)Lipids
- c)Nucleic acids
- d)Carbohydrates
Correct answer is option 'B'. Can you explain this answer?
Ester linkages occur in
a)
Proteins
b)
Lipids
c)
Nucleic acids
d)
Carbohydrates
|
|
Anjali Iyer answered |
Lipids are actually triglycerides. A triglyceride consists of glycerol and fatty acids which are held together by ester linkages.
The oils have- a)High melting point
- b)Low melting point
- c)Optimum melting point
- d)No melting point
Correct answer is option 'B'. Can you explain this answer?
The oils have
a)
High melting point
b)
Low melting point
c)
Optimum melting point
d)
No melting point
|
|
Geetika Shah answered |
Oils have lower melting point (e.g., gingely oil) and hence remain as oil in winters.
A living state is in- a)Equilibrium always
- b)Sometimes in equilibrium and sometimes in non-equilibrium
- c)Constant stable state
- d)Non-equilibrium always
Correct answer is 'D'. Can you explain this answer?
A living state is in
a)
Equilibrium always
b)
Sometimes in equilibrium and sometimes in non-equilibrium
c)
Constant stable state
d)
Non-equilibrium always
|
|
Rohan Singh answered |
Living State : The living state is a non-equilibrium steady-state to be able to perform work.(a) The living process is a constant effort to prevent falling into equilibrium and is achieved by energy input.(b) The metabolism provides a mechanism for the production of energy.The living state and metabolism are synonymous, without metabolism there cannot exist a living : state.(d) A living organism works continuously so it cannotafford to reach equillibrium because a system at equilibrium cannot perform the work
A nucleoside differs from a nucleotide in not having a- a)Phosphate group
- b)Glucose
- c)Sugar
- d)Nitrogen base
Correct answer is option 'A'. Can you explain this answer?
A nucleoside differs from a nucleotide in not having a
a)
Phosphate group
b)
Glucose
c)
Sugar
d)
Nitrogen base

|
Aliya Agrawal answered |
Phosphate group...
because nucleoside = sugar + nitrogen base...
while nucleotides = sugar + nitrogen base + phosphate group.....
because nucleoside = sugar + nitrogen base...
while nucleotides = sugar + nitrogen base + phosphate group.....
Double hydrogen bond occurs in DNA between- a)Adenine and guanine
- b)Thymine and cytosine
- c)Adenine and thymine
- d)Uracil and thymine
Correct answer is option 'C'. Can you explain this answer?
Double hydrogen bond occurs in DNA between
a)
Adenine and guanine
b)
Thymine and cytosine
c)
Adenine and thymine
d)
Uracil and thymine
|
|
Gaurav Kumar answered |
The complementary base pairs of guanine with cytosine and adenine with thymine connect to one another using hydrogen bonds. In addition to holding the DNA strands together, the hydrogen bonding between the complementary bases also sequester the bases in the interior of the double helix. Since, the option of Guanine and Cytosine is not provided. Hence, the correct option is Option C.
A segment of DNA has 120 adenine and 120 cytosine bases. The total number of nucleotides present in the segment is- a)480
- b)240
- c)60
- d)120
Correct answer is option 'A'. Can you explain this answer?
A segment of DNA has 120 adenine and 120 cytosine bases. The total number of nucleotides present in the segment is
a)
480
b)
240
c)
60
d)
120
|
|
Pooja Shah answered |
According to Chargaff’s rule, the amount of adenine is always equal to that of thymine and the amount of guanine is always equal to that of cytosine.
A = T(120), G = C(120)
The total number of nucleotides would be 120 × 4 = 480.
Which is a reducing sugar?- a)Cellulose
- b)Maltose
- c)Starch
- d)Sucrose
Correct answer is option 'B'. Can you explain this answer?
Which is a reducing sugar?
a)
Cellulose
b)
Maltose
c)
Starch
d)
Sucrose
|
|
Prem Darade answered |
The sugar that is capable of acting as a reducing sugar because it has free aldehyde group or a free ketone group ...
The enzyme which cuts DNA is- a)DNA polymerase
- b)DNA ligase
- c)Restriction endonucleases
- d)DNA lyase
Correct answer is option 'C'. Can you explain this answer?
The enzyme which cuts DNA is
a)
DNA polymerase
b)
DNA ligase
c)
Restriction endonucleases
d)
DNA lyase
|
|
Baby Ghosh answered |
Restriction Endonucleases are enzyme which scan DNA molecules for a particular nucleotide sequence. These are called Recognition Sequences.Once the Endonuclease finds this sequence it halts ans cuts the strand.Thus,the correct option is "C".
Enzyme amylase belongs to- a)Hydrolases
- b)Transferases
- c)Isomerases
- d)Oxidoreductases
Correct answer is option 'A'. Can you explain this answer?
Enzyme amylase belongs to
a)
Hydrolases
b)
Transferases
c)
Isomerases
d)
Oxidoreductases
|
|
Ragini Shukla answered |
Hydrolase are the enzyme which breaks down the large molecules into smaller ones with the help of hydrogen and hydroxyl groups of water molecule this phenomena is known as hydrolysis. Amylase is the enzyme produced by the salivary gland and helps in breaking down the starch into glucose. Amylase catalysis the hydrolysis of starch into sugar (glucose).
Amylase is also found in germinating seeds.
Amylase is also found in germinating seeds.
The plant cell wall are made up of- a)Cellulose
- b)Starch
- c)Glycogen Bacteria
- d)Inulin
Correct answer is option 'A'. Can you explain this answer?
The plant cell wall are made up of
a)
Cellulose
b)
Starch
c)
Glycogen Bacteria
d)
Inulin

|
Srishti Sen answered |
Plant cell walls are made of cellulose. Paper made from plant pulp is cellulose.
DNA nucleotides are attached by
- a)Hydrogen bond
- b)Covalent bond
- c)Van der Waals bond
- d)Electrovalent bond
Correct answer is option 'A'. Can you explain this answer?
DNA nucleotides are attached by
a)
Hydrogen bond
b)
Covalent bond
c)
Van der Waals bond
d)
Electrovalent bond
|
|
Gopikas S answered |
Explanation: DNA nucleotides are attached by the Hydrogen bond. A nucleotide is the basic unit of polynucleotide chain of DNA (deoxyribonucleic acid) or RNA (Ribonucleic acid).
The nitrogenous bases are found in the strand's inward direction. The nitrogenous bases of the two antiparallel strands form hydrogen bonds, resulting in the formation of two helical strands.
The nitrogenous bases used in DNA (double-stranded helical structure) are adenine (A), cytosine (C), guanine (G), and thymine (T).
Adenine is joined to thymine with two hydrogen bonds, whereas guanine is joined to cytosine by three hydrogen bonds.
Thus, DNA nucleotides are attached by Hydrogen bond.
Process in which activation or inhibition of an enzyme by a small regulatory molecule is called- a)enzyme control
- b)allosteric control
- c)Specificity control
- d)Enzyme action
Correct answer is option 'B'. Can you explain this answer?
Process in which activation or inhibition of an enzyme by a small regulatory molecule is called
a)
enzyme control
b)
allosteric control
c)
Specificity control
d)
Enzyme action
|
|
Rajat Kapoor answered |
Allosteric control refers to a type of enzyme regulation involving the binding of a non-substrate molecule, known as the allosteric effector, at locations on the enzyme other than the active site.
The enzyme found functional in lysosome is- a)Acid phosphataseLyases
- b)Lyases
- c)Oxidoreductase
- d)Basic phosphatase
Correct answer is option 'B'. Can you explain this answer?
The enzyme found functional in lysosome is
a)
Acid phosphatase
Lyases
b)
Lyases
c)
Oxidoreductase
d)
Basic phosphatase
|
|
Vijay Bansal answered |
A lysosome is a membrane-bound organelle found in nearly all animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules.
Enormous diversity of protein molecules is due to- a)R groups of amino acids
- b)Sequence of amino acids
- c)Peptide bonds
- d)Amino groups of amino acids
Correct answer is option 'B'. Can you explain this answer?
Enormous diversity of protein molecules is due to
a)
R groups of amino acids
b)
Sequence of amino acids
c)
Peptide bonds
d)
Amino groups of amino acids
|
|
Rajat Kapoor answered |
The third is tertiary; this is the folding of the secondary structures into the final 3D structure of a protein. Amino acids have properties that guide this; some interact easily with water (hydrophilic) and these orient themselves on the outside of a protein, while others don't interact well with water (hydrophobic) and will try to get themselves on the inside of the folded structure where they will be protected. Hydrophobicity/philicity is the major driving force in protein folding but other bonds will also be formed between amino acids like S-S linkages, other ionic bonds and HYDROGEN BONDS (tons of these are made). These smaller interactions generally stabilize the protein and keep it folded in the most ideal conformation possible.
The nucleotide chemical components are- a)Heterocyclic compounds, sugar and phosphate
- b)Sugar and Phosphate
- c)Heterocyclic compounds and sugar
- d)Phosphate and heterocyclic compounds
Correct answer is option 'A'. Can you explain this answer?
The nucleotide chemical components are
a)
Heterocyclic compounds, sugar and phosphate
b)
Sugar and Phosphate
c)
Heterocyclic compounds and sugar
d)
Phosphate and heterocyclic compounds

|
Akshat Chavan answered |
The nucleotide has three chemically distinct components. One is a heterocyclic compound, the second is a monosaccharide and the third a phosphoric acid or phosphate.
Which of the following carbohydrates is not a disaccharide?- a)Lactose
- b)Maltose
- c)Sucrose
- d)Galactose
Correct answer is option 'D'. Can you explain this answer?
Which of the following carbohydrates is not a disaccharide?
a)
Lactose
b)
Maltose
c)
Sucrose
d)
Galactose
|
|
Gaurav Kumar answered |
Galactose is a monosaccharide. When combined with glucose (monosaccharide), through a condensation reaction, the result is the disaccharide lactose. The hydrolysis of lactose to glucose and galactose is catalyzed by the enzymes lactase and β-galactosidase.
Chemical formula: C6H12O6
Solubility in water: 650 g/L (20 °C)
Chemical formula: C6H12O6
Solubility in water: 650 g/L (20 °C)
Which enzyme is concerned with the transfer of electrons?
- a)Dehydrogenase
- b)Transaminase
- c)Hydrolase
- d) Desmolase
Correct answer is option 'A'. Can you explain this answer?
Which enzyme is concerned with the transfer of electrons?
a)
Dehydrogenase
b)
Transaminase
c)
Hydrolase
d)
Desmolase
|
|
Leelu Bhai answered |
Transaminase are the enzymes concerned with transfer of atoms or group of atoms or electrons.. so, option B is correct not A
Proteins perform many physiological functions. For example, some function as enzymes. Which one of the following represents an additional function which some proteins discharge?- a)Antibiotics
- b)Pigments making colours of flowers
- c)Hormones
- d)Pigments conferring colour to skin
Correct answer is option 'C'. Can you explain this answer?
Proteins perform many physiological functions. For example, some function as enzymes. Which one of the following represents an additional function which some proteins discharge?
a)
Antibiotics
b)
Pigments making colours of flowers
c)
Hormones
d)
Pigments conferring colour to skin
|
|
Anjali Iyer answered |
Proteins perform many physiological functions. For example, some proteins function as enzymes. Hormones represents an additional function that some proteins discharge (like insulin).
Lactose is made of
- a)Glucose + Fructose
- b)Fructose + Fructose
- c)Glucose + Glucose
- d)Glucose + Galactose
Correct answer is option 'D'. Can you explain this answer?
Lactose is made of
a)
Glucose + Fructose
b)
Fructose + Fructose
c)
Glucose + Glucose
d)
Glucose + Galactose

|
Ciel Knowledge answered |
Glucose+Galactose
- Lactose is a disaccharide that breaks down into two saccharides, glucose and galactose on hydrolysis.
- Both saccharides are joined by a beta glycosidic link.
- It is a natural sugar found in milk in amounts ranging from 2 to 8%.
- Lactose is a reducing sugar because it contains one free hemiacetal hydroxide.
- Hence, it is the correct option.
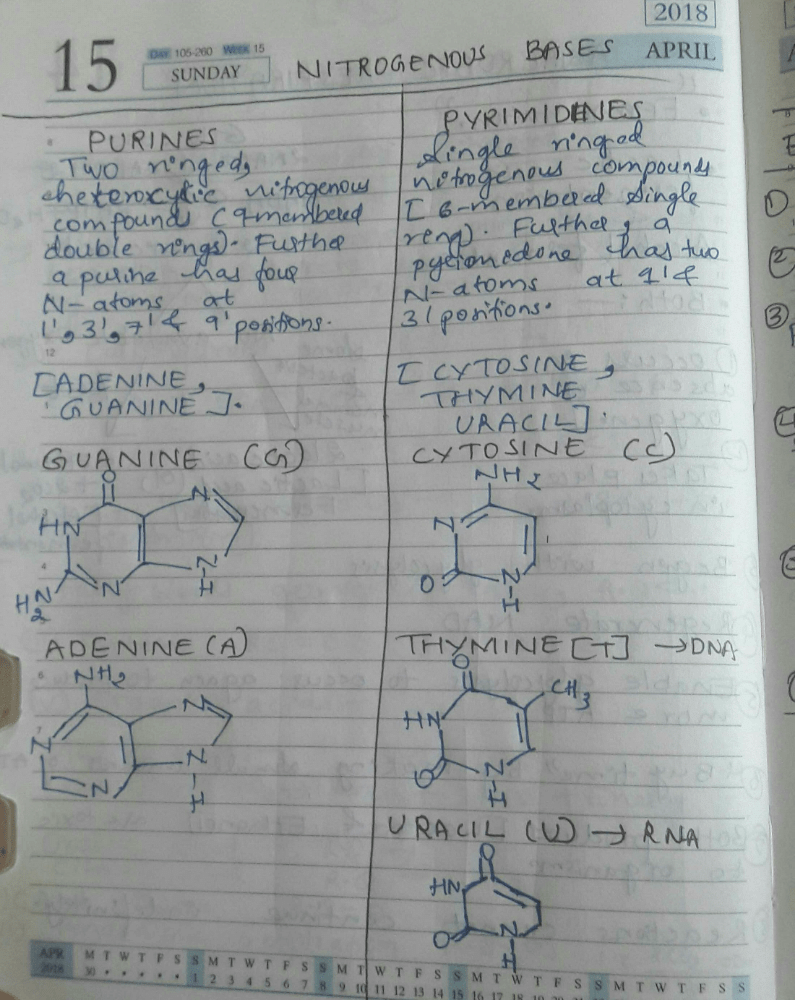
Which one of the following pairs of nitrogenous bases of nucleic acids is wrongly matched with the category mentioned against it?- a)Adenine, Thymine – Purines
- b)Uracil, Cytosine – Pyrimidines
- c)Guanine, Adenine – Purines
- d)Thymine, Uracil – Pyrimidines
Correct answer is option 'A'. Can you explain this answer?
Which one of the following pairs of nitrogenous bases of nucleic acids is wrongly matched with the category mentioned against it?
a)
Adenine, Thymine – Purines
b)
Uracil, Cytosine – Pyrimidines
c)
Guanine, Adenine – Purines
d)
Thymine, Uracil – Pyrimidines

|
Aastha Kulshreshtha answered |
Answer= a
As,
•purines= Adenine and Guanine.
•pyrimidine= Cytosine, Uracil and Thymine.
therefore the wrongly matched pair would be A.
As,
•purines= Adenine and Guanine.
•pyrimidine= Cytosine, Uracil and Thymine.
therefore the wrongly matched pair would be A.
Fehling’s solution is used for the detection of- a)Fats
- b)Starch
- c)Glucose
- d)All carbohydrates
Correct answer is option 'C'. Can you explain this answer?
Fehling’s solution is used for the detection of
a)
Fats
b)
Starch
c)
Glucose
d)
All carbohydrates
|
|
Athira Mukherjee answered |
Fehling is a chemical reagent used to test for the presence of aldehyde functional groups. It consists of two separate solutions, Fehling's A and Fehling's B, which are mixed together before use. When an aldehyde is added to Fehling's solution and heated, a red precipitate of copper(I) oxide is formed, indicating the presence of the aldehyde. Fehling's reagent is commonly used in organic chemistry laboratories to identify and distinguish aldehydes from other types of compounds.
Which of the following is not a conjugated protein?- a)Peptone
- b)Glycoprotein
- c)Chromoprotein
- d)Lipoprotein
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not a conjugated protein?
a)
Peptone
b)
Glycoprotein
c)
Chromoprotein
d)
Lipoprotein
|
|
Nandini Patel answered |
A conjugated protein is a protein that functions in interaction with other (non-polypeptide) chemical groups attached by covalent bonding or weak interactions. Many proteins contain only amino acids and no other chemical groups, and they are called simple proteins.
In yeast, during fermentation the glycolysis pathway leads to- a)Production of glucose
- b)Production of oxygen
- c)Production of pyruvic acid
- d)Production of ethanol
Correct answer is option 'D'. Can you explain this answer?
In yeast, during fermentation the glycolysis pathway leads to
a)
Production of glucose
b)
Production of oxygen
c)
Production of pyruvic acid
d)
Production of ethanol

|
Jatin Chakraborty answered |
In yeast, during fermentation, the same pathway leads to the production of ethanol(alcohol).
DNA differs from RNA in having- a)Thymine but no uracil
- b)Uracil but no thymine
- c)Thymine but no cytosine
- d)Cytosine but no guanine
Correct answer is option 'A'. Can you explain this answer?
DNA differs from RNA in having
a)
Thymine but no uracil
b)
Uracil but no thymine
c)
Thymine but no cytosine
d)
Cytosine but no guanine
|
|
Om Desai answered |
Uracil is energetically less expensive to produce than thymine, which may account for its use in RNA. In DNA, however, uracil is readily produced by chemical degradation of cytosine, so having thymine as the normal base makes detection and repair of such incipient mutations more efficient.
Which is the most abundant chemical for the living organisms?- a)Lipids
- b)Proteins
- c)Ions
- d)Water
Correct answer is option 'D'. Can you explain this answer?
Which is the most abundant chemical for the living organisms?
a)
Lipids
b)
Proteins
c)
Ions
d)
Water

|
Anand Jain answered |
Water is the most abundant chemical for the living organisms.
Assertion (A): Competitive inhibitors bind to the active site of an enzyme, preventing substrate binding. Reason (R): Competitive inhibition can be overcome by increasing the concentration of the substrate.- a)If both Assertion and Reason are true and Reason is the correct explanation of Assertion
- b)If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
- c)If Assertion is true but Reason is false
- d)If both Assertion and Reason are false
Correct answer is option 'A'. Can you explain this answer?
Assertion (A): Competitive inhibitors bind to the active site of an enzyme, preventing substrate binding.
Reason (R): Competitive inhibition can be overcome by increasing the concentration of the substrate.
a)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion
b)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion
c)
If Assertion is true but Reason is false
d)
If both Assertion and Reason are false
|
|
Sreemoyee Iyer answered |
Understanding Competitive Inhibition
Competitive inhibitors play a crucial role in enzyme activity and regulation. Let's break down the assertion and reason provided.
Assertion (A): Competitive inhibitors bind to the active site of an enzyme, preventing substrate binding.
- Competitive inhibitors are molecules that resemble the substrate and compete for binding at the enzyme's active site.
- When a competitive inhibitor occupies the active site, the actual substrate cannot bind, leading to decreased enzyme activity.
Reason (R): Competitive inhibition can be overcome by increasing the concentration of the substrate.
- Increasing substrate concentration effectively outcompetes the inhibitor for the active site.
- As the substrate concentration rises, more substrate molecules can bind to the enzyme, thereby restoring enzyme activity and overcoming the inhibition.
Conclusion: Why Option A is Correct
- Both the assertion and reason are true. Competitive inhibitors indeed bind to the active site, and their effects can be mitigated by increasing substrate concentration.
- The reason provided correctly explains the assertion, as the mechanism of competitive inhibition directly relates to the ability to overcome it by increasing substrate levels.
Summary
- Competitive inhibitors block substrate binding at the active site.
- Increased substrate concentration can alleviate the effects of competitive inhibition.
- Therefore, both assertion and reason are true, with the reason serving as the correct explanation for the assertion.
In conclusion, option 'A' is the correct choice as it reflects the relationship between competitive inhibition and substrate concentration effectively.
Competitive inhibitors play a crucial role in enzyme activity and regulation. Let's break down the assertion and reason provided.
Assertion (A): Competitive inhibitors bind to the active site of an enzyme, preventing substrate binding.
- Competitive inhibitors are molecules that resemble the substrate and compete for binding at the enzyme's active site.
- When a competitive inhibitor occupies the active site, the actual substrate cannot bind, leading to decreased enzyme activity.
Reason (R): Competitive inhibition can be overcome by increasing the concentration of the substrate.
- Increasing substrate concentration effectively outcompetes the inhibitor for the active site.
- As the substrate concentration rises, more substrate molecules can bind to the enzyme, thereby restoring enzyme activity and overcoming the inhibition.
Conclusion: Why Option A is Correct
- Both the assertion and reason are true. Competitive inhibitors indeed bind to the active site, and their effects can be mitigated by increasing substrate concentration.
- The reason provided correctly explains the assertion, as the mechanism of competitive inhibition directly relates to the ability to overcome it by increasing substrate levels.
Summary
- Competitive inhibitors block substrate binding at the active site.
- Increased substrate concentration can alleviate the effects of competitive inhibition.
- Therefore, both assertion and reason are true, with the reason serving as the correct explanation for the assertion.
In conclusion, option 'A' is the correct choice as it reflects the relationship between competitive inhibition and substrate concentration effectively.
Directions : In the following question, the Assertions (A) and Reason (R) have been put forward. Read both the statements and choose the correct option from the following.Assertion : Glutamate pyruvate transaminase is a transferase enzyme.Reason : They transfer group from one molecule to another.- a)Both A and R are true, and R is the correct explanation of A.
- b)Both A and R are true, but R is not the correct explanation of A.
- c)A is true but R is false.
- d)Both A and R are false.
Correct answer is option 'A'. Can you explain this answer?
Directions : In the following question, the Assertions (A) and Reason (R) have been put forward. Read both the statements and choose the correct option from the following.
Assertion : Glutamate pyruvate transaminase is a transferase enzyme.
Reason : They transfer group from one molecule to another.
a)
Both A and R are true, and R is the correct explanation of A.
b)
Both A and R are true, but R is not the correct explanation of A.
c)
A is true but R is false.
d)
Both A and R are false.
|
|
Dev Patel answered |
Transferases transfer group from one molecule to another e.g., glutamate pyruvate transaminase.
Which of the following is not obtained on hydrolysis of nucleic acid?- a)Purine
- b)Phosphoric acid
- c)Pyrimidine
- d)Pentose sugar
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not obtained on hydrolysis of nucleic acid?
a)
Purine
b)
Phosphoric acid
c)
Pyrimidine
d)
Pentose sugar

|
Ruchi Chopra answered |
Hydrolysis of nucleic acid (DNA and RNA) produces pentose sugar (ribose or deoxyribose) purine and pyrimidine. Phosphoric acid is not released on hydrolysis of DNA or RNA.
The energy currency of cell is—- a)GDP
- b)ATP
- c)ADP
- d)NAD
Correct answer is option 'B'. Can you explain this answer?
The energy currency of cell is—
a)
GDP
b)
ATP
c)
ADP
d)
NAD
|
|
Rohan Singh answered |
When the ATP converts to ADP, the ATP is said to be spent. he molecule is used like a battery within cells and allows the consumption of one of its phosphorous molecules.The energy currency used by all cells from bacteria to man is adenosine triphosphate (ATP).
Which one is not a denaturing factor for protein?- a)High energy radiation
- b)High pressure
- c)Drastic change in pH
- d)High temperature
Correct answer is option 'B'. Can you explain this answer?
Which one is not a denaturing factor for protein?
a)
High energy radiation
b)
High pressure
c)
Drastic change in pH
d)
High temperature

|
Prasenjit Pillai answered |
Protein molecules get denatured due to high temperature, very high or low pH and high energy radiation but there is no effect due to high pressure.
The rate of energy metabolism is equivalent to the- a)Rate of carbon dioxide consumption
- b)Rate of nitrogen consumption
- c)Rate of hydrogen consumption
- d)Rate of oxygen consumption
Correct answer is option 'D'. Can you explain this answer?
The rate of energy metabolism is equivalent to the
a)
Rate of carbon dioxide consumption
b)
Rate of nitrogen consumption
c)
Rate of hydrogen consumption
d)
Rate of oxygen consumption
|
|
Anjana Sharma answered |
The energy equivalent for converting rate of oxygen consumption into rate of heat production (Qox cal mg-1 oxygen consumed) is 3.53 cal mg-1 for carbohydrate oxidation, 3.28 cal mg-1 (range 3.22–3.32) for fat oxidation. Qox values for the respiration of standard protein are the same at 3.25 cal mg-1 for ureotelic and uricotelic animals, and about 2% less at 3.20 cal mg-1 for ammoniotelic animals. The energy equivalent for converting rate of oxygen consumption into rate of energy loss in excreta (Qex cal mg-1) varies considerably with different excretory products. Values for standard protein are 0.62 cal mg-1 for ammonioteles, 0.58 cal mg-1 for ureoteles, and 0.94 cal mg-1 for uricoteles.
. __________ is a globular protein of 6 kDa consisting of 51 amino acids arranged in 2 polypeptide chains held together by a disulphide bridge- a)Fibrinogen
- b)Keratin
- c)Insulin
- d)Glucagon
Correct answer is option 'C'. Can you explain this answer?
. __________ is a globular protein of 6 kDa consisting of 51 amino acids arranged in 2 polypeptide chains held together by a disulphide bridge
a)
Fibrinogen
b)
Keratin
c)
Insulin
d)
Glucagon
|
|
Pooja Mehta answered |
Human insulin is a peptide hormone composed of 51 ammo acids and has a molecular weight of 5805 Da.(∼6 Kda). In this molecule, there are two polypeptide chains (A and B) held together by disulphide bridge.
A polysaccharide present as storehouse of energy of plant tissues- a)Chitin
- b)Starch
- c)Hemi cellulose
- d)Cellulose
Correct answer is option 'B'. Can you explain this answer?
A polysaccharide present as storehouse of energy of plant tissues
a)
Chitin
b)
Starch
c)
Hemi cellulose
d)
Cellulose
|
|
Jyoti Kapoor answered |
Polysaccharide
A polysaccharide is a large molecule made of many smaller monosaccharides. Monosaccharides are simple sugars, like glucose. Special enzymes bind these small monomers together creating large sugar polymers, or polysaccharides. A polysaccharide is also called a glycan. A polysaccharide can be a homopolysaccharide, in which all the monosaccharides are the same, or a heteropolysaccharide in which the monosaccharides vary. Depending on which monosaccharides are connected, and which carbons in the monosaccharides connects, polysaccharides take on a variety of forms. A molecule with a straight chain of monosaccharides is called a linear polysaccharide, while a chain that has arms and turns is known as a branched polysaccharide.
One turn of the DNA double helix spans a distance of- a)3.4 nm
- b)4.26 nm
- c)4.56 nm
- d)2.46 nm
Correct answer is option 'A'. Can you explain this answer?
One turn of the DNA double helix spans a distance of
a)
3.4 nm
b)
4.26 nm
c)
4.56 nm
d)
2.46 nm
|
|
Suresh Kumar answered |
Refer biomolecules chapter in Ncert .Its mentioned in that chapter...
Which one of the following is fibrous protein?- a)Collagen
- b)Ribozymes
- c)Haemoglobin
- d)Hemicellulose
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is fibrous protein?
a)
Collagen
b)
Ribozymes
c)
Haemoglobin
d)
Hemicellulose

|
Shanaya Rane answered |
Collagen is a fibrous protein. It is the main structural protein in the extracellular space in thevarious connective tissues. It is the most abundant protein in mammals.
The part of enzyme bound to the protein part by a covalent bond is called- a)Holoenzyme
- b)Cofactor
- c)Prosthetic group
- d)Apoenzyme
Correct answer is option 'C'. Can you explain this answer?
The part of enzyme bound to the protein part by a covalent bond is called
a)
Holoenzyme
b)
Cofactor
c)
Prosthetic group
d)
Apoenzyme

|
Imk Pathsala answered |
A prosthetic group is a tightly covalently bound, specific non-polypeptide unit required for the biological function of some proteins. The prosthetic group may be organic (such as a vitamin, sugar, or lipid) or inorganic (such as a metal ion), but is not composed of amino acids.
Chapter doubts & questions for Biomolecules - Biology for ACT 2025 is part of ACT exam preparation. The chapters have been prepared according to the ACT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for ACT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Biomolecules - Biology for ACT in English & Hindi are available as part of ACT exam.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Biology for ACT
208 videos|226 docs|136 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup