All Exams >
BMAT >
Biology for BMAT (Section 2) >
All Questions
All questions of Enzymes for BMAT Exam
Which is true about enzymes?a)All enzymes are ... moreproteins.b)All proteins are enzymes.c)All enzymes are not proteins.d)All enzymes are vitamins.Correct answer is option 'C'. Can you explain this answer?
|
|
Gaurav Kumar answered |
Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. The latter are called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures.
Which one is true for ATP?- a)ATP is organic ions of enzyme.
- b)ATP is a coenzyme.
- c)ATP is an enzyme
- d)ATP is a prosthetic part of an enzyme.
Correct answer is option 'B'. Can you explain this answer?
Which one is true for ATP?
a)
ATP is organic ions of enzyme.
b)
ATP is a coenzyme.
c)
ATP is an enzyme
d)
ATP is a prosthetic part of an enzyme.
|
|
Hitakshi HTG answered |
Adenosine triphosphate, also known as molecular unit of currency, is a coenzyme of vast importance in the transfer of chemical energy derived from biochemical oxidations and it also transports energy within cells for metabolism. Thus, option 'B' is the right answer.
The enzyme which cuts DNA is- a)DNA polymerase
- b)DNA ligase
- c)Restriction endonucleases
- d)DNA lyase
Correct answer is option 'C'. Can you explain this answer?
The enzyme which cuts DNA is
a)
DNA polymerase
b)
DNA ligase
c)
Restriction endonucleases
d)
DNA lyase
|
|
Baby Ghosh answered |
Restriction Endonucleases are enzyme which scan DNA molecules for a particular nucleotide sequence. These are called Recognition Sequences.Once the Endonuclease finds this sequence it halts ans cuts the strand.Thus,the correct option is "C".
The enzyme found functional in lysosome is- a)Acid phosphataseLyases
- b)Lyases
- c)Oxidoreductase
- d)Basic phosphatase
Correct answer is option 'B'. Can you explain this answer?
The enzyme found functional in lysosome is
a)
Acid phosphatase
Lyases
b)
Lyases
c)
Oxidoreductase
d)
Basic phosphatase
|
|
Vijay Bansal answered |
A lysosome is a membrane-bound organelle found in nearly all animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules.
Enzyme amylase belongs to- a)Hydrolases
- b)Transferases
- c)Isomerases
- d)Oxidoreductases
Correct answer is option 'A'. Can you explain this answer?
Enzyme amylase belongs to
a)
Hydrolases
b)
Transferases
c)
Isomerases
d)
Oxidoreductases
|
|
Ragini Shukla answered |
Hydrolase are the enzyme which breaks down the large molecules into smaller ones with the help of hydrogen and hydroxyl groups of water molecule this phenomena is known as hydrolysis. Amylase is the enzyme produced by the salivary gland and helps in breaking down the starch into glucose. Amylase catalysis the hydrolysis of starch into sugar (glucose).
Amylase is also found in germinating seeds.
Amylase is also found in germinating seeds.
Which enzyme is concerned with the transfer of electrons?
- a)Dehydrogenase
- b)Transaminase
- c)Hydrolase
- d) Desmolase
Correct answer is option 'A'. Can you explain this answer?
Which enzyme is concerned with the transfer of electrons?
a)
Dehydrogenase
b)
Transaminase
c)
Hydrolase
d)
Desmolase
|
|
Leelu Bhai answered |
Transaminase are the enzymes concerned with transfer of atoms or group of atoms or electrons.. so, option B is correct not A
Enzymes with slightly different molecular structure but performing identical activity are- a)Isoenzymes
- b)Apoenzymes
- c)Holoenzyme
- d)Coenzymes
Correct answer is option 'A'. Can you explain this answer?
Enzymes with slightly different molecular structure but performing identical activity are
a)
Isoenzymes
b)
Apoenzymes
c)
Holoenzyme
d)
Coenzymes
|
|
Mira Tiwari answered |
Enzymes with slightly different molecular structure but performing identical activity are called isoenzymes.
Explanation:
Enzymes are biological catalysts that facilitate and speed up biochemical reactions in living organisms. They are usually proteins with a specific three-dimensional structure that allows them to bind to specific substrates and convert them into products. However, enzymes can also have slightly different molecular structures while performing the same catalytic activity. These enzymes are called isoenzymes.
Definition of Isoenzymes:
Isoenzymes, also known as isozymes, are enzymes that catalyze the same chemical reaction but have slightly different amino acid sequences or molecular structures. They are encoded by different genes but perform identical functions in the same organism or different tissues. Isoenzymes are often found in multiple forms and can be distinguished from each other through various methods such as electrophoresis or immunoassay.
Reasons for Different Molecular Structures:
1. Genetic Differences: Isoenzymes are encoded by different genes, which can result in variations in the amino acid sequence and overall structure of the enzyme.
2. Post-translational Modifications: Isoenzymes may undergo different post-translational modifications, such as phosphorylation or glycosylation, which can alter their structure and function.
3. Tissue-Specific Expression: Different tissues within an organism may require slightly different versions of an enzyme to perform the same activity optimally. These tissue-specific isoenzymes may have distinct molecular structures.
Importance of Isoenzymes:
1. Diagnostic Tool: Isoenzymes can be used as diagnostic markers for certain diseases or conditions. For example, the measurement of different forms of creatine kinase isoenzymes can help diagnose heart attacks.
2. Tissue-Specific Function: Isoenzymes may have different kinetic properties or substrate specificities, allowing them to perform specialized functions in specific tissues.
3. Evolutionary Adaptation: Isoenzymes can evolve to perform similar functions under different conditions or in different organisms, allowing for adaptation and survival in diverse environments.
In conclusion, isoenzymes refer to enzymes with slightly different molecular structures but identical catalytic activities. They play important roles in various biological processes and have diagnostic and evolutionary significance.
Explanation:
Enzymes are biological catalysts that facilitate and speed up biochemical reactions in living organisms. They are usually proteins with a specific three-dimensional structure that allows them to bind to specific substrates and convert them into products. However, enzymes can also have slightly different molecular structures while performing the same catalytic activity. These enzymes are called isoenzymes.
Definition of Isoenzymes:
Isoenzymes, also known as isozymes, are enzymes that catalyze the same chemical reaction but have slightly different amino acid sequences or molecular structures. They are encoded by different genes but perform identical functions in the same organism or different tissues. Isoenzymes are often found in multiple forms and can be distinguished from each other through various methods such as electrophoresis or immunoassay.
Reasons for Different Molecular Structures:
1. Genetic Differences: Isoenzymes are encoded by different genes, which can result in variations in the amino acid sequence and overall structure of the enzyme.
2. Post-translational Modifications: Isoenzymes may undergo different post-translational modifications, such as phosphorylation or glycosylation, which can alter their structure and function.
3. Tissue-Specific Expression: Different tissues within an organism may require slightly different versions of an enzyme to perform the same activity optimally. These tissue-specific isoenzymes may have distinct molecular structures.
Importance of Isoenzymes:
1. Diagnostic Tool: Isoenzymes can be used as diagnostic markers for certain diseases or conditions. For example, the measurement of different forms of creatine kinase isoenzymes can help diagnose heart attacks.
2. Tissue-Specific Function: Isoenzymes may have different kinetic properties or substrate specificities, allowing them to perform specialized functions in specific tissues.
3. Evolutionary Adaptation: Isoenzymes can evolve to perform similar functions under different conditions or in different organisms, allowing for adaptation and survival in diverse environments.
In conclusion, isoenzymes refer to enzymes with slightly different molecular structures but identical catalytic activities. They play important roles in various biological processes and have diagnostic and evolutionary significance.
Which of these enzymes are not proteinaceous?- a)Ribozymes
- b)Endonucleases
- c)Ligases
- d)Kinases
Correct answer is option 'A'. Can you explain this answer?
Which of these enzymes are not proteinaceous?
a)
Ribozymes
b)
Endonucleases
c)
Ligases
d)
Kinases
|
|
Gaurav Basu answered |
Explanation:
Enzymes are biological catalysts that speed up chemical reactions in living organisms. They are typically protein molecules, but there are exceptions.
Ribozymes
Ribozymes are RNA molecules that exhibit catalytic activity. Unlike most enzymes, which are proteinaceous, ribozymes are composed of RNA. They were first discovered in the 1980s and have since been found to play important roles in various biological processes. Ribozymes can catalyze a wide range of reactions, including cleavage, ligation, and isomerization reactions.
Endonucleases
Endonucleases are enzymes that cleave the phosphodiester bonds within a nucleic acid chain. They are involved in processes such as DNA replication, repair, recombination, and transcription. Endonucleases can be either proteinaceous or ribozymes.
Ligases
Ligases are enzymes that catalyze the joining of two molecules by forming a new chemical bond, usually in the presence of ATP. They are involved in DNA repair, DNA replication, and the synthesis of RNA and proteins. Ligases are proteinaceous enzymes.
Kinases
Kinases are enzymes that catalyze the transfer of a phosphate group from ATP to a substrate molecule, a process known as phosphorylation. This phosphorylation can regulate the activity of the substrate molecule. Kinases are proteinaceous enzymes.
Conclusion
Among the enzymes listed, ribozymes are not proteinaceous. Ribozymes are RNA molecules that exhibit catalytic activity and can perform a wide range of enzymatic reactions. This discovery challenged the long-held belief that all enzymes are proteinaceous. Ribozymes have since been found to play important roles in various biological processes, contributing to our understanding of the diversity and complexity of enzymatic reactions in living organisms.
Enzymes are biological catalysts that speed up chemical reactions in living organisms. They are typically protein molecules, but there are exceptions.
Ribozymes
Ribozymes are RNA molecules that exhibit catalytic activity. Unlike most enzymes, which are proteinaceous, ribozymes are composed of RNA. They were first discovered in the 1980s and have since been found to play important roles in various biological processes. Ribozymes can catalyze a wide range of reactions, including cleavage, ligation, and isomerization reactions.
Endonucleases
Endonucleases are enzymes that cleave the phosphodiester bonds within a nucleic acid chain. They are involved in processes such as DNA replication, repair, recombination, and transcription. Endonucleases can be either proteinaceous or ribozymes.
Ligases
Ligases are enzymes that catalyze the joining of two molecules by forming a new chemical bond, usually in the presence of ATP. They are involved in DNA repair, DNA replication, and the synthesis of RNA and proteins. Ligases are proteinaceous enzymes.
Kinases
Kinases are enzymes that catalyze the transfer of a phosphate group from ATP to a substrate molecule, a process known as phosphorylation. This phosphorylation can regulate the activity of the substrate molecule. Kinases are proteinaceous enzymes.
Conclusion
Among the enzymes listed, ribozymes are not proteinaceous. Ribozymes are RNA molecules that exhibit catalytic activity and can perform a wide range of enzymatic reactions. This discovery challenged the long-held belief that all enzymes are proteinaceous. Ribozymes have since been found to play important roles in various biological processes, contributing to our understanding of the diversity and complexity of enzymatic reactions in living organisms.
An organic substance bound to an enzyme and essential for its activity is called- a)Isoenzyme
- b)Coenzyme
- c)Holoenzyme
- d)Apoenzyme
Correct answer is option 'B'. Can you explain this answer?
An organic substance bound to an enzyme and essential for its activity is called
a)
Isoenzyme
b)
Coenzyme
c)
Holoenzyme
d)
Apoenzyme
|
|
Rohan Singh answered |
Coenzyme is an organic nonprotein molecule that associates with an enzyme molecule in catalsying biochemical reactions. It usually participates in the substrate-enzyme interaction by donating or accepting certain chemical groups. Holoenzyme is a complex comprising of enzyme molecule and its cofactor. The enzyme is catalytically active in this state. Apoenzyme is an inactive enzyme that must associate with a specific cofactor molecule in order to function. Isoenzyme or isozyme is one of the several forms of an enzyme that catalyse the same reaction but differ from each other in such properties as substrate affinity and maximum rates of enzyme-substrate reaction.
Some enzymes require the presence of a nonprotein molecule to behave catalytically. An enzyme devoid of this molecule is called a(n):- a)holoenzyme
- b)apoenzyme
- c)coenzyme
- d)zymoenzyme
Correct answer is option 'B'. Can you explain this answer?
Some enzymes require the presence of a nonprotein molecule to behave catalytically. An enzyme devoid of this molecule is called a(n):
a)
holoenzyme
b)
apoenzyme
c)
coenzyme
d)
zymoenzyme

|
Elite Coaching Classes answered |
An enzyme devoid of its necessary cofactor is called an apoenzyme and is catalytically inactive.
At which part of the enzyme does the substrate fit in?- a)Active site
- b)Right end
- c)Left end
- d)Binding site
Correct answer is option 'A'. Can you explain this answer?
At which part of the enzyme does the substrate fit in?
a)
Active site
b)
Right end
c)
Left end
d)
Binding site
|
|
Megha Basu answered |
Active site:
The substrate fits into the active site of an enzyme. The active site is a specific region on the enzyme where the substrate binds and undergoes a chemical reaction. This site has a unique shape and chemical properties that complement the structure of the substrate molecule.
Key points:
- The active site is like a lock and key, where the substrate (key) fits into the active site (lock) with precision.
- The active site provides a microenvironment that is conducive to the chemical reaction between the enzyme and substrate.
- The binding of the substrate to the active site induces a conformational change in the enzyme, which helps facilitate the reaction.
- The active site may contain specific amino acid residues that directly participate in the catalytic process.
In conclusion, the substrate fits into the active site of an enzyme, where it undergoes a chemical reaction facilitated by the enzyme's specific structure and properties.
The substrate fits into the active site of an enzyme. The active site is a specific region on the enzyme where the substrate binds and undergoes a chemical reaction. This site has a unique shape and chemical properties that complement the structure of the substrate molecule.
Key points:
- The active site is like a lock and key, where the substrate (key) fits into the active site (lock) with precision.
- The active site provides a microenvironment that is conducive to the chemical reaction between the enzyme and substrate.
- The binding of the substrate to the active site induces a conformational change in the enzyme, which helps facilitate the reaction.
- The active site may contain specific amino acid residues that directly participate in the catalytic process.
In conclusion, the substrate fits into the active site of an enzyme, where it undergoes a chemical reaction facilitated by the enzyme's specific structure and properties.
The active site of an enzyme E, which catalyzes a reaction X, is partially denatured: which of the following quantities associated with X is most likely to be affected by the partial denaturation of E compared to the native form of E?- a)the equilibrium constant Keq
- b)the rate-constant k
- c)the boltzmann constant kB
- d)the heat of reaction ΔH
Correct answer is option 'B'. Can you explain this answer?
The active site of an enzyme E, which catalyzes a reaction X, is partially denatured: which of the following quantities associated with X is most likely to be affected by the partial denaturation of E compared to the native form of E?
a)
the equilibrium constant Keq
b)
the rate-constant k
c)
the boltzmann constant kB
d)
the heat of reaction ΔH

|
Elite Coaching Classes answered |
Enzymes play a crucial role in catalyzing chemical reactions by providing an environment in the active site that facilitates the conversion of reactants into products. The active site of an enzyme is typically formed by a specific three-dimensional arrangement of amino acids, and any changes or disruptions to this structure can impact the enzyme's catalytic activity.
Partial denaturation of enzyme E can lead to alterations in the active site, such as changes in the shape, charge distribution, or flexibility of the active site residues. These changes can directly affect the binding of substrate molecules and the formation of the enzyme-substrate complex. Consequently, the rate at which the reaction X proceeds, as reflected by the rate-constant k, is likely to be influenced by the partial denaturation of enzyme E.
The equilibrium constant Keq, the Boltzmann constant kB, and the heat of reaction ΔH are properties associated with the thermodynamics and equilibrium state of a reaction, and they are generally independent of the specific enzyme catalyzing the reaction. Thus, these quantities are less likely to be directly affected by the partial denaturation of enzyme E compared to the rate-constant k.
Enzymes increase the rate of a reaction by:- a)decreasing the activation energy.
- b)increasing the overall free energy change of the reaction.
- c)increasing the activation energy.
- d)decreasing the overall free energy change of the reaction.
Correct answer is option 'A'. Can you explain this answer?
Enzymes increase the rate of a reaction by:
a)
decreasing the activation energy.
b)
increasing the overall free energy change of the reaction.
c)
increasing the activation energy.
d)
decreasing the overall free energy change of the reaction.

|
Elite Coaching Classes answered |
Enzymes increase the rate of a reaction by decreasing the activation energy. They do not affect the overall free energy, ΔG, of the reaction.
The conversion of ATP to cyclic AMP and inorganic phosphate is most likely catalyzed by which class of enzyme?- a)Ligase
- b)Hydrolase
- c)Lyase
- d)Transferase
Correct answer is option 'C'. Can you explain this answer?
The conversion of ATP to cyclic AMP and inorganic phosphate is most likely catalyzed by which class of enzyme?
a)
Ligase
b)
Hydrolase
c)
Lyase
d)
Transferase

|
Elite Coaching Classes answered |
Lyases are responsible for the breakdown of a single molecule into two molecules without the addition of water or the transfer of electrons. Lyases often form cyclic compounds or double bonds in the products to accommodate this. Water was not a reactant, and no cofactor was mentioned; thus lyase, choice (C), remains the best answer choice.
In order to analyze the catalytic effect of two different enzymes on the same chemical reaction, it is best to compare which of the following quantities?- a)The differences in entropy between the reactants and products
- b)The differences in enthalpy between the reactants and products
- c)The differences in free energy between the reactants and products
- d)The difference between transition state energies
Correct answer is option 'D'. Can you explain this answer?
In order to analyze the catalytic effect of two different enzymes on the same chemical reaction, it is best to compare which of the following quantities?
a)
The differences in entropy between the reactants and products
b)
The differences in enthalpy between the reactants and products
c)
The differences in free energy between the reactants and products
d)
The difference between transition state energies

|
Elite Coaching Classes answered |
The transition state is an intermediate state that the reactants must pass through during a chemical reaction before forming the products. It represents the highest energy point along the reaction pathway. Enzymes facilitate reactions by stabilizing the transition state, lowering its energy and reducing the activation energy required for the reaction to proceed.
By comparing the difference between transition state energies in the presence of different enzymes, one can assess their catalytic effectiveness. A larger difference in transition state energies suggests a more significant catalytic effect, as the enzyme is more effective in stabilizing the transition state and lowering the activation energy barrier.
Local conditions can affect the specificity of an enzyme for its substrate, and thus the enzymes catalytic ability: which of the following alterations would most likely not affect an enzyme in this manner?- a)Increased substrate concentration
- b)Increased temperature
- c)Increased concentration of OH−
- d)Increased concentration of H+
Correct answer is option 'A'. Can you explain this answer?
Local conditions can affect the specificity of an enzyme for its substrate, and thus the enzymes catalytic ability: which of the following alterations would most likely not affect an enzyme in this manner?
a)
Increased substrate concentration
b)
Increased temperature
c)
Increased concentration of OH−
d)
Increased concentration of H+

|
Elite Coaching Classes answered |
Increased substrate concentration is more likely to enhance the catalytic ability of an enzyme rather than affect its specificity. When the substrate concentration is increased, the enzyme has a greater chance of encountering and binding to the substrate molecules, leading to an increase in the rate of the enzymatic reaction. However, it does not directly alter the enzyme's specificity for its substrate.
On the other hand, options B, C, and D can potentially affect the enzyme's specificity and catalytic ability:
- Increased temperature (option B) can denature the enzyme and disrupt its active site, leading to a loss of specificity and a decrease in catalytic ability.
- Increased concentration of OH- (option C) or H+ (option D) can alter the pH of the environment, which can affect the ionization state of amino acid residues in the enzyme's active site. This change in ionization state can impact the electrostatic interactions and binding affinity between the enzyme and substrate, thereby affecting specificity and catalytic ability.
Therefore, the alteration that would least likely affect the enzyme's specificity is option A, increased substrate concentration.
An organic substance bound to an enzyme and essential for its activity is called- a)Isoenzyme
- b)Coenzyme
- c)Holoenzyme
- d)Apoenzyme
Correct answer is option 'B'. Can you explain this answer?
An organic substance bound to an enzyme and essential for its activity is called
a)
Isoenzyme
b)
Coenzyme
c)
Holoenzyme
d)
Apoenzyme

|
Arathy Arab answered |
B. coenzyme
Coenzyme is an organic nonprotein molecule that associates with an enzymes molecule in catalysing biochemical reactions. It usually participates in the substrate-enzyme interaction by donating or accepting certain chemical groups.
Apoenzyme is an inactive enzyme that must associate with a specific cofactor molecule in order to function.
Isoenzyme or isozyme is one of the several forms of an enzyme that catalyse the same reaction but differ from each other in such properties as substrate affinity and maximum rates of enzymes-substrate reaction.
Coenzyme is an organic nonprotein molecule that associates with an enzymes molecule in catalysing biochemical reactions. It usually participates in the substrate-enzyme interaction by donating or accepting certain chemical groups.
Apoenzyme is an inactive enzyme that must associate with a specific cofactor molecule in order to function.
Isoenzyme or isozyme is one of the several forms of an enzyme that catalyse the same reaction but differ from each other in such properties as substrate affinity and maximum rates of enzymes-substrate reaction.
How are enzymes different from catalysts?- a)Enzymes are active at high temperatures
- b)Catalysts are active at subzero temperatures
- c)Catalysts are efficient at high temperatures and high pressures
- d)Enzymes are denatured at room temperature
Correct answer is option 'C'. Can you explain this answer?
How are enzymes different from catalysts?
a)
Enzymes are active at high temperatures
b)
Catalysts are active at subzero temperatures
c)
Catalysts are efficient at high temperatures and high pressures
d)
Enzymes are denatured at room temperature

|
Top Rankers answered |
- Enzymes are organic catalysts or biocatalysts.
- However, inorganic catalysts work at high temperatures and high pressures efficiently.
- Enzymes, being proteinaceous in nature, get denatured under such conditions.
Which of the following enzyme types catalyzes the formation of a single bond between two substrates through the elimination of H2O.?- a)Isomerase
- b)Hydrolase
- c)Oxidoreductase
- d)Ligase
Correct answer is option 'D'. Can you explain this answer?
Which of the following enzyme types catalyzes the formation of a single bond between two substrates through the elimination of H2O.?
a)
Isomerase
b)
Hydrolase
c)
Oxidoreductase
d)
Ligase

|
Elite Coaching Classes answered |
Ligases are enzymes that catalyze the formation of a covalent bond between two substrates using ATP or another high-energy molecule as a source of energy. They are involved in the joining of two molecules, typically with the simultaneous release of a small molecule such as water (H2O). This type of reaction is often referred to as a condensation or dehydration reaction, as it involves the elimination of water.
Option A, Isomerase, is an enzyme type that catalyzes the interconversion of isomers, which involves rearranging atoms within a molecule but does not typically involve the formation or breaking of covalent bonds.
Option B, Hydrolase, is an enzyme type that catalyzes the cleavage of a covalent bond by the addition of water (hydrolysis). Hydrolases break down substrates by adding a water molecule, resulting in the formation of two separate molecules.
Option C, Oxidoreductase, is an enzyme type that catalyzes oxidation-reduction reactions. These enzymes are involved in the transfer of electrons between substrates, rather than the formation of single bonds through the elimination of water.
Therefore, the enzyme type that catalyzes the formation of a single bond between two substrates through the elimination of H2O is a Ligase.
The active site model (also called the lock and key model) of enzyme-substrate binding differs from the induced fit model in which of the following ways?- a)The induced fit model holds that the shape of the substrate is irrelevant to enzyme-substrate binding
- b)The induced fit model holds that the shape of the active site is altered during the course of substrate binding
- c)The induced fit model holds that the shape of the active site is permanently altered by substrate binding
- d)The induced fit model holds that enzyme-substrate binding does not take place at the enzyme’s active site
Correct answer is option 'B'. Can you explain this answer?
The active site model (also called the lock and key model) of enzyme-substrate binding differs from the induced fit model in which of the following ways?
a)
The induced fit model holds that the shape of the substrate is irrelevant to enzyme-substrate binding
b)
The induced fit model holds that the shape of the active site is altered during the course of substrate binding
c)
The induced fit model holds that the shape of the active site is permanently altered by substrate binding
d)
The induced fit model holds that enzyme-substrate binding does not take place at the enzyme’s active site

|
Elite Coaching Classes answered |
The correct answer is B. The induced fit model holds that the shape of the active site is altered during the course of substrate binding.
The active site model (lock and key model) and the induced fit model are two different models that describe the binding between an enzyme and its substrate.
The active site model proposes that the active site of the enzyme has a rigid shape that perfectly matches the shape of the substrate. It suggests that the substrate fits into the active site like a key into a lock, and the binding is based on complementary shapes.
In contrast, the induced fit model proposes that the active site of the enzyme is not fully rigid but rather dynamic. It suggests that the binding of the substrate induces a conformational change in the enzyme, leading to a more precise fit between the active site and the substrate. In other words, the shape of the active site can be altered during the course of substrate binding to accommodate the substrate more effectively.
Option A is incorrect because the induced fit model does consider the shape of the substrate as relevant to enzyme-substrate binding.
Option C is incorrect because the induced fit model does not suggest that the shape of the active site is permanently altered by substrate binding. The conformational changes are reversible, and the enzyme can return to its original shape after the substrate is released.
Option D is incorrect because the induced fit model still holds that enzyme-substrate binding takes place at the enzyme's active site. The model emphasizes that the active site undergoes changes to accommodate the substrate, but it does not suggest that binding occurs elsewhere.
Enzymes undergo a decrease in catalytic efficiency in the presence of excess temperature, but can regain this efficiency once temperature returns to normal: this suggests that increased temperature does not disrupt which of the following aspects of enzyme structure?- a)Van der Waal’s forces
- b)Peptide bonds
- c)Hydrophobic interactions
- d)Hydrogen bonds
Correct answer is option 'B'. Can you explain this answer?
Enzymes undergo a decrease in catalytic efficiency in the presence of excess temperature, but can regain this efficiency once temperature returns to normal: this suggests that increased temperature does not disrupt which of the following aspects of enzyme structure?
a)
Van der Waal’s forces
b)
Peptide bonds
c)
Hydrophobic interactions
d)
Hydrogen bonds

|
Elite Coaching Classes answered |
Peptide bonds are covalent bonds that link amino acids together to form the backbone of proteins. They are relatively stable and not easily disrupted by changes in temperature. Therefore, increased temperature does not significantly affect the integrity of peptide bonds in enzyme structure.
In contrast, increased temperature can disrupt other non-covalent interactions, such as hydrogen bonds, van der Waals forces, and hydrophobic interactions, which contribute to the overall structure and stability of enzymes. Disruption of these interactions can lead to changes in the active site conformation and decrease in catalytic efficiency.
Therefore, option B, peptide bonds, is not disrupted by increased temperature, while the other options (A, C, and D) can be affected.
Which of the following molecules cannot be classified as an enzymatic cofactor?- a)Mg2+
- b)Valine
- c)Heme
- d)Flavin adenine dinucleotide (FAD)
Correct answer is option 'B'. Can you explain this answer?
Which of the following molecules cannot be classified as an enzymatic cofactor?
a)
Mg2+
b)
Valine
c)
Heme
d)
Flavin adenine dinucleotide (FAD)

|
Elite Coaching Classes answered |
Valine is an amino acid and is not classified as an enzymatic cofactor. Enzymatic cofactors are non-protein molecules that are required for the proper functioning of enzymes. They can be inorganic ions, such as Mg2+ in option A, or organic molecules, such as heme in option C and flavin adenine dinucleotide (FAD) in option D. These cofactors assist enzymes in catalyzing chemical reactions by providing necessary functional groups, aiding in substrate binding, or participating in electron transfer processes. Valine, on the other hand, is one of the 20 standard amino acids that are the building blocks of proteins and is not involved in the catalytic activity of enzymes as a cofactor.
Feedback inhibition of enzymes is affected by which of the following:- a)Enzyme
- b)End-products
- c)Substrate
- d)Intermediate and products
Correct answer is option 'B'. Can you explain this answer?
Feedback inhibition of enzymes is affected by which of the following:
a)
Enzyme
b)
End-products
c)
Substrate
d)
Intermediate and products
|
|
Jananignanamurugan Janan answered |
Yes option B is correct
when the end products levels are more,products will bind to enzymes and reduces its activity.After the body utilises products and the level decreases and becomes zero means then enzymes become free from inhibitors(products) and then again the enzymes catalyse the reaction and form products
when the end products levels are more,products will bind to enzymes and reduces its activity.After the body utilises products and the level decreases and becomes zero means then enzymes become free from inhibitors(products) and then again the enzymes catalyse the reaction and form products
Which of the following is NOT a method by which enzymes decrease the activation energy for biological reactions?- a)Modifying the local charge environment
- b)Forming transient covalent bonds
- c)Acting as electron donors or receptors
- d)Breaking bonds in the enzyme to provide energy
Correct answer is option 'D'. Can you explain this answer?
Which of the following is NOT a method by which enzymes decrease the activation energy for biological reactions?
a)
Modifying the local charge environment
b)
Forming transient covalent bonds
c)
Acting as electron donors or receptors
d)
Breaking bonds in the enzyme to provide energy

|
Elite Coaching Classes answered |
Enzymes are not altered by the process of catalysis. A molecule that breaks intramolecular bonds to provide activation energy would not be able to be reused.
How does the ideal temperature for a reaction change with and without an enzyme catalyst?- a)The ideal temperature is generally higher with a catalyst than without.
- b)The ideal temperature is generally lower with a catalyst than without.
- c)The ideal temperature is characteristic of the reaction, not the enzyme.
- d)No conclusion can be made without knowing the enzyme type.
Correct answer is option 'B'. Can you explain this answer?
How does the ideal temperature for a reaction change with and without an enzyme catalyst?
a)
The ideal temperature is generally higher with a catalyst than without.
b)
The ideal temperature is generally lower with a catalyst than without.
c)
The ideal temperature is characteristic of the reaction, not the enzyme.
d)
No conclusion can be made without knowing the enzyme type.

|
Elite Coaching Classes answered |
The rate of reaction increases with temperature because of the increased kinetic energy of the reactants, but reaches a peak temperature because the enzyme denatures with the disruption of hydrogen bonds at excessively high temperatures. In the absence of enzyme, this peak temperature is generally much hotter. Heating a reaction provides molecules with an increased chance of achieving the activation energy, but the enzyme catalyst would typically reduce activation energy. Keep in mind that thermodynamics and kinetics are not interchangeable, so we are not considering the impact of heat on the equilibrium position.
The induced fit model of enzyme binding states that which of the following molecules alters the enzyme active site to more closely match the shape of the substrate?- a)The substrate
- b)A cofactor
- c)A coenzyme
- d)An allosteric effector
Correct answer is option 'A'. Can you explain this answer?
The induced fit model of enzyme binding states that which of the following molecules alters the enzyme active site to more closely match the shape of the substrate?
a)
The substrate
b)
A cofactor
c)
A coenzyme
d)
An allosteric effector

|
Elite Coaching Classes answered |
The induced fit model of enzyme binding describes the dynamic interaction between an enzyme and its substrate. According to this model, the active site of the enzyme undergoes a conformational change upon binding to the substrate. The initial active site may not perfectly match the shape of the substrate, but as the substrate binds, it induces a conformational change in the enzyme, leading to a more precise fit between the active site and the substrate.
The binding of the substrate induces changes in the enzyme's structure, such as alterations in the orientation of amino acid side chains, which allows for optimal interaction and formation of the enzyme-substrate complex. This conformational change enhances the catalytic activity of the enzyme and promotes the conversion of the substrate to product.
Cofactors and coenzymes (options B and C) are additional molecules that can assist enzyme activity by providing necessary chemical groups or aiding in electron transfer, but they do not directly alter the enzyme's active site to match the substrate. Allosteric effectors (option D) can modulate enzyme activity by binding to regulatory sites distinct from the active site, but they do not directly induce a conformational change in the active site to match the substrate.
Therefore, the substrate itself is the molecule that alters the enzyme active site to more closely match its shape in the induced fit model of enzyme binding.
Which of the following best describes the role of an enzyme in a biological reaction?- a)To increase the activation energy required for the reaction
- b)To decrease the activation energy required for the reaction
- c)To change the equilibrium constant of the reaction
- d)To change the free energy change (∆G) of the reaction
Correct answer is option 'B'. Can you explain this answer?
Which of the following best describes the role of an enzyme in a biological reaction?
a)
To increase the activation energy required for the reaction
b)
To decrease the activation energy required for the reaction
c)
To change the equilibrium constant of the reaction
d)
To change the free energy change (∆G) of the reaction

|
Elite Coaching Classes answered |
Enzymes are biological catalysts that facilitate chemical reactions by lowering the activation energy required for the reaction to occur. By binding to the substrate and creating an enzyme-substrate complex, enzymes stabilize the transition state and provide an alternative reaction pathway with lower activation energy. This allows the reaction to proceed more rapidly at physiological conditions. Option B is the correct answer as it accurately describes the role of enzymes in biological reactions.
Which of the following factors can influence the rate of an enzyme-catalyzed reaction?- a)pH and temperature
- b)Substrate concentration
- c)Enzyme concentration
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
Which of the following factors can influence the rate of an enzyme-catalyzed reaction?
a)
pH and temperature
b)
Substrate concentration
c)
Enzyme concentration
d)
All of the above

|
Elite Coaching Classes answered |
The rate of an enzyme-catalyzed reaction can be influenced by various factors, including pH, temperature, substrate concentration, and enzyme concentration.
pH: Enzymes have an optimal pH at which they exhibit maximum activity. Deviations from this optimal pH can disrupt the enzyme's structure and affect its catalytic ability.
Temperature: Enzymes also have an optimal temperature at which they function most efficiently. Changes in temperature can impact the enzyme's structure and alter the rate of the reaction. High temperatures can denature the enzyme, while low temperatures can decrease the kinetic energy of the molecules, slowing down the reaction.
Substrate concentration: The rate of an enzyme-catalyzed reaction initially increases with an increase in substrate concentration, as more substrate molecules are available for binding to the enzyme. However, beyond a certain point, the reaction rate reaches a maximum (Vmax) as all enzyme active sites become saturated with substrate.
Enzyme concentration: The rate of the reaction can be influenced by the amount of enzyme present. Generally, increasing the enzyme concentration leads to an increase in the reaction rate until all substrate molecules are bound to enzyme active sites.
Therefore, option D is the correct answer as all of these factors (pH, temperature, substrate concentration, and enzyme concentration) can affect the rate of an enzyme-catalyzed reaction.
Consider a biochemical reaction A → B, which is catalyzed by A–B dehydrogenase. Which of the following statements is true?- a)The reaction will proceed until the enzyme concentration decreases.
- b)The reaction will be most favorable at 0°
- c)A component of the enzyme is transferred from A to B.
- d)The free energy change (ΔG) of the catalyzed reaction is the same as for the uncatalyzed reaction.
Correct answer is option 'D'. Can you explain this answer?
Consider a biochemical reaction A → B, which is catalyzed by A–B dehydrogenase. Which of the following statements is true?
a)
The reaction will proceed until the enzyme concentration decreases.
b)
The reaction will be most favorable at 0°
c)
A component of the enzyme is transferred from A to B.
d)
The free energy change (ΔG) of the catalyzed reaction is the same as for the uncatalyzed reaction.

|
Elite Coaching Classes answered |
Enzymes catalyze reactions by lowering their activation energy, and are not changed or consumed during the course of the reaction. While the activation energy is lowered, the free energy of the reaction, ΔG, remains unchanged in the presence of an enzyme. A reaction will continue to occur in the presence or absence of an enzyme; it simply runs slower without the enzyme, eliminating choice (A). Most physiological reactions are optimized at body temperature, 37°C, eliminating choice (B). Finally, dehydrogenases catalyze oxidation–reduction reactions, not transfer reactions, eliminating choice (C).
A certain cooperative enzyme has four subunits, two of which are bound to substrate. Which of the following statements can be made?- a)The affinity of the enzyme for the substrate has just increased.
- b)The affinity of the enzyme for the substrate has just decreased.
- c)The affinity of the enzyme for the substrate is at the average for this enzyme class.
- d)The affinity of the enzyme for the substrate is greater than with one substrate bound.
Correct answer is option 'D'. Can you explain this answer?
A certain cooperative enzyme has four subunits, two of which are bound to substrate. Which of the following statements can be made?
a)
The affinity of the enzyme for the substrate has just increased.
b)
The affinity of the enzyme for the substrate has just decreased.
c)
The affinity of the enzyme for the substrate is at the average for this enzyme class.
d)
The affinity of the enzyme for the substrate is greater than with one substrate bound.

|
Elite Coaching Classes answered |
Cooperative enzymes demonstrate a change in affinity for the substrate depending on how many substrate molecules are bound and whether the last change was accomplished because a substrate molecule was bound or left the active site of the enzyme. Because we cannot determine whether the most recent reaction was binding or dissociation, choices (A) and (B) are eliminated. We can make absolute comparisons though. The unbound enzyme has the lowest affinity for substrate, and the enzyme with all but one subunit bound has the highest. The increase in affinity is not linear, and therefore choice (C) is not necessarily true. An enzyme with two subunits occupied must have a higher affinity for the substrate than the same enzyme with only one subunit occupied; thus, choice (D) is correct.
The graph below shows kinetic data obtained for flu virus enzyme activity as a function of substrate concentration in the presence and absence of two antiviral drugs.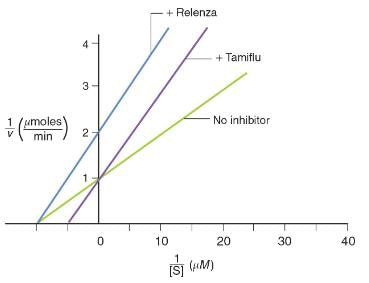 Based on the graph, which of the following statements is correct?
Based on the graph, which of the following statements is correct?- a)Both drugs are noncompetitive inhibitors of the viral enzyme.
- b)Tamiflu increases the Km value for the substrate compared to Relenza.
- c)Relenza increases the vmax value for the substrate compared to Tamiflu.
- d)Both drugs are competitive inhibitors of the viral enzyme.
Correct answer is option 'B'. Can you explain this answer?
The graph below shows kinetic data obtained for flu virus enzyme activity as a function of substrate concentration in the presence and absence of two antiviral drugs.

Based on the graph, which of the following statements is correct?
a)
Both drugs are noncompetitive inhibitors of the viral enzyme.
b)
Tamiflu increases the Km value for the substrate compared to Relenza.
c)
Relenza increases the vmax value for the substrate compared to Tamiflu.
d)
Both drugs are competitive inhibitors of the viral enzyme.

|
Elite Coaching Classes answered |
Based on the graph, when the substrate is present, Tamiflu results in the same vmax and a higher Km compared to when no inhibitor is added. These are hallmarks of competitive inhibitors. Noncompetitive inhibitors result in decreased vmax and the same Km as the uninhibited reaction, which is shown by the Relenza line in the graph. Because the question is only comparing the values between the two inhibitors, and not the enzyme without inhibitor, the mechanism of inhibition is less important to determine than the values of Km and vmax. This is a great example of why previewing the answer choices works well in the sciences.
In the equation below, substrate C is an allosteric inhibitor to enzyme 1. Which of the following is another mechanism necessarily caused by substrate C?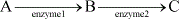
- a)Competitive inhibition
- b)Irreversible inhibition
- c)Feedback enhancement
- d)Negative feedback
Correct answer is option 'D'. Can you explain this answer?
In the equation below, substrate C is an allosteric inhibitor to enzyme 1. Which of the following is another mechanism necessarily caused by substrate C?
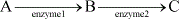
a)
Competitive inhibition
b)
Irreversible inhibition
c)
Feedback enhancement
d)
Negative feedback

|
Elite Coaching Classes answered |
By limiting the activity of enzyme 1, the rest of the pathway is slowed, which is the definition of negative feedback. Choice (A) is incorrect because there is no competition for the active site with allosteric interactions. While many products do indeed competitively inhibit an enzyme in the pathway that creates them, this is an example of an allosterically inhibited enzyme. There is not enough information for choice (B) to be correct because we aren’t told whether the inhibition is reversible. In general, allosteric interactions are temporary. Choice (C) is incorrect because it is the opposite of what occurs when enzyme 1 activity is reduced.
Which of the following is LEAST likely to be required for a series of metabolic reactions?- a)Triglyceride acting as a coenzyme
- b)Oxidoreductase enzymes
- c)Magnesium acting as a cofactor
- d)Transferase enzymes
Correct answer is option 'A'. Can you explain this answer?
Which of the following is LEAST likely to be required for a series of metabolic reactions?
a)
Triglyceride acting as a coenzyme
b)
Oxidoreductase enzymes
c)
Magnesium acting as a cofactor
d)
Transferase enzymes

|
Elite Coaching Classes answered |
Triglycerides are unlikely to act as coenzymes for a few reasons, including their large size, neutral charge, and ubiquity in cells. Cofactors and coenzymes tend to be small in size, such as metal ions like choice (C) or small organic molecules. They can usually carry a charge by ionization, protonation, or deprotonation. Finally, they are usually in low, tightly regulated concentrations within cells. Metabolic pathways would be expected to include both oxidation–reduction reactions and movement of functional groups, thus eliminating choices (B) and (D).
Chapter doubts & questions for Enzymes - Biology for BMAT (Section 2) 2025 is part of BMAT exam preparation. The chapters have been prepared according to the BMAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for BMAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Enzymes - Biology for BMAT (Section 2) in English & Hindi are available as part of BMAT exam.
Download more important topics, notes, lectures and mock test series for BMAT Exam by signing up for free.
Biology for BMAT (Section 2)
1 videos|51 docs|17 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup