All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Alkanamines(Amines) for JAMB Exam
The following reaction takes place in the presence of 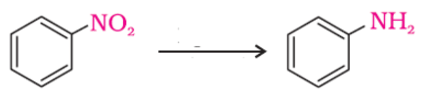
- a)NaOH/Pd
- b)H2/Pd
- c)HCl/Pd
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
The following reaction takes place in the presence of
a)
NaOH/Pd
b)
H2/Pd
c)
HCl/Pd
d)
None of these
|
|
Anaya Patel answered |
−NO2 group is reduced to –NH2 using H2/Pd.
The molecular formula of ethyl acetate is- a)C4H8O
- b)C4H8O2
- c)C5H10O2
- d)C5H8O2
Correct answer is option 'B'. Can you explain this answer?
The molecular formula of ethyl acetate is
a)
C4H8O
b)
C4H8O2
c)
C5H10O2
d)
C5H8O2

|
Tejas Singh answered |
Its molecular formula is C4H8O2
The main product formed by treating an alkyl or benzyl halide with excess ammonia- a)Mixed
- b)Tertiary
- c)Secondary
- d)Primary
Correct answer is option 'D'. Can you explain this answer?
The main product formed by treating an alkyl or benzyl halide with excess ammonia
a)
Mixed
b)
Tertiary
c)
Secondary
d)
Primary
|
|
Amita Das answered |
The N in ammonia functions as the nucleophile and attacks the electrophilic C of the alkyl halide displacing the bromide and creating the new C-N bond.
Step 2: An acid/base reaction. The base (excess ammonia) deprotonates the positive N (ammonium) center creating the alkylation product, the primary amine.
The Hofmann elimination proceeds via a(n) __________ pathway.- a)SN2
- b)E2
- c)E1
- d)SN1
Correct answer is option 'B'. Can you explain this answer?
The Hofmann elimination proceeds via a(n) __________ pathway.
a)
SN2
b)
E2
c)
E1
d)
SN1
|
|
Rajat Kapoor answered |
The Hofmann Elimination is an elimination reaction that forms C-C double (pi) bonds that specifically occurs when the leaving group is NR3 [note] It proceeds through an E2 mechanism. Although the key concepts are no different than one learns in the chapter on elimination from way back in Org 1, it is often included in Org 2 as part of the grab-bag chapter on amines. Because, well… nitrogen.
Which one of the following is used to increase blood pressure- a)Ephedrine
- b)Novocain
- c)Benadryl
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is used to increase blood pressure
a)
Ephedrine
b)
Novocain
c)
Benadryl
d)
None of these
|
|
Anjali Iyer answered |
Correct answer is d) none of these:
1. Fludrocortisone is a medication that seems to help most types of low blood pressure. It works by promoting sodium retention by the kidney, thereby causing fluid retention and some swelling, which is necessary to improve blood pressure.
2. Midodrine activates receptors on the smallest arteries and veins to produce an increase in blood pressure. It is used to help increase standing blood pressure in people with postural hypotension related to nervous system dysfunction.
Diazonium salts are used in the preparation of- a)Hormones
- b)Vitamins
- c)Dyes
- d)Proteins
Correct answer is option 'C'. Can you explain this answer?
Diazonium salts are used in the preparation of
a)
Hormones
b)
Vitamins
c)
Dyes
d)
Proteins
|
|
Vijay Bansal answered |
A diazonium salt is an organic compound that contains a nitrogen-nitrogen triple bond and some other generic side group that could be either alkyl (an alkane derivative) or aryl (benzene ring). The 'salt' portion of the name comes from the fact that the diazo (meaning 'di-nitrogen') portion of the compound is present as its ionic salt, with a chloride ion being a typical counter-ion for the positively charged nitrogen atom.
Which of the following amines is most soluble in water?- a)(CH3CH2CH2)2NH
- b)ethylamine
- c)pyrrolidine
- d)(CH3CH3)3N
Correct answer is option 'B'. Can you explain this answer?
Which of the following amines is most soluble in water?
a)
(CH3CH2CH2)2NH
b)
ethylamine
c)
pyrrolidine
d)
(CH3CH3)3N

|
Sankar Gupta answered |
Because to maximum hydrogen bonding.
For producing amines, the reaction of nitro compounds with iron scrap is preferred because- a)HCl is very cheap
- b)HCl is formed on hydrolysis of FeCl2
- c)Not too much HCl is formed in the reaction
- d)Nitro compounds are easily available
Correct answer is option 'B'. Can you explain this answer?
For producing amines, the reaction of nitro compounds with iron scrap is preferred because
a)
HCl is very cheap
b)
HCl is formed on hydrolysis of FeCl2
c)
Not too much HCl is formed in the reaction
d)
Nitro compounds are easily available

|
Kavya Das answered |
This reaction is preferred because on reduction of nitro compouns to amine , Fe will get oxidize to Fe(II) a which on hydrolysis will produce HCl which is required in the reaction
Aniline does not undergo Friedel – Crafts reaction- a)Anilium ion deactivates any further reaction.
- b)aluminium chloride, the catalyst reacts with NH2 group
- c)nitrogen of aniline acquires positive charge
- d)all of these
Correct answer is option 'D'. Can you explain this answer?
Aniline does not undergo Friedel – Crafts reaction
a)
Anilium ion deactivates any further reaction.
b)
aluminium chloride, the catalyst reacts with NH2 group
c)
nitrogen of aniline acquires positive charge
d)
all of these

|
Gauri Sharma answered |
NH2 has lp of electron which reacts with AlCl3 or other catalyst that we add to form anilium ion.
Hinsberg’s reagent reacts with primary and secondary amines to form sulphonamides. This reagent is also known as- a)N Methylbenzamide
- b)p – toluenesulphonyl chloride
- c)None of these
- d)Benzenesulphonyl chloride
Correct answer is option 'D'. Can you explain this answer?
Hinsberg’s reagent reacts with primary and secondary amines to form sulphonamides. This reagent is also known as
a)
N Methylbenzamide
b)
p – toluenesulphonyl chloride
c)
None of these
d)
Benzenesulphonyl chloride

|
Mohit Patel answered |
C6H5SO2Cl this is benzenesulphonyl chloride or hinsberg reagent.
Aniline does not undergo Friedel – Crafts reaction- a)Anilium ion deactivates any further reaction.
- b)aluminium chloride, the catalyst reacts with NH2 group
- c)nitrogen of aniline acquires positive charge
- d)all of these
Correct answer is option 'D'. Can you explain this answer?
Aniline does not undergo Friedel – Crafts reaction
a)
Anilium ion deactivates any further reaction.
b)
aluminium chloride, the catalyst reacts with NH2 group
c)
nitrogen of aniline acquires positive charge
d)
all of these

|
Aryan Dasgupta answered |
NH2 has lp of electron which reacts with AlCl3 or other catalyst that we add to form anilium ion.
Direct nitration of aniline yields significant amount of meta derivative. To obtain more p – nitro derivative, one or more of the below can be done- a)reacting with acetic anhydride
- b)acetylation reaction
- c)controlling the nitration reaction
- d)all of these
Correct answer is option 'D'. Can you explain this answer?
Direct nitration of aniline yields significant amount of meta derivative. To obtain more p – nitro derivative, one or more of the below can be done
a)
reacting with acetic anhydride
b)
acetylation reaction
c)
controlling the nitration reaction
d)
all of these

|
Janhavi Kaur answered |
Meta isomer form because of converstion of aniline to anilium ion which is META director. This can be prevented by acetylation of anilie by reaction with acetyl chloride or acetylanhydride.
Arrange the following in order of increasing basicity: aniline, p – nitroaniline, p – toluidine,and p – methoxyaniline- a)p – methoxyaniline p – nitroaniline < aniline < p – toluidine
- b)p – nitroaniline < aniline < p – toluidine < p – methoxyaniline
- c)aniline < p – methoxyaniline p – nitroaniline < p – toluidine
- d)p – nitroaniline < aniline< p – methoxyaniline < p – toluidine
Correct answer is option 'B'. Can you explain this answer?
Arrange the following in order of increasing basicity: aniline, p – nitroaniline, p – toluidine,and p – methoxyaniline
a)
p – methoxyaniline p – nitroaniline < aniline < p – toluidine
b)
p – nitroaniline < aniline < p – toluidine < p – methoxyaniline
c)
aniline < p – methoxyaniline p – nitroaniline < p – toluidine
d)
p – nitroaniline < aniline< p – methoxyaniline < p – toluidine

|
Nilesh Goyal answered |
-OMe group at p position will increase the basicity more tha normal –CH3 group at p position. While presence of –NO2 at position will decrease the basicity.
Which of the following is a tertiary amine?- a)Diphenhydramine
- b)Adrenaline
- c)Ephedrine
- d)Novocaine
Correct answer is option 'A'. Can you explain this answer?
Which of the following is a tertiary amine?
a)
Diphenhydramine
b)
Adrenaline
c)
Ephedrine
d)
Novocaine
|
|
Subhankar Choudhary answered |
Diphenhydramine is an antihistamine mainly used to treat allergies. It can also be used for insomnia, symptoms of the common cold, tremor in parkinsonism, and nausea. It is used by mouth, injection into a vein, injection into a muscle, or applied to the skin.

The general formula of a primary amine is:- a)R-NH2
- b)R-NH-R
- c)R-NH-R1-R2
- d)R-NH3+
Correct answer is option 'A'. Can you explain this answer?
The general formula of a primary amine is:
a)
R-NH2
b)
R-NH-R
c)
R-NH-R1-R2
d)
R-NH3+
|
|
Deepak Iyer answered |
The general formula of a primary amine is R-NH2, where R represents an alkyl or aryl group. This formula indicates that the nitrogen atom is bonded to one alkyl or aryl group and one hydrogen atom.
Which of the following statements about amines is true?- a)Amines can only be synthesized from ammonia.
- b)Amines have a higher boiling point than their corresponding alcohols.
- c)Amines cannot form hydrogen bonds.
- d)Amines are always liquids at room temperature.
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements about amines is true?
a)
Amines can only be synthesized from ammonia.
b)
Amines have a higher boiling point than their corresponding alcohols.
c)
Amines cannot form hydrogen bonds.
d)
Amines are always liquids at room temperature.
|
|
Deepak Iyer answered |
Amines have higher boiling points than their corresponding alcohols of similar molecular weight. This is due to the presence of intermolecular hydrogen bonding in amines, whereas alcohols can form both intermolecular and intramolecular hydrogen bonds.
How many hydrogen atoms are directly bonded to the nitrogen atom in a tertiary amine?- a)0
- b)1
- c)2
- d)3
Correct answer is option 'A'. Can you explain this answer?
How many hydrogen atoms are directly bonded to the nitrogen atom in a tertiary amine?
a)
0
b)
1
c)
2
d)
3
|
|
Deepak Iyer answered |
In a tertiary amine, the nitrogen atom is bonded to three alkyl or aryl groups and does not have any hydrogen atoms directly attached to it. Tertiary amines have the general formula R3N.
Which of the following compounds is a tertiary amine?- a)CH3NH2
- b)CH3NHCH3
- c)(CH3)3N
- d)(CH3)2NH
Correct answer is option 'C'. Can you explain this answer?
Which of the following compounds is a tertiary amine?
a)
CH3NH2
b)
CH3NHCH3
c)
(CH3)3N
d)
(CH3)2NH
|
|
Deepak Iyer answered |
(CH3)3N is a tertiary amine because it has three methyl groups attached to the nitrogen atom. It does not have any hydrogen atoms directly bonded to the nitrogen.
Amines are derivatives of which of the following functional groups?- a)Alcohols
- b)Aldehydes
- c)Carboxylic acids
- d)Ammonia
Correct answer is option 'D'. Can you explain this answer?
Amines are derivatives of which of the following functional groups?
a)
Alcohols
b)
Aldehydes
c)
Carboxylic acids
d)
Ammonia
|
|
Deepak Iyer answered |
Amines are derivatives of ammonia (NH3) in which one or more of the hydrogen atoms are replaced by alkyl or aryl groups. They are formed by replacing one or more hydrogen atoms in ammonia with alkyl or aryl groups.
Which of the following is a secondary amine?- a)Methylamine
- b)Diethylamine
- c)Trimethylamine
- d)Ethylamine
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a secondary amine?
a)
Methylamine
b)
Diethylamine
c)
Trimethylamine
d)
Ethylamine
|
|
Deepak Iyer answered |
Diethylamine is a secondary amine because it has two alkyl groups (ethyl groups) attached to the nitrogen atom. In secondary amines, the nitrogen atom is bonded to two alkyl groups and one hydrogen atom.
The process of converting a primary amine to a secondary amine is called:- a)Substitution
- b)Dehydration
- c)Dehydrogenation
- d)N-alkylation
Correct answer is option 'D'. Can you explain this answer?
The process of converting a primary amine to a secondary amine is called:
a)
Substitution
b)
Dehydration
c)
Dehydrogenation
d)
N-alkylation
|
|
Deepak Iyer answered |
The process of converting a primary amine to a secondary amine is called N-alkylation. It involves the substitution of an alkyl group for one of the hydrogen atoms bonded to the nitrogen atom.
Which of the following is an example of a primary amine?- a)Dimethylamine
- b)Diethylamine
- c)Ethylamine
- d)Trimethylamine
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of a primary amine?
a)
Dimethylamine
b)
Diethylamine
c)
Ethylamine
d)
Trimethylamine
|
|
Deepak Iyer answered |
Ethylamine is a primary amine because it has only one alkyl group (ethyl group) attached to the nitrogen atom. In primary amines, the nitrogen atom is bonded to one alkyl group and two hydrogen atoms.
Which of the following reagents can be used to distinguish between primary, secondary, and tertiary amines?- a)Sodium hydroxide
- b)Hydrochloric acid
- c)Sodium nitrite
- d)Hinsberg reagent
Correct answer is option 'D'. Can you explain this answer?
Which of the following reagents can be used to distinguish between primary, secondary, and tertiary amines?
a)
Sodium hydroxide
b)
Hydrochloric acid
c)
Sodium nitrite
d)
Hinsberg reagent
|
|
Deepak Iyer answered |
Hinsberg reagent (benzenesulfonyl chloride) can be used to distinguish between primary, secondary, and tertiary amines. It reacts differently with each type of amine, forming distinct products that can be identified. Primary amines give N-alkylbenzenesulfonamide, secondary amines give N,N-dialkylbenzenesulfonamide, and tertiary amines do not react with Hinsberg reagent.
Which of the following amines is a strong base?- a)Aniline
- b)Ethylamine
- c)Dimethylamine
- d)Pyrrole
Correct answer is option 'B'. Can you explain this answer?
Which of the following amines is a strong base?
a)
Aniline
b)
Ethylamine
c)
Dimethylamine
d)
Pyrrole
|
|
Deepak Iyer answered |
Ethylamine is a strong base because it readily accepts a proton (H+) to form the ethylammonium ion (C2H5NH3+). It has a lone pair of electrons on the nitrogen atom, which can easily accept a proton.
Chapter doubts & questions for Alkanamines(Amines) - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Alkanamines(Amines) - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup















