All Exams >
JAMB >
Physics for JAMB >
All Questions
All questions of Heat Transfer for JAMB Exam
Fluorescent lamps are more efficient than incandescent lamps in converting electrical energy to visible light because- a)they produce more white light
- b)they do not use uv radiations
- c)they do not waste as much energy producing (invisible) infrared photons
- d)they do not waste as much energy producing visible photons
Correct answer is option 'C'. Can you explain this answer?
Fluorescent lamps are more efficient than incandescent lamps in converting electrical energy to visible light because
a)
they produce more white light
b)
they do not use uv radiations
c)
they do not waste as much energy producing (invisible) infrared photons
d)
they do not waste as much energy producing visible photons
|
|
Riya Banerjee answered |
The phosphor fluoresces to produce light. A fluorescent bulb produces less heat, so it is much more efficient. This makes fluorescent bulbs four to six times more efficient than incandescent bulbs. That's why you can buy a 15-watt fluorescent bulb that produces the same amount of light as a 60-watt incandescent bulb.
Emission line spectra of different elements- a)is not different
- b)is the same if both elements are at the same temperature
- c)is the same if both elements are in liquid form
- d)is different
Correct answer is option 'D'. Can you explain this answer?
Emission line spectra of different elements
a)
is not different
b)
is the same if both elements are at the same temperature
c)
is the same if both elements are in liquid form
d)
is different

|
Adithya Shasan answered |
Option D : Emission line spectra of different elements is different..
In Geiger-Marsden experiment very small deflection of the beam was expected because- a)there are no electrical forces at work
- b)positive charge and the negative electrons are distributed through the whole atom reducing electric field inside the atom
- c)particles are collimated by lead screens
- d)most particles pass through
Correct answer is option 'B'. Can you explain this answer?
In Geiger-Marsden experiment very small deflection of the beam was expected because
a)
there are no electrical forces at work
b)
positive charge and the negative electrons are distributed through the whole atom reducing electric field inside the atom
c)
particles are collimated by lead screens
d)
most particles pass through
|
|
Shraddha Choudhury answered |
Explanation:
The Geiger-Marsden experiment was conducted to study the structure of an atom. In this experiment, a beam of alpha particles was directed towards a thin gold foil. The alpha particles were expected to pass through the gold foil with little or no deflection, as it was believed that the positive charge and the negative electrons in an atom are distributed uniformly, reducing the electric field inside the atom. However, the results of the experiment were surprising, as some of the alpha particles were deflected at large angles, and some even bounced back.
Reasons for very small deflection of the beam:
- Electrical forces: According to Coulomb's law, any two charged particles exert a force on each other. In an atom, the positively charged nucleus and the negatively charged electrons are attracted to each other by electrical forces. However, the electrons are in constant motion, creating a cloud of negative charge around the nucleus. This cloud of negative charge reduces the electric field inside the atom, making it difficult for the alpha particles to be deflected.
- Distribution of charge: The positive charge in an atom is concentrated in the nucleus, while the negative charge is distributed throughout the atom. This distribution of charge makes the electric field inside the atom more uniform, reducing the chances of the alpha particles being deflected.
- Collimation of particles: The alpha particles were collimated by lead screens before they were directed towards the gold foil. This was done to ensure that the particles were traveling in a straight line and were not scattered by other particles or objects in the environment.
- Most particles pass through: Despite the above factors, it was still expected that some of the alpha particles would be deflected at small angles due to the random nature of the collisions between the particles and the atoms in the gold foil. However, it was not expected that some of the particles would be deflected at large angles or bounce back, as this implied that the positive charge in an atom was not uniformly distributed.
Conclusion:
In conclusion, the very small deflection of the beam was expected in the Geiger-Marsden experiment due to the distribution of charge in an atom and the reduction of electric field inside the atom. However, the unexpected results of the experiment led to the discovery of the nucleus and the development of the modern atomic model.
The Geiger-Marsden experiment was conducted to study the structure of an atom. In this experiment, a beam of alpha particles was directed towards a thin gold foil. The alpha particles were expected to pass through the gold foil with little or no deflection, as it was believed that the positive charge and the negative electrons in an atom are distributed uniformly, reducing the electric field inside the atom. However, the results of the experiment were surprising, as some of the alpha particles were deflected at large angles, and some even bounced back.
Reasons for very small deflection of the beam:
- Electrical forces: According to Coulomb's law, any two charged particles exert a force on each other. In an atom, the positively charged nucleus and the negatively charged electrons are attracted to each other by electrical forces. However, the electrons are in constant motion, creating a cloud of negative charge around the nucleus. This cloud of negative charge reduces the electric field inside the atom, making it difficult for the alpha particles to be deflected.
- Distribution of charge: The positive charge in an atom is concentrated in the nucleus, while the negative charge is distributed throughout the atom. This distribution of charge makes the electric field inside the atom more uniform, reducing the chances of the alpha particles being deflected.
- Collimation of particles: The alpha particles were collimated by lead screens before they were directed towards the gold foil. This was done to ensure that the particles were traveling in a straight line and were not scattered by other particles or objects in the environment.
- Most particles pass through: Despite the above factors, it was still expected that some of the alpha particles would be deflected at small angles due to the random nature of the collisions between the particles and the atoms in the gold foil. However, it was not expected that some of the particles would be deflected at large angles or bounce back, as this implied that the positive charge in an atom was not uniformly distributed.
Conclusion:
In conclusion, the very small deflection of the beam was expected in the Geiger-Marsden experiment due to the distribution of charge in an atom and the reduction of electric field inside the atom. However, the unexpected results of the experiment led to the discovery of the nucleus and the development of the modern atomic model.
Absorption line spectrum is obtained- a)If we pass off-white (discrete-spectrum) light through a cool gas
- b)If we pass white (continuous-spectrum) light through a hot gas
- c)If we pass off-white (discrete-spectrum) light through a hot gas
- d)If we pass white (continuous-spectrum) light through a cool gas
Correct answer is option 'D'. Can you explain this answer?
Absorption line spectrum is obtained
a)
If we pass off-white (discrete-spectrum) light through a cool gas
b)
If we pass white (continuous-spectrum) light through a hot gas
c)
If we pass off-white (discrete-spectrum) light through a hot gas
d)
If we pass white (continuous-spectrum) light through a cool gas
|
|
Nishant Sharma answered |
Answer is D
Fluorescence is- a)it consists of accelerated atoms/molecules striking suitable material
- b)it consists only of atoms going into stable excited states
- c)what happens in a fluorescent lamp
- d)it consists of a molecule, atom or nanostructure relaxing to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy
Correct answer is option 'D'. Can you explain this answer?
Fluorescence is
a)
it consists of accelerated atoms/molecules striking suitable material
b)
it consists only of atoms going into stable excited states
c)
what happens in a fluorescent lamp
d)
it consists of a molecule, atom or nanostructure relaxing to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy
|
|
Nikita Singh answered |
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. The most striking example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, while the emitted light is in the visible region, which gives the fluorescent substance a distinct color that can be seen only when exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after.
Which of these statements about Bohr model hypothesis is correct?- a)velocity of electron is quantized
- b)mass of electron is quantized
- c)radius of electron is quantized
- d)angular momentum of electron is quantized
Correct answer is option 'D'. Can you explain this answer?
Which of these statements about Bohr model hypothesis is correct?
a)
velocity of electron is quantized
b)
mass of electron is quantized
c)
radius of electron is quantized
d)
angular momentum of electron is quantized
|
|
Krishna Iyer answered |
Bohr never assumed stable electron orbits with the electronic angular momentum quantized as l=mvr=(nh/2π) Quantization of angular momentum means that the radius of the orbit and the energy will be quantized as well. Bohr assumed that the discrete lines seen in the spectrum of the hydrogen atom were due to transitions of an electron from one allowed orbit/energy to another.
In Geiger-Marsden experiment, at the point of closest approach- a)the kinetic energy is not zero and the electrical potential is less than the initial kinetic energy supplied
- b)the kinetic energy is not zero and the electrical potential equals the initial kinetic energy supplied
- c)the kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
- d)the kinetic energy is not zero and the electrical potential is greater than the initial kinetic energy supplied
Correct answer is option 'C'. Can you explain this answer?
In Geiger-Marsden experiment, at the point of closest approach
a)
the kinetic energy is not zero and the electrical potential is less than the initial kinetic energy supplied
b)
the kinetic energy is not zero and the electrical potential equals the initial kinetic energy supplied
c)
the kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
d)
the kinetic energy is not zero and the electrical potential is greater than the initial kinetic energy supplied
|
|
Dino James answered |
The kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
A triply ionized beryllium ion Be3+, (a beryllium atom with three electrons removed), behaves very much like a hydrogen atom except that the nuclear charge is four times as great. For the hydrogen atom, the wavelength of the photon emitted in the n =2 to n=1 to transition is 122 nm. What is the wavelength of the photon emitted when a Be3+ ion undergoes this transition?- a)7.60 nm
- b)10.60 nm
- c)9.60 nm
- d)8.60 nm
Correct answer is option 'A'. Can you explain this answer?
A triply ionized beryllium ion Be3+, (a beryllium atom with three electrons removed), behaves very much like a hydrogen atom except that the nuclear charge is four times as great. For the hydrogen atom, the wavelength of the photon emitted in the n =2 to n=1 to transition is 122 nm. What is the wavelength of the photon emitted when a Be3+ ion undergoes this transition?
a)
7.60 nm
b)
10.60 nm
c)
9.60 nm
d)
8.60 nm
|
|
Suresh Iyer answered |
1/ λ =Z2. (both have same transition so same value of n)
λ /122=1/16
λ =122/16
=7.62nm
λ /122=1/16
λ =122/16
=7.62nm
Find the longest wavelength present in the Balmer series of hydrogen, corresponding to the H- line.- a)656 nm
- b)676 nm
- c)666 nm
- d)686 nm
Correct answer is option 'A'. Can you explain this answer?
Find the longest wavelength present in the Balmer series of hydrogen, corresponding to the H- line.
a)
656 nm
b)
676 nm
c)
666 nm
d)
686 nm
|
|
Arun Khanna answered |
If that is so grant us some suggestion! Or Balmer sequence (2nd sequence from electrons laying off to n = 2 from bigger than 2) longest wavelength [velocityconstant = frequency x wavelength] is smallest frequency [power = hconstant x frequency] is least enegy is from transition n = 3 to n = 2 or Google it 656.3 nm
Which of these statements about Bohr model applied to hydrogen atom correct?- a)hydrogen atom in its ground level has no magnetic moment due to orbital motion
- b)hydrogen atom in its ground level has magnetic moment due to orbital motion
- c)hydrogen atom in some orbits radiates electromagnetic waves
- d)hydrogen atom in quantized orbits radiates electromagnetic waves
Correct answer is option 'B'. Can you explain this answer?
Which of these statements about Bohr model applied to hydrogen atom correct?
a)
hydrogen atom in its ground level has no magnetic moment due to orbital motion
b)
hydrogen atom in its ground level has magnetic moment due to orbital motion
c)
hydrogen atom in some orbits radiates electromagnetic waves
d)
hydrogen atom in quantized orbits radiates electromagnetic waves
|
|
Swati Verma answered |
A hydrogen atom has magnetic properties because the motion of the electron acts as a current loop. The energy levels of a hydrogen atom associated with orbital angular momentum are split by an external magnetic field because the orbital angular magnetic moment interacts with the field.
An electron collides with a hydrogen atom in its ground state and excites it to a state of n = 3. How much energy was given to the hydrogen atom in this inelastic collision?- a)
15.1 eV - b)13.1 eV
- c)12.1 eV
- d)14.1 eV
Correct answer is option 'C'. Can you explain this answer?
An electron collides with a hydrogen atom in its ground state and excites it to a state of n = 3. How much energy was given to the hydrogen atom in this inelastic collision?
a)
15.1 eV
b)
13.1 eV
c)
12.1 eV
d)
14.1 eV
|
|
Ritu Singh answered |
Energy at ground state E1=−13.6 eV
Energy at n=3: E3=−13.6/9=1.5 eV
To excite it to n=3 energy given to electron is E3−E1=12.1 eV
Energy at n=3: E3=−13.6/9=1.5 eV
To excite it to n=3 energy given to electron is E3−E1=12.1 eV
Average angle of deflection of α-particles by a thin gold foil predicted by Thomson’s model is- a)about the same as predicted by Rutherford’s model
- b)incomparable to Rutherford’s model
- c)about the more than predicted by Rutherford’s model
- d)about the less than predicted by Rutherford’s model
Correct answer is option 'A'. Can you explain this answer?
Average angle of deflection of α-particles by a thin gold foil predicted by Thomson’s model is
a)
about the same as predicted by Rutherford’s model
b)
incomparable to Rutherford’s model
c)
about the more than predicted by Rutherford’s model
d)
about the less than predicted by Rutherford’s model
|
|
Priya Patel answered |
About the same
The average angle of deflection of α-particles by a thin gold foil predicted by Thomson’s model is about the same size as predicted by Rutherford’s model. This is because the average angle was taken in both models.
According to ‘plum pudding model’ atoms on the whole are electrically neutral because- a)positive charge is concentrated at one place and negative charge is elsewhere.
- b)the negative charge of the atom is uniformly distributed throughout the volume of the atom and the positively charged electrons are embedded in it
- c)the positive charge of the atom is uniformly distributed throughout the volume of the atom and the negatively charged electrons are embedded in it
- d)the positive charge of the atom is uniformly distributed throughout the volume of the electron and the negative charge of electrons balances positive parts
Correct answer is option 'C'. Can you explain this answer?
According to ‘plum pudding model’ atoms on the whole are electrically neutral because
a)
positive charge is concentrated at one place and negative charge is elsewhere.
b)
the negative charge of the atom is uniformly distributed throughout the volume of the atom and the positively charged electrons are embedded in it
c)
the positive charge of the atom is uniformly distributed throughout the volume of the atom and the negatively charged electrons are embedded in it
d)
the positive charge of the atom is uniformly distributed throughout the volume of the electron and the negative charge of electrons balances positive parts
|
|
Manoj Chauhan answered |
The plum pudding model is one of several scientific models of the atom. According to J.J. Thomson atomic models the positive particles in the atom form something like the "batter" in a plum pudding, while the negative electrons are scattered through this "batter".
Each element is associated with- a)with a characteristic spectrum of radiation
- b)with a characteristic absorption of monochromatic light
- c)no light radiation or absorption
- d)with a characteristic radiation of monochromatic light
Correct answer is option 'A'. Can you explain this answer?
Each element is associated with
a)
with a characteristic spectrum of radiation
b)
with a characteristic absorption of monochromatic light
c)
no light radiation or absorption
d)
with a characteristic radiation of monochromatic light
|
|
Navya Banerjee answered |
Explanation:
The given question is related to the concept of atomic spectra. Atomic spectra is a characteristic property of every element which is used to identify the element. It is produced when an atom absorbs or emits energy as electromagnetic radiation.
The correct option is A, i.e., each element is associated with a characteristic spectrum of radiation. This means that every element emits or absorbs radiation of a unique wavelength that is specific to that particular element.
The characteristic spectrum of radiation is the set of wavelengths at which an element emits or absorbs radiation. There are three types of atomic spectra:
1. Continuous spectrum: A continuous spectrum is produced when a solid, liquid or dense gas is heated and the atoms in the material emit radiation at all wavelengths. This type of spectrum is not specific to any particular element.
2. Emission spectrum: An emission spectrum is produced when an element is heated or excited with energy, and the electrons in the atom jump to higher energy levels. When these electrons fall back to their original energy levels, they emit energy as radiation. The wavelengths of this radiation are specific to that particular element.
3. Absorption spectrum: An absorption spectrum is produced when an element absorbs certain wavelengths of radiation. When white light is passed through a sample of a specific element, certain wavelengths of light are absorbed by the atoms in the material, leaving dark lines in the spectrum. These dark lines are specific to that particular element.
In summary, the correct option is A because every element is associated with a characteristic spectrum of radiation, which is used to identify the element.
The given question is related to the concept of atomic spectra. Atomic spectra is a characteristic property of every element which is used to identify the element. It is produced when an atom absorbs or emits energy as electromagnetic radiation.
The correct option is A, i.e., each element is associated with a characteristic spectrum of radiation. This means that every element emits or absorbs radiation of a unique wavelength that is specific to that particular element.
The characteristic spectrum of radiation is the set of wavelengths at which an element emits or absorbs radiation. There are three types of atomic spectra:
1. Continuous spectrum: A continuous spectrum is produced when a solid, liquid or dense gas is heated and the atoms in the material emit radiation at all wavelengths. This type of spectrum is not specific to any particular element.
2. Emission spectrum: An emission spectrum is produced when an element is heated or excited with energy, and the electrons in the atom jump to higher energy levels. When these electrons fall back to their original energy levels, they emit energy as radiation. The wavelengths of this radiation are specific to that particular element.
3. Absorption spectrum: An absorption spectrum is produced when an element absorbs certain wavelengths of radiation. When white light is passed through a sample of a specific element, certain wavelengths of light are absorbed by the atoms in the material, leaving dark lines in the spectrum. These dark lines are specific to that particular element.
In summary, the correct option is A because every element is associated with a characteristic spectrum of radiation, which is used to identify the element.
Reason why there are many lines in an atomic spectrum is because- a)All atoms are in the same excited state and make transition to same state
- b)There are many atoms in different states of excitation making transition to the same state
- c)There are many atoms in different states of excitation making transition to different states
- d)All atoms are in the same excited state and make transition to different states
Correct answer is option 'C'. Can you explain this answer?
Reason why there are many lines in an atomic spectrum is because
a)
All atoms are in the same excited state and make transition to same state
b)
There are many atoms in different states of excitation making transition to the same state
c)
There are many atoms in different states of excitation making transition to different states
d)
All atoms are in the same excited state and make transition to different states
|
|
Nishtha Dasgupta answered |
Lines in the spectrum were due to transitions in which an electron moved from a higher-energy orbit with a larger radius to a lower-energy orbit with smaller radius. The orbit closest to the nucleus represented the ground state of the atom and was most stable; orbits farther away were higher-energy excited states.
Which of these statements correctly describe the atomic model according to classical electromagnetic theory ?- a)The electrons would spiral inwards and fall into the nucleus
- b)The waves emitted by electrons are discrete
- c)Electrons do not radiate electromagnetic waves
- d)The positive charge and negative electron cancel and no waves are radiated
Correct answer is option 'A'. Can you explain this answer?
Which of these statements correctly describe the atomic model according to classical electromagnetic theory ?
a)
The electrons would spiral inwards and fall into the nucleus
b)
The waves emitted by electrons are discrete
c)
Electrons do not radiate electromagnetic waves
d)
The positive charge and negative electron cancel and no waves are radiated
|
|
Avantika Dasgupta answered |
In classical electromagnetic theory, atoms and molecules are considered to contain electrical charges (i.e. electrons, ions) which are regarded as oscillating about positions of equilibrium, each with its appropriate natural frequency, v0 . When placed in a radiation field of frequency v , each oscillator in the atom or molecule is set into forced vibration with the same frequency as that of the radiation. The amplitude of the forced vibration is small, but as v approaches v0 , the amplitude of the forced vibration increases rapidly. To account for the absorption of energy from the radiation field, it is necessary to assume that the oscillator in the atom or molecule must overcome some frictional force proportional to its velocity during its forced motion. For small amplitudes of forced oscillation, the frictional force, and therefore the absorption of energy, is negligible. Near resonance , the amplitude of oscillation becomes large, with a correspondingly large absorption of energy to overcome the frictional force. Therefore, the radiation of frequencies near the natural frequency of the oscillator corresponds to an absorption band.
Which of these statements about Bohr model hypothesis is correct?- a)angular momentum is not quantized
- b)electron in a stable orbit does not radiate electromagnetic waves
- c)velocity of electron is quantized
- d)electron in a stable orbit emit quanta of light
Correct answer is option 'B'. Can you explain this answer?
Which of these statements about Bohr model hypothesis is correct?
a)
angular momentum is not quantized
b)
electron in a stable orbit does not radiate electromagnetic waves
c)
velocity of electron is quantized
d)
electron in a stable orbit emit quanta of light
|
|
Shraddha Choudhury answered |
Bohr Model Hypothesis and Stable Electron Orbit
Bohr model hypothesis is a model of the atom proposed by Niels Bohr in 1913. It was one of the earliest attempts to explain the structure of atoms and their behavior. The model was based on the assumption that electrons orbit the nucleus in circular paths.
The correct statement about Bohr model hypothesis is:
- Electron in a stable orbit does not radiate electromagnetic waves.
Explanation:
- Electrons in atoms can exist only in certain discrete energy levels. According to Bohr's model, electrons in atoms move around the nucleus in stable orbits, each with a specific energy level.
- Electrons in stable orbits do not emit electromagnetic waves because they are in a stable state and have a fixed amount of energy.
- However, when an electron transitions from a higher energy level to a lower energy level, it emits a photon of light.
- This is because the energy lost by the electron is emitted as a photon of light. The energy of the photon is equal to the difference in energy between the two energy levels.
- Therefore, the Bohr model hypothesis proposed that the electron does not continuously lose energy as it moves in a circular orbit around the nucleus, but instead only loses energy when it transitions between energy levels.
Conclusion:
Bohr model hypothesis is an important model that helps explain the behavior of atoms. The model is based on the assumption that electrons move around the nucleus in stable orbits and only emit electromagnetic waves when they transition between energy levels. The correct statement about the model is that electrons in a stable orbit do not radiate electromagnetic waves.
Bohr model hypothesis is a model of the atom proposed by Niels Bohr in 1913. It was one of the earliest attempts to explain the structure of atoms and their behavior. The model was based on the assumption that electrons orbit the nucleus in circular paths.
The correct statement about Bohr model hypothesis is:
- Electron in a stable orbit does not radiate electromagnetic waves.
Explanation:
- Electrons in atoms can exist only in certain discrete energy levels. According to Bohr's model, electrons in atoms move around the nucleus in stable orbits, each with a specific energy level.
- Electrons in stable orbits do not emit electromagnetic waves because they are in a stable state and have a fixed amount of energy.
- However, when an electron transitions from a higher energy level to a lower energy level, it emits a photon of light.
- This is because the energy lost by the electron is emitted as a photon of light. The energy of the photon is equal to the difference in energy between the two energy levels.
- Therefore, the Bohr model hypothesis proposed that the electron does not continuously lose energy as it moves in a circular orbit around the nucleus, but instead only loses energy when it transitions between energy levels.
Conclusion:
Bohr model hypothesis is an important model that helps explain the behavior of atoms. The model is based on the assumption that electrons move around the nucleus in stable orbits and only emit electromagnetic waves when they transition between energy levels. The correct statement about the model is that electrons in a stable orbit do not radiate electromagnetic waves.
The model that best explains the results of Geiger-Marsden experiment is- a)Thomson model
- b)Thomson model
- c)Rutherford model
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
The model that best explains the results of Geiger-Marsden experiment is
a)
Thomson model
b)
Thomson model
c)
Rutherford model
d)
None of the above
|
|
Sankar Banerjee answered |
When Rutherford saw the results of the experiment by Geiger and Marsden, he said:
“It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Rutherford used the results of this experiment to develop a new model for the atom. This model proposed a central nucleus with a positive charge. It was this positively charged nucleus that was responsible for the strong backward deflection of the positively charged alpha particles.
The model also proposed that negatively charged electrons surrounded this nucleus. However, as most of the alpha particles passed through the gold foil with no deflection at all, Rutherford realised that most of the atom was empty space. So, his model placed the electrons at some distance from the nucleus.
“It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Rutherford used the results of this experiment to develop a new model for the atom. This model proposed a central nucleus with a positive charge. It was this positively charged nucleus that was responsible for the strong backward deflection of the positively charged alpha particles.
The model also proposed that negatively charged electrons surrounded this nucleus. However, as most of the alpha particles passed through the gold foil with no deflection at all, Rutherford realised that most of the atom was empty space. So, his model placed the electrons at some distance from the nucleus.
In which of the models An atom has a nearly continuous mass distribution?- a)Thomson’s model
- b)Bohr model
- c)Rutherford’s model
- d)No model
Correct answer is option 'A'. Can you explain this answer?
In which of the models An atom has a nearly continuous mass distribution?
a)
Thomson’s model
b)
Bohr model
c)
Rutherford’s model
d)
No model
|
|
Naina Datta answered |
An atom has a nearly continuous mass distribution in Thomson’s model, but has a highly non-uniform mass distribution in Rutherford’s model.
Suppose you are given a chance to repeat the alpha-particle scattering experiment using a thin sheet of solid hydrogen in place of the gold foil. (Hydrogen is a solid at temperatures below 14 K.) What results do you expect?- a)there would be scattering at all angles
- b)there would be no scattering
- c)there would be scattering at 90∘
- d)there would be no large-angle scattering
Correct answer is option 'D'. Can you explain this answer?
Suppose you are given a chance to repeat the alpha-particle scattering experiment using a thin sheet of solid hydrogen in place of the gold foil. (Hydrogen is a solid at temperatures below 14 K.) What results do you expect?
a)
there would be scattering at all angles
b)
there would be no scattering
c)
there would be scattering at 90∘
d)
there would be no large-angle scattering

|
Yash Kumar answered |
In the alpha-particle scattering experiment, if a thin sheet of solid hydrogen is used in place of a gold foil, then the scattering angle would not be large enough. This is because the mass of hydrogen (1.67 x 10 −^27 kg) is less than the mass of incident a−particles (6.64 x 10 ^−27 kg). Thus, the mass of the scattering particle is more than the target nucleus (hydrogen).
As a result, the α−particles would not bounce back if solid hydrogen is used in the α-particle scattering experiment.
As a result, the α−particles would not bounce back if solid hydrogen is used in the α-particle scattering experiment.
Probability of backward scattering (i.e., scattering of α -particles at angles greater than 90∘) predicted by Thomson’s model is- a)much less than predicted by Rutherford’s model
- b)much more than predicted by Rutherford’s model
- c)same as predicted by Rutherford’s model
- d)more than predicted by Rutherford’s model
Correct answer is option 'A'. Can you explain this answer?
Probability of backward scattering (i.e., scattering of α -particles at angles greater than 90∘) predicted by Thomson’s model is
a)
much less than predicted by Rutherford’s model
b)
much more than predicted by Rutherford’s model
c)
same as predicted by Rutherford’s model
d)
more than predicted by Rutherford’s model
|
|
Sounak Mukherjee answered |
In Rutherford's model we have a large massive core called the nucleus whereas, in Thomson's model we do not have. Thus the probability of backward scattering by Thomson's model is much less than that predicted by Rutherford model.
Which of these statements about Bohr model is correct?- a)Bohr model postulates wavy paths around the nucleus
- b)Bohr model combines classical and early quantum concepts
- c)Bohr model is based classical electromagnetic theory
- d)Bohr model is pure quantum mechanical theory
Correct answer is option 'B'. Can you explain this answer?
Which of these statements about Bohr model is correct?
a)
Bohr model postulates wavy paths around the nucleus
b)
Bohr model combines classical and early quantum concepts
c)
Bohr model is based classical electromagnetic theory
d)
Bohr model is pure quantum mechanical theory
|
|
Isha Rane answered |
Bohr model of the hydrogen atom attempts to plug in certain gaps as suggested by Rutherford’s model by including ideas from the newly developing Quantum hypothesis. According to Rutherford’s model, an atom has a central nucleus and electron/s revolve around it like the sun-planet system.
However, the fundamental difference between the two is that, while the planetary system is held in place by the gravitational force, the nucleus-electron system interacts by Coulomb’s Law of Force. This is because the nucleus and electrons are charged particles. Also, an object moving in a circle undergoes constant acceleration due to the centripetal force.
Further, electromagnetic theory teaches us that an accelerating charged particle emits radiation in the form of electromagnetic waves. Therefore, the energy of such an electron should constantly decrease and the electron should collapse into the nucleus. This would make the atom unstable.
The classical electromagnetic theory also states that the frequency of the electromagnetic waves emitted by an accelerating electron is equal to the frequency of revolution. This would mean that, as the electron spirals inwards, it would emit electromagnetic waves of changing frequencies. In other words, it would emit a continuous spectrum. However, actual observation tells us that the electron emits a line spectrum.
However, the fundamental difference between the two is that, while the planetary system is held in place by the gravitational force, the nucleus-electron system interacts by Coulomb’s Law of Force. This is because the nucleus and electrons are charged particles. Also, an object moving in a circle undergoes constant acceleration due to the centripetal force.
Further, electromagnetic theory teaches us that an accelerating charged particle emits radiation in the form of electromagnetic waves. Therefore, the energy of such an electron should constantly decrease and the electron should collapse into the nucleus. This would make the atom unstable.
The classical electromagnetic theory also states that the frequency of the electromagnetic waves emitted by an accelerating electron is equal to the frequency of revolution. This would mean that, as the electron spirals inwards, it would emit electromagnetic waves of changing frequencies. In other words, it would emit a continuous spectrum. However, actual observation tells us that the electron emits a line spectrum.
The rate of heat transfer per unit area is called:- a)Temperature gradient
- b)Thermal conductivity
- c)Heat flux
- d)Specific heat
Correct answer is option 'C'. Can you explain this answer?
The rate of heat transfer per unit area is called:
a)
Temperature gradient
b)
Thermal conductivity
c)
Heat flux
d)
Specific heat
|
|
Adebisi Ekpe answered |
The correct answer is option 'C' - heat flux.
Heat transfer is the process by which thermal energy is exchanged between objects or systems due to a temperature difference. The rate at which heat is transferred per unit area is known as the heat flux.
Heat flux is a measure of how much heat is transferred through a given area in a given amount of time. It is expressed in units of watts per square meter (W/m²) or calories per square centimeter per second (cal/cm²/s).
Now let's break down the options and understand why heat flux is the correct answer:
a) Temperature gradient: The temperature gradient refers to the change in temperature per unit length or distance. It represents how temperature changes with respect to position. While temperature gradient is related to heat transfer, it does not directly measure the rate of heat transfer per unit area.
b) Thermal conductivity: Thermal conductivity is a property of a material that describes how well it conducts heat. It is the measure of the ability of a material to conduct heat through it. While thermal conductivity is important in determining the rate of heat transfer, it is not the rate of heat transfer per unit area itself.
c) Heat flux: Heat flux is the rate of heat transfer per unit area. It quantifies the amount of heat energy transferred across a given surface area in a given time. It represents the flow of heat through a surface and is directly related to the temperature difference across the surface and the thermal conductivity of the material.
d) Specific heat: Specific heat is the amount of heat energy required to raise the temperature of a unit mass of a substance by a certain amount. It is an intrinsic property of a substance and is not directly related to the rate of heat transfer per unit area.
In summary, the rate of heat transfer per unit area is called heat flux. It represents the flow of heat through a surface and is determined by the temperature difference and thermal conductivity.
Heat transfer is the process by which thermal energy is exchanged between objects or systems due to a temperature difference. The rate at which heat is transferred per unit area is known as the heat flux.
Heat flux is a measure of how much heat is transferred through a given area in a given amount of time. It is expressed in units of watts per square meter (W/m²) or calories per square centimeter per second (cal/cm²/s).
Now let's break down the options and understand why heat flux is the correct answer:
a) Temperature gradient: The temperature gradient refers to the change in temperature per unit length or distance. It represents how temperature changes with respect to position. While temperature gradient is related to heat transfer, it does not directly measure the rate of heat transfer per unit area.
b) Thermal conductivity: Thermal conductivity is a property of a material that describes how well it conducts heat. It is the measure of the ability of a material to conduct heat through it. While thermal conductivity is important in determining the rate of heat transfer, it is not the rate of heat transfer per unit area itself.
c) Heat flux: Heat flux is the rate of heat transfer per unit area. It quantifies the amount of heat energy transferred across a given surface area in a given time. It represents the flow of heat through a surface and is directly related to the temperature difference across the surface and the thermal conductivity of the material.
d) Specific heat: Specific heat is the amount of heat energy required to raise the temperature of a unit mass of a substance by a certain amount. It is an intrinsic property of a substance and is not directly related to the rate of heat transfer per unit area.
In summary, the rate of heat transfer per unit area is called heat flux. It represents the flow of heat through a surface and is determined by the temperature difference and thermal conductivity.
According to Bohr model radiation takes place when- a)there is transition from one of the stable orbits of definite energy to another of higher energy
- b)there is transition from one of the unstable orbits of definite energy to another of higher energy
- c)there is transition from one of the unstable orbits of definite energy to another of same energy
- d)there is transition from one of the stable orbits of definite energy to another of lower energy. hν = Ef−Ei
Correct answer is option 'D'. Can you explain this answer?
According to Bohr model radiation takes place when
a)
there is transition from one of the stable orbits of definite energy to another of higher energy
b)
there is transition from one of the unstable orbits of definite energy to another of higher energy
c)
there is transition from one of the unstable orbits of definite energy to another of same energy
d)
there is transition from one of the stable orbits of definite energy to another of lower energy. hν = Ef−Ei
|
|
Kalyan Chavan answered |
In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus. To remedy the stability problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump.
hν = Ef−Ei
hν = Ef−Ei
In the ground state of which model electrons are in stable equilibrium with zero net force?- a)Bohr model
- b)No model
- c)Rutherford’s model
- d)Thomson’s model
Correct answer is option 'D'. Can you explain this answer?
In the ground state of which model electrons are in stable equilibrium with zero net force?
a)
Bohr model
b)
No model
c)
Rutherford’s model
d)
Thomson’s model
|
|
Jyoti Kapoor answered |
In Thomson's model, the atom is composed of electrons surrounded by a soup of positive charge to balance the electrons' negative charges, like negatively charged “plums” surrounded by positively charged “pudding”. The 1904 Thomson model was disproved by Hans Geiger's and Ernest Marsden's 1909 gold foil experiment.
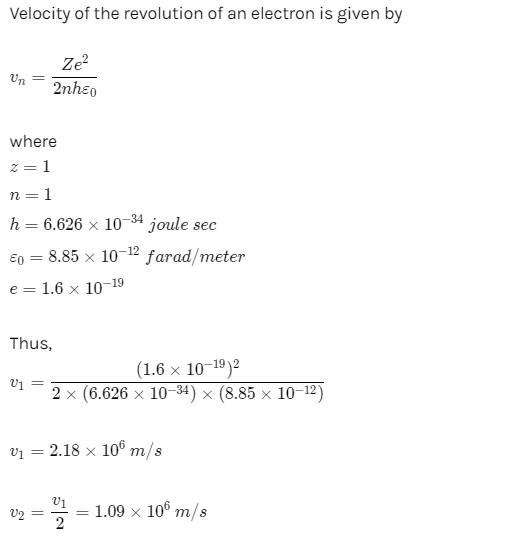
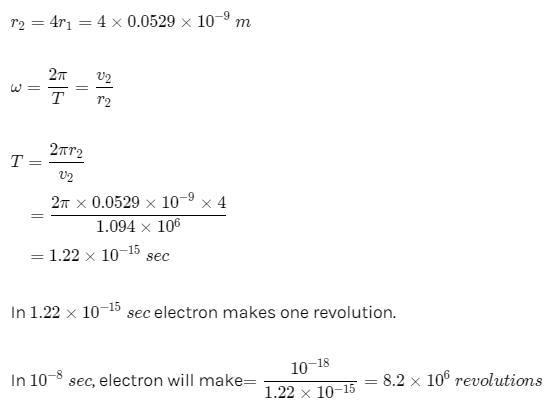
The average lifetime of the first excited level of a hydrogen atom is 1.0 ×10−8 s. In the Bohr model, how many orbits does an electron in the n = 2 level complete before returning to the ground level?- a)9.7 ×106
- b)8.2 ×106
- c)9.2 ×106
- d)8.7 ×106
Correct answer is option 'B'. Can you explain this answer?
The average lifetime of the first excited level of a hydrogen atom is 1.0 ×10−8 s. In the Bohr model, how many orbits does an electron in the n = 2 level complete before returning to the ground level?
a)
9.7 ×106
b)
8.2 ×106
c)
9.2 ×106
d)
8.7 ×106
|
|
Sankar Singh answered |
To produce an emission spectrum of hydrogen- a)It needs to be in glowing gaseous form
- b)It needs to be cold and white light shining through
- c)It needs to be cool liquid form
- d)It needs to be hot and white light shining through
Correct answer is option 'A'. Can you explain this answer?
To produce an emission spectrum of hydrogen
a)
It needs to be in glowing gaseous form
b)
It needs to be cold and white light shining through
c)
It needs to be cool liquid form
d)
It needs to be hot and white light shining through

|
Ishita Reddy answered |
Answer:
To produce an emission spectrum of hydrogen, the hydrogen gas needs to be in a glowing gaseous form. This is because the emission spectrum of an element is produced when the electrons in the atoms of that element are excited to higher energy levels and then fall back to lower energy levels, emitting photons of specific wavelengths in the process.
Glowing Gaseous Form
When hydrogen gas is in a glowing gaseous form, the atoms are excited by an external energy source such as an electric discharge or a flame. This excitation causes the electrons in the hydrogen atoms to move to higher energy levels. As the excited electrons return to their original energy levels, they release energy in the form of photons. The photons emitted have specific wavelengths corresponding to the energy difference between the excited and ground states of the hydrogen atom.
Emission Spectrum
The emitted photons create a spectrum of discrete lines, known as an emission spectrum. These lines are unique to each element and can be used to identify the presence of that element. In the case of hydrogen, the emission spectrum consists of several series of lines, with each series corresponding to a different energy transition within the hydrogen atom.
Other Options
The other options mentioned in the question, such as cold and white light shining through, cool liquid form, and hot and white light shining through, are not suitable for producing an emission spectrum of hydrogen.
- Cold and white light shining through: White light is a combination of all visible wavelengths, and shining it through a cold hydrogen gas would not result in the emission of specific wavelengths characteristic of hydrogen.
- Cool liquid form: When hydrogen is in a cool liquid form, the atoms are not excited, and therefore, no emission spectrum is produced.
- Hot and white light shining through: Similar to the cold and white light scenario, shining white light through hot hydrogen gas would not produce specific wavelengths characteristic of hydrogen.
Conclusion
In conclusion, to produce an emission spectrum of hydrogen, the hydrogen gas must be in a glowing gaseous form. This allows for the excitation of the hydrogen atoms and the subsequent emission of photons with specific wavelengths, creating the characteristic emission spectrum of hydrogen.
Introduction
To produce an emission spectrum of hydrogen, the hydrogen gas needs to be in a glowing gaseous form. This is because the emission spectrum of an element is produced when the electrons in the atoms of that element are excited to higher energy levels and then fall back to lower energy levels, emitting photons of specific wavelengths in the process.
Explanation
Glowing Gaseous Form
When hydrogen gas is in a glowing gaseous form, the atoms are excited by an external energy source such as an electric discharge or a flame. This excitation causes the electrons in the hydrogen atoms to move to higher energy levels. As the excited electrons return to their original energy levels, they release energy in the form of photons. The photons emitted have specific wavelengths corresponding to the energy difference between the excited and ground states of the hydrogen atom.
Emission Spectrum
The emitted photons create a spectrum of discrete lines, known as an emission spectrum. These lines are unique to each element and can be used to identify the presence of that element. In the case of hydrogen, the emission spectrum consists of several series of lines, with each series corresponding to a different energy transition within the hydrogen atom.
Other Options
The other options mentioned in the question, such as cold and white light shining through, cool liquid form, and hot and white light shining through, are not suitable for producing an emission spectrum of hydrogen.
- Cold and white light shining through: White light is a combination of all visible wavelengths, and shining it through a cold hydrogen gas would not result in the emission of specific wavelengths characteristic of hydrogen.
- Cool liquid form: When hydrogen is in a cool liquid form, the atoms are not excited, and therefore, no emission spectrum is produced.
- Hot and white light shining through: Similar to the cold and white light scenario, shining white light through hot hydrogen gas would not produce specific wavelengths characteristic of hydrogen.
Conclusion
In conclusion, to produce an emission spectrum of hydrogen, the hydrogen gas must be in a glowing gaseous form. This allows for the excitation of the hydrogen atoms and the subsequent emission of photons with specific wavelengths, creating the characteristic emission spectrum of hydrogen.
The transfer of heat through electromagnetic waves is called:- a)Conduction
- b)Convection
- c)Radiation
- d)Expansion
Correct answer is option 'C'. Can you explain this answer?
The transfer of heat through electromagnetic waves is called:
a)
Conduction
b)
Convection
c)
Radiation
d)
Expansion
|
|
Deepak Iyer answered |
Radiation is the transfer of heat energy through electromagnetic waves. Unlike conduction and convection, radiation does not require a medium for heat transfer. Examples of radiation include feeling the warmth of the sun on your face or the heat emitted by a fire.
The transfer of heat through a material by direct contact of particles is called:- a)Conduction
- b)Convection
- c)Radiation
- d)Expansion
Correct answer is option 'A'. Can you explain this answer?
The transfer of heat through a material by direct contact of particles is called:
a)
Conduction
b)
Convection
c)
Radiation
d)
Expansion
|
|
Deepak Iyer answered |
Conduction refers to the transfer of heat through a material by direct contact of particles. When heat is applied to one end of a material, the particles at that end gain energy and start vibrating. These vibrating particles collide with neighboring particles, transferring the heat energy from one particle to another.
It is found experimentally that for small thickness t, the number of α-particles scattered at moderate angles is proportional to t. What clue does this linear dependence on t provide?- a)scattering is predominantly due to a single collision
- b)scattering is predominantly due to multiple collisions
- c)scattering is predominantly due to deflecting fields being proportional to t
- d)scattering is predominantly due to no collison
Correct answer is option 'A'. Can you explain this answer?
It is found experimentally that for small thickness t, the number of α-particles scattered at moderate angles is proportional to t. What clue does this linear dependence on t provide?
a)
scattering is predominantly due to a single collision
b)
scattering is predominantly due to multiple collisions
c)
scattering is predominantly due to deflecting fields being proportional to t
d)
scattering is predominantly due to no collison
|
|
Nisha Pillai answered |
Linear Dependence on Thickness t in Particle Scattering
Particle scattering refers to the process of a particle being deflected or redirected from its original trajectory after interacting with another particle or a field. In experiments involving particle scattering, the number of particles scattered at moderate angles is found to be proportional to the thickness t of the scattering material for small t values. This linear dependence on t provides a clue about the nature of the scattering process.
Predominance of Single Collision
The linear dependence of particle scattering on small thickness t suggests that the scattering is predominantly due to a single collision between the incident particle and the scattering material. This means that the incident particle interacts with only one scattering center or atom in the material and is deflected by a small angle. The probability of the incident particle interacting with multiple scattering centers and undergoing multiple collisions is low and does not significantly contribute to the overall scattering.
Explanation of Option A
Option A states that scattering is predominantly due to a single collision. This is consistent with the linear dependence of scattering on small thickness t, as explained above. Therefore, option A is the correct answer.
Conclusion
The linear dependence of particle scattering on small thickness t provides a clue about the nature of the scattering process. In particular, it suggests that scattering is predominantly due to a single collision between the incident particle and the scattering material. This information can be useful in designing experiments and understanding the behavior of particles in various materials.
Particle scattering refers to the process of a particle being deflected or redirected from its original trajectory after interacting with another particle or a field. In experiments involving particle scattering, the number of particles scattered at moderate angles is found to be proportional to the thickness t of the scattering material for small t values. This linear dependence on t provides a clue about the nature of the scattering process.
Predominance of Single Collision
The linear dependence of particle scattering on small thickness t suggests that the scattering is predominantly due to a single collision between the incident particle and the scattering material. This means that the incident particle interacts with only one scattering center or atom in the material and is deflected by a small angle. The probability of the incident particle interacting with multiple scattering centers and undergoing multiple collisions is low and does not significantly contribute to the overall scattering.
Explanation of Option A
Option A states that scattering is predominantly due to a single collision. This is consistent with the linear dependence of scattering on small thickness t, as explained above. Therefore, option A is the correct answer.
Conclusion
The linear dependence of particle scattering on small thickness t provides a clue about the nature of the scattering process. In particular, it suggests that scattering is predominantly due to a single collision between the incident particle and the scattering material. This information can be useful in designing experiments and understanding the behavior of particles in various materials.
The ability of a material to conduct heat is determined by its:- a)Temperature gradient
- b)Thermal conductivity
- c)Heat flux
- d)Specific heat
Correct answer is option 'B'. Can you explain this answer?
The ability of a material to conduct heat is determined by its:
a)
Temperature gradient
b)
Thermal conductivity
c)
Heat flux
d)
Specific heat
|
|
Deepak Iyer answered |
Thermal conductivity is the property of a material that determines its ability to conduct heat. It represents how well a material can transfer heat through conduction. Materials with higher thermal conductivity can transfer heat more efficiently than materials with lower thermal conductivity.
Which material has the lowest thermal conductivity?- a)Copper
- b)Air
- c)Steel
- d)Silver
Correct answer is option 'B'. Can you explain this answer?
Which material has the lowest thermal conductivity?
a)
Copper
b)
Air
c)
Steel
d)
Silver
|
|
Deepak Iyer answered |
Air has a low thermal conductivity compared to copper, steel, and silver. It is considered an insulator when it comes to heat transfer. Air-filled gaps or spaces are often used as insulation materials to reduce heat transfer through conduction or convection.
Which material has the highest thermal conductivity?- a)Wood
- b)Glass
- c)Aluminum
- d)Rubber
Correct answer is option 'C'. Can you explain this answer?
Which material has the highest thermal conductivity?
a)
Wood
b)
Glass
c)
Aluminum
d)
Rubber
|
|
Deepak Iyer answered |
Aluminum has higher thermal conductivity compared to wood, glass, and rubber. It is an excellent conductor of heat and is widely used in various applications where efficient heat transfer is required, such as in heat sinks and cooking utensils.
The temperature difference across a material per unit length is called:- a)Temperature gradient
- b)Thermal conductivity
- c)Heat flux
- d)Specific heat
Correct answer is option 'A'. Can you explain this answer?
The temperature difference across a material per unit length is called:
a)
Temperature gradient
b)
Thermal conductivity
c)
Heat flux
d)
Specific heat
|
|
Deepak Iyer answered |
The temperature gradient is the change in temperature per unit length across a material. It represents the rate at which the temperature changes along a specific direction. A steeper temperature gradient indicates a more rapid change in temperature over a given distance.
The energy radiated or absorbed by a surface depends on its:- a)Mass
- b)Volume
- c)Temperature
- d)Color
Correct answer is option 'C'. Can you explain this answer?
The energy radiated or absorbed by a surface depends on its:
a)
Mass
b)
Volume
c)
Temperature
d)
Color
|
|
Deepak Iyer answered |
The energy radiated or absorbed by a surface depends primarily on its temperature. According to Stefan-Boltzmann's law, the rate of radiation emitted by a surface is proportional to the fourth power of its absolute temperature. The higher the temperature, the greater the energy radiated by the surface.
Chapter doubts & questions for Heat Transfer - Physics for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Heat Transfer - Physics for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Physics for JAMB
259 videos|253 docs|230 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup