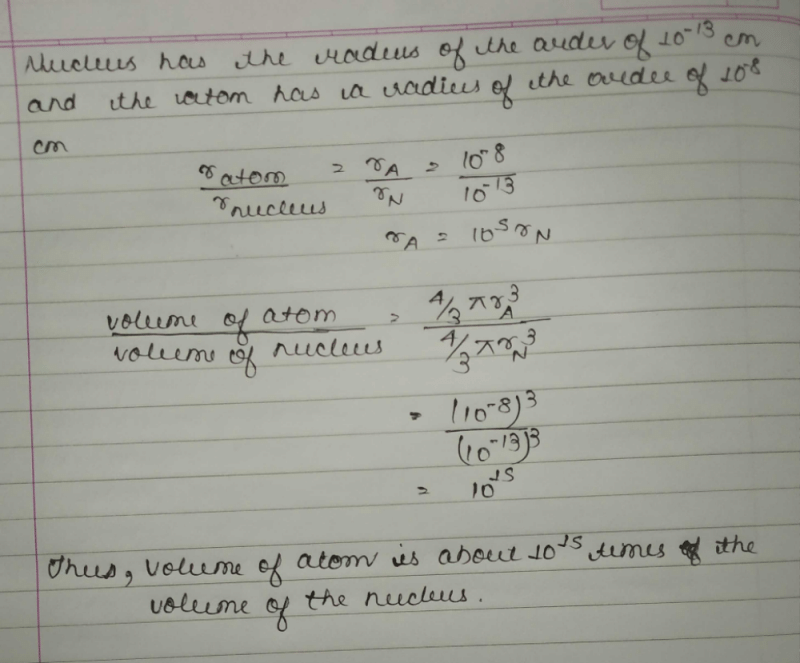All Exams >
Grade 12 >
Physics for Grade 12 >
All Questions
All questions of Nuclear Physics for Grade 12 Exam
Nucleus ”a” contains 5 protons and 5 neutrons and has radius R. The radius of nucleus ”b”, which contains 35 protons and 45 neutrons, is closest to:- a)2R
- b)8R
- c)1.4R
- d)R
Correct answer is option 'A'. Can you explain this answer?
Nucleus ”a” contains 5 protons and 5 neutrons and has radius R. The radius of nucleus ”b”, which contains 35 protons and 45 neutrons, is closest to:
a)
2R
b)
8R
c)
1.4R
d)
R
|
|
Gaurav Kumar answered |
R∝A1/3
A(mass no.)=n+p
R/x=(10/80)1/3
R/x=(13/23)1/3
R/x=1/2
X=2R
A(mass no.)=n+p
R/x=(10/80)1/3
R/x=(13/23)1/3
R/x=1/2
X=2R
Number of alpha particles N scattered at an angle θ during Rutherford’s alpha scattering experiment is :- a)
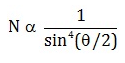
- b)
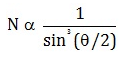
- c)
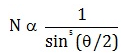
- d)
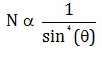
Correct answer is option 'A'. Can you explain this answer?
Number of alpha particles N scattered at an angle θ during Rutherford’s alpha scattering experiment is :
a)
b)
c)
d)

|
Sushil Kumar answered |
Answer :- a
Solution :- For a single scatterer, such as a single gold nucleus within a thin gold foil layer, the differential scattering cross section is defined as follows [2]:
dσ(θ, φ)/dΩ = flux scattered into element dΩ at angles (θ, φ)/incident flux per unit area
dσ/dΩ = (Iθ × A)/ (dΩ × I0 × NAvo × ρ × x(foil))........... (1)
where NAvo is Avogadro’s number, xfoil is the thickness of the target foil, A is the atomic mass of the material in the target foil, dΩ is the solid-angle of the detector, I0 is the unattenuated intensity of the alpha particle beam.
dσ/dΩ = [(ZZ0 e^2/4E )^2]/(1/sin^4(θ/2 ))
dσ/dΩ is directly proportional to 1/sin^4(θ/2 )
Select an incorrect alternative:
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
- a)Statement i
- b)Statement iv
- c)Statement ii
- d)Statement iii
Correct answer is option 'C'. Can you explain this answer?
Select an incorrect alternative:
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
a)
Statement i
b)
Statement iv
c)
Statement ii
d)
Statement iii
|
|
Hansa Sharma answered |
Statement i. Radius of Bohr's orbit of hydrogen atom is given by
r= n2h2/4π2mKze2
or, r=(0.59A˚)(n2/z)
So, from expression we found r∝n2
Hence the 1st statement is correct.
Statement ii.
We know that
En=-13.6 x z2/n2
So, En ∝1/n2
Hence the 2nd statement is wrong.
Statement iii.Bohr defined these stable orbits in his second postulate. According to this postulate:
r= n2h2/4π2mKze2
or, r=(0.59A˚)(n2/z)
So, from expression we found r∝n2
Hence the 1st statement is correct.
Statement ii.
We know that
En=-13.6 x z2/n2
So, En ∝1/n2
Hence the 2nd statement is wrong.
Statement iii.Bohr defined these stable orbits in his second postulate. According to this postulate:
- An electron revolves around the nucleus in orbits
- The angular momentum of revolution is an integral multiple of h/2π – where Planck’s constant [h = 6.6 x 10-34 J-s].
- Hence, the angular momentum (L) of the orbiting electron is: L = nh/2 π
Hence the 3rd statement is correct.
Statement iv.According to Bohr's theory
Angular momentum of electron in an orbit will be Integral multiple of (h/2π)
Magnitude of potential energy is twice of kinetic energy of electron in an orbit
∣P.E∣=2∣K.E∣
K.E=(13.6ev)( z2/n2)
Hence, The 4th statement is correct.
Statement iv.According to Bohr's theory
Angular momentum of electron in an orbit will be Integral multiple of (h/2π)
Magnitude of potential energy is twice of kinetic energy of electron in an orbit
∣P.E∣=2∣K.E∣
K.E=(13.6ev)( z2/n2)
Hence, The 4th statement is correct.
A sample of radioactive material contains 1018 atoms. The half life of the material is 2 days, then the activity of the sample is- a)3.5 x 1014 Bq
- b)3.5 x 1012 Bq
- c)7 x 1011 Bq
- d)7 x 1016 Bq
Correct answer is option 'B'. Can you explain this answer?
A sample of radioactive material contains 1018 atoms. The half life of the material is 2 days, then the activity of the sample is
a)
3.5 x 1014 Bq
b)
3.5 x 1012 Bq
c)
7 x 1011 Bq
d)
7 x 1016 Bq
|
|
Jyoti Sengupta answered |
To find activity of the sample --->which is the rate of disintegration.
Since radioactivity comes under 1o kinetics.
[R]=k[A] [A]-->amount of initial sample 1018 atoms
Given,
Half-life=2days
K=0.693/2x24x60x60 sec
R=(0.693/2x24x60x60)x1018
R≈3.5x1012 Bq
Rutherford’s experiments on scattering of alpha particles proved that:- a)atoms contain electrons
- b)number of positive charges is equal to the number of negative charges
- c)atom is mostly empty
- d)positive charge is uniformly distributed in the atom
Correct answer is option 'C'. Can you explain this answer?
Rutherford’s experiments on scattering of alpha particles proved that:
a)
atoms contain electrons
b)
number of positive charges is equal to the number of negative charges
c)
atom is mostly empty
d)
positive charge is uniformly distributed in the atom

|
Divey Sethi answered |
Most of the α-particle passed through the foil straight without suffering any change in their direction. This shows that most of the space inside the atom is empty or hollow.
A small fraction of α-particles was deflected through small angles and a few through larger angles. For this to happen α- particles (positively charged) must approach a heavy positively charged core inside the atom (like charges repel each other). This heavy positively charged core inside the atom was named as the nucleus.
A small fraction of α-particles was deflected through small angles and a few through larger angles. For this to happen α- particles (positively charged) must approach a heavy positively charged core inside the atom (like charges repel each other). This heavy positively charged core inside the atom was named as the nucleus.
A radioactive material decays by simultaneous emission of two particles with respective half lives 1620 and 810 years. The time in years, after which one fourth of the material remains is- a)4860
- b)2340
- c)1080.0
- d)3240
Correct answer is option 'C'. Can you explain this answer?
A radioactive material decays by simultaneous emission of two particles with respective half lives 1620 and 810 years. The time in years, after which one fourth of the material remains is
a)
4860
b)
2340
c)
1080.0
d)
3240
|
|
Nikita Singh answered |
Since, from Rutherford-Soddy law, the number of atoms left after half-lives is given by
N=N0(1/2)n
where, N0 is the original number of atoms.
The number of half-lives, n= time of decay/effective half−life
Relation between effective disintegration constant (λ) and half-life (T)
λ=ln2/T
∴λ1+λ2= (ln2/ T1)+ (ln2/ T2)
Effective half-life,
1/T=1/T1+1/T2=(1/1620)+(1/810)
1/T=1+2/1620 ⇒T=540yr
∴n=T/540
∴N=N0(1/2)t/540⇒N/N0=(1/2)2=(1/2)t/540
⇒t/540=2⇒t=2×540=1080yr
N=N0(1/2)n
where, N0 is the original number of atoms.
The number of half-lives, n= time of decay/effective half−life
Relation between effective disintegration constant (λ) and half-life (T)
λ=ln2/T
∴λ1+λ2= (ln2/ T1)+ (ln2/ T2)
Effective half-life,
1/T=1/T1+1/T2=(1/1620)+(1/810)
1/T=1+2/1620 ⇒T=540yr
∴n=T/540
∴N=N0(1/2)t/540⇒N/N0=(1/2)2=(1/2)t/540
⇒t/540=2⇒t=2×540=1080yr
Cadmium rods are used in a nuclear reactor for- a)absorbing neutrons
- b)speeding up slow neutrons
- c)regulating the power level of the reactor.
- d)slowing down fast neutrons
Correct answer is option 'A'. Can you explain this answer?
Cadmium rods are used in a nuclear reactor for
a)
absorbing neutrons
b)
speeding up slow neutrons
c)
regulating the power level of the reactor.
d)
slowing down fast neutrons

|
Rohini Bharti answered |
Cadium rod is used to control the fission rate of uranium
The number of electrons in an atom X of atomic number Z and mass number A is- a)Zero
- b)A
- c)Z
- d)A-Z
Correct answer is option 'C'. Can you explain this answer?
The number of electrons in an atom X of atomic number Z and mass number A is
a)
Zero
b)
A
c)
Z
d)
A-Z

|
Sushil Kumar answered |
No of neutrons are given by: (A−Z)
Given an atomic number (Z) and mass number (A), you can find the number of protons, neutrons, and electrons in a neutral atom. For example, a lithium atom (Z=3,A=7 amu) contains three protons (found from Z), three electrons (as the number of protons is equal to the number of electrons in an atom), and four neutrons (7–3=4).
Given an atomic number (Z) and mass number (A), you can find the number of protons, neutrons, and electrons in a neutral atom. For example, a lithium atom (Z=3,A=7 amu) contains three protons (found from Z), three electrons (as the number of protons is equal to the number of electrons in an atom), and four neutrons (7–3=4).
In hydrogen atom the kinetic energy of electron in an orbit of radius r is given by- a)
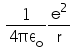
- b)
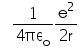
- c)
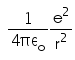
- d)
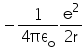
Correct answer is option 'B'. Can you explain this answer?
In hydrogen atom the kinetic energy of electron in an orbit of radius r is given by
a)
b)
c)
d)

|
Sushil Kumar answered |
K.E. of nth orbit
=> (1/k) Ze2/2r
For H atom,
K.E.=(1/4πε) x (e2/2r)
=> (1/k) Ze2/2r
For H atom,
K.E.=(1/4πε) x (e2/2r)
All nuclides with same mass number A are called- a)isobars
- b)isoclines
- c)isotones
- d)isotopes
Correct answer is option 'A'. Can you explain this answer?
All nuclides with same mass number A are called
a)
isobars
b)
isoclines
c)
isotones
d)
isotopes
|
|
Rocky Handsome answered |
Isobars are atoms of different elements with the same mass number but different atomic numbers.
• Isotones are atomic nuclei with the same number of neutrons (N) and different number of protons(Z)
• Isotones are atomic nuclei with the same number of neutrons (N) and different number of protons(Z)
90% of a radioactive sample is left undisintegrated after time τ has elapsed, what percentage of initial sample will decay in a total time2τ?- a)9%
- b)38%
- c)19%
- d)62%
Correct answer is option 'C'. Can you explain this answer?
90% of a radioactive sample is left undisintegrated after time τ has elapsed, what percentage of initial sample will decay in a total time2τ?
a)
9%
b)
38%
c)
19%
d)
62%
|
|
Krishna Iyer answered |
Given that 90% is left un-decayed after time 't'.
Hence, 10% decays in time 't'.
Initially assume that the amount of substance is 'x'
After time 't' 10% is decayed.
i.e. Amount of substance left =0.9x
After further time 't' another 10% is decayed.
i.e. 0.1×0.9x is decayed
Leaving behind 0.81x.
Hence after time 2t we see that 0.19x has decayed, which is 19%.
Hence, 10% decays in time 't'.
Initially assume that the amount of substance is 'x'
After time 't' 10% is decayed.
i.e. Amount of substance left =0.9x
After further time 't' another 10% is decayed.
i.e. 0.1×0.9x is decayed
Leaving behind 0.81x.
Hence after time 2t we see that 0.19x has decayed, which is 19%.
In Rutherford’s experiment, a thin gold foil was bombarded with alpha particles. According to Thomson’s “plum-pudding” model of the atom, what should have happened?- a)All the alpha particles would have been deflected by the foil.
- b)All the alpha particles should have bounced straight back from the foil.
- c)Alpha particles should have passed through the foil with little or no deflection.
- d)Alpha particles should have become embedded in the foil.
Correct answer is option 'C'. Can you explain this answer?
In Rutherford’s experiment, a thin gold foil was bombarded with alpha particles. According to Thomson’s “plum-pudding” model of the atom, what should have happened?
a)
All the alpha particles would have been deflected by the foil.
b)
All the alpha particles should have bounced straight back from the foil.
c)
Alpha particles should have passed through the foil with little or no deflection.
d)
Alpha particles should have become embedded in the foil.

|
Snehal Gosavi answered |
Correct Option C ===>ΔΔΔ
ΔAlfa particles are massive particles and they have speed when they bombarded....
ΔAccording plum pudding model if atom given by Thomson protons and electrons are equally distributed....
Δthat's why massive alpha particles will pass through the foil with little deflection (due to protons)......
ΔBcoz here protons are like spreaded cloud and not in nucleus ( massive part or atom) ....
Δand alpha particle is more massive than single proton....so they will not deflect due to protons and will pass through foil....
The nuclide 92U238 has all the following except- a)92 protons
- b)146 neutrons
- c)238 nucleons
- d)92 neutrons
Correct answer is option 'D'. Can you explain this answer?
The nuclide 92U238 has all the following except
a)
92 protons
b)
146 neutrons
c)
238 nucleons
d)
92 neutrons

|
Sushil Kumar answered |
The nuclide (nucleus) consists of neutrons and protons (when combined called nucleons).
Thus,
No. of protons in 92U238 = 92,
No. of neutrons = 146 (238 – 92)
No. of nucleons = 238 (146 + 92)
Thus,
No. of protons in 92U238 = 92,
No. of neutrons = 146 (238 – 92)
No. of nucleons = 238 (146 + 92)
We know that the Rutherford model of the atom is superior to the Thompson model because when alpha particles are scattered from atoms:- a)the deflected angle is always large
- b)they are usually observed with kinetic energy between 5 and 10 MeV
- c)some alpha particles are deflected to large angles
- d)the deflected angle is usually small
Correct answer is option 'C'. Can you explain this answer?
We know that the Rutherford model of the atom is superior to the Thompson model because when alpha particles are scattered from atoms:
a)
the deflected angle is always large
b)
they are usually observed with kinetic energy between 5 and 10 MeV
c)
some alpha particles are deflected to large angles
d)
the deflected angle is usually small
|
|
Tanuja Kapoor answered |
In ruther ford experiment he suggest that all the positive charge and mass are concentrated at the centre when he bombarded the alpha partical which is dipositive in nature and when it is more close to centre it get deflect to a large angle and with increase of closenes to centre its deflection angle increase and some alpha partical deflect to 180 degree so it prove that all the positive charge and mass are concentrated at the centre where as acccording to thomson atom is hard solid sphere in which its total +ve charge and mass uniformalyy distributed on the surface and electrone reside as seed in watermelon ( plum pudding model)
α-rays are- a)helium nuclei
- b)heavy nuclei
- c)lithium nuclei
- d)hydrogen nuclei
Correct answer is option 'A'. Can you explain this answer?
α-rays are
a)
helium nuclei
b)
heavy nuclei
c)
lithium nuclei
d)
hydrogen nuclei
|
|
Ræjû Bhæï answered |
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways.
Fluorescent lamps are more efficient than incandescent lamps in converting electrical energy to visible light because- a)they produce more white light
- b)they do not use uv radiations
- c)they do not waste as much energy producing (invisible) infrared photons
- d)they do not waste as much energy producing visible photons
Correct answer is option 'C'. Can you explain this answer?
Fluorescent lamps are more efficient than incandescent lamps in converting electrical energy to visible light because
a)
they produce more white light
b)
they do not use uv radiations
c)
they do not waste as much energy producing (invisible) infrared photons
d)
they do not waste as much energy producing visible photons
|
|
Riya Banerjee answered |
The phosphor fluoresces to produce light. A fluorescent bulb produces less heat, so it is much more efficient. This makes fluorescent bulbs four to six times more efficient than incandescent bulbs. That's why you can buy a 15-watt fluorescent bulb that produces the same amount of light as a 60-watt incandescent bulb.
At a given time there are 25% undecayed nuclei in a sample. After 10 seconds number of undecayed nuclei reduces to 12.5%. Then mean life of the nuclei will be about- a)22 sec
- b)10 sec
- c)12 sec
- d)15 sec
Correct answer is option 'D'. Can you explain this answer?
At a given time there are 25% undecayed nuclei in a sample. After 10 seconds number of undecayed nuclei reduces to 12.5%. Then mean life of the nuclei will be about
a)
22 sec
b)
10 sec
c)
12 sec
d)
15 sec
|
|
Lavanya Menon answered |
Half-life of radioactive sample, i.e., the time in which the number of undecayed nuclei becomes half (T) is 10 s.
Mean life, τ=T/loge2=10s/0.693=1.443×10=14.43s ≈ 15s
Mean life, τ=T/loge2=10s/0.693=1.443×10=14.43s ≈ 15s
The half life of radon is 3.8 days. After how many days will only one twentieth of a radon sample be left over?
a)10.00 daysb)5.45 daysc)15.45 daysd)16.45 daysCorrect answer is option 'D'. Can you explain this answer?
|
|
Suresh Iyer answered |
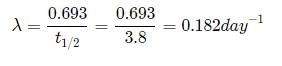 Let the initial amount of radon be N0 and the amount left after t days be N which is equal to N0/2
Let the initial amount of radon be N0 and the amount left after t days be N which is equal to N0/2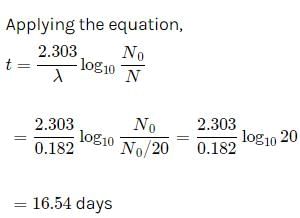
Which of these is true?- a)The alpha particles used in Rutherford’s experiment are positively charged particles
- b)Dalton assumed that atoms are made up of electrons, protons, and neutrons
- c)In Rutherford’s alpha scattering experiment, all of the alpha particles passed through the gold foil.
- d)JJ Thomson determined the charge and mass of electrons
Correct answer is 'A'. Can you explain this answer?
Which of these is true?
a)
The alpha particles used in Rutherford’s experiment are positively charged particles
b)
Dalton assumed that atoms are made up of electrons, protons, and neutrons
c)
In Rutherford’s alpha scattering experiment, all of the alpha particles passed through the gold foil.
d)
JJ Thomson determined the charge and mass of electrons
|
|
Shalini Basu answered |
**Explanation:**
The correct answer is **a) The alpha particles used in Rutherford's experiment are positively charged particles**.
Rutherford's experiment, also known as the gold foil experiment, was conducted in 1909 by Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden. The experiment aimed to understand the structure of the atom and investigate the distribution of positive charge within it.
In this experiment, Rutherford and his team bombarded a thin gold foil with a beam of alpha particles. Alpha particles are positively charged particles that consist of two protons and two neutrons, which are the same as helium nuclei. They are emitted from a radioactive source, such as radium or polonium.
Rutherford observed that while most of the alpha particles passed straight through the gold foil, some of them were deflected at different angles, and a very small fraction bounced back in the direction opposite to the source. This unexpected result led to the discovery of the atomic nucleus and the concept of a mostly empty space within the atom.
Based on the observations from the experiment, Rutherford proposed a new atomic model known as the nuclear model. According to this model, atoms have a dense, positively charged nucleus at the center, which contains most of the atom's mass. The electrons, which are negatively charged particles, orbit around the nucleus in specific energy levels.
Therefore, the correct answer is a) The alpha particles used in Rutherford's experiment are positively charged particles.
The correct answer is **a) The alpha particles used in Rutherford's experiment are positively charged particles**.
Rutherford's experiment, also known as the gold foil experiment, was conducted in 1909 by Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden. The experiment aimed to understand the structure of the atom and investigate the distribution of positive charge within it.
In this experiment, Rutherford and his team bombarded a thin gold foil with a beam of alpha particles. Alpha particles are positively charged particles that consist of two protons and two neutrons, which are the same as helium nuclei. They are emitted from a radioactive source, such as radium or polonium.
Rutherford observed that while most of the alpha particles passed straight through the gold foil, some of them were deflected at different angles, and a very small fraction bounced back in the direction opposite to the source. This unexpected result led to the discovery of the atomic nucleus and the concept of a mostly empty space within the atom.
Based on the observations from the experiment, Rutherford proposed a new atomic model known as the nuclear model. According to this model, atoms have a dense, positively charged nucleus at the center, which contains most of the atom's mass. The electrons, which are negatively charged particles, orbit around the nucleus in specific energy levels.
Therefore, the correct answer is a) The alpha particles used in Rutherford's experiment are positively charged particles.
In the following reaction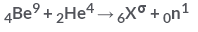 What is following value of a?
What is following value of a?- a)14
- b)10
- c)16
- d)12
Correct answer is option 'D'. Can you explain this answer?
In the following reaction
What is following value of a?
a)
14
b)
10
c)
16
d)
12
|
|
Tanuja Kapoor answered |
The sum of the atomic no. and atomic mass no. on the reactant and product should be equal .
therefore 9+4 = a+1
a = 12
therefore 9+4 = a+1
a = 12
Emission line spectra of different elements- a)is not different
- b)is the same if both elements are at the same temperature
- c)is the same if both elements are in liquid form
- d)is different
Correct answer is option 'D'. Can you explain this answer?
Emission line spectra of different elements
a)
is not different
b)
is the same if both elements are at the same temperature
c)
is the same if both elements are in liquid form
d)
is different

|
Adithya Shasan answered |
Option D : Emission line spectra of different elements is different..
What is the main source of energy of the sun?- a)Nuclear fission of heavier unstable elements in the sun
- b)Combustion of pure carbon present in the sun
- c)Gravitational energy liberated during the slow contraction of the sun.
- d)Nuclear fusion of lighter elements in the sun.
Correct answer is option 'D'. Can you explain this answer?
What is the main source of energy of the sun?
a)
Nuclear fission of heavier unstable elements in the sun
b)
Combustion of pure carbon present in the sun
c)
Gravitational energy liberated during the slow contraction of the sun.
d)
Nuclear fusion of lighter elements in the sun.

|
Kanika S answered |
Option d
When H and He in sun undergoes fusion large amount of heat is released.
When H and He in sun undergoes fusion large amount of heat is released.
The radius of a nucleus is directly proportional to (A=mass number)- a)A1/3
- b)A2
- c)A3
- d)A1/2
Correct answer is option 'A'. Can you explain this answer?
The radius of a nucleus is directly proportional to (A=mass number)
a)
A1/3
b)
A2
c)
A3
d)
A1/2
|
|
Riya Banerjee answered |
For A nucleons
R=RoA1/3 [Ro=constant]
So, R∝A1/3
R=RoA1/3 [Ro=constant]
So, R∝A1/3
The atomic number Z of the nucleus is- a)Number of deutrons.
- b)Number of neutrons in it.
- c)Number if electrons in it.
- d)Number of protons in it.
Correct answer is option 'D'. Can you explain this answer?
The atomic number Z of the nucleus is
a)
Number of deutrons.
b)
Number of neutrons in it.
c)
Number if electrons in it.
d)
Number of protons in it.
|
|
Shraddha Dey answered |
**Explanation:**
The atomic number (Z) of an atom refers to the number of protons in the nucleus. Here, we will discuss why the correct answer is option 'D' and explain the significance of atomic number in an atom.
**Atomic Number (Z):**
The atomic number of an atom is a fundamental property that determines its identity and place in the periodic table. It is denoted by the symbol 'Z'. Each element on the periodic table has a unique atomic number.
**Protons in the Nucleus:**
Protons are subatomic particles that carry a positive charge. They are located in the nucleus of an atom, which is the central core of the atom. The number of protons in the nucleus is equal to the atomic number of the atom.
**Electrons in the Atom:**
Electrons are subatomic particles that carry a negative charge. They orbit around the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons, ensuring that the atom has a balanced charge overall.
**Neutrons in the Nucleus:**
Neutrons are subatomic particles that have no charge (they are electrically neutral). They are also located in the nucleus along with protons. The number of neutrons in an atom can vary, resulting in different isotopes of the same element. Isotopes have the same atomic number (same number of protons) but different mass numbers (different number of neutrons).
**Significance of Atomic Number:**
The atomic number is a crucial characteristic of an atom because it determines the element's identity. Elements are organized in increasing order of their atomic numbers on the periodic table. For example, hydrogen has an atomic number of 1, helium has an atomic number of 2, and so on.
The atomic number defines the unique properties and behavior of an element. It determines the number of electrons in the atom, which influences the atom's chemical reactivity and bonding. It also provides information about the element's position in the periodic table, its atomic mass, and its isotopes.
Therefore, the correct answer to the given question is option 'D' – the atomic number (Z) of the nucleus represents the number of protons in it.
The atomic number (Z) of an atom refers to the number of protons in the nucleus. Here, we will discuss why the correct answer is option 'D' and explain the significance of atomic number in an atom.
**Atomic Number (Z):**
The atomic number of an atom is a fundamental property that determines its identity and place in the periodic table. It is denoted by the symbol 'Z'. Each element on the periodic table has a unique atomic number.
**Protons in the Nucleus:**
Protons are subatomic particles that carry a positive charge. They are located in the nucleus of an atom, which is the central core of the atom. The number of protons in the nucleus is equal to the atomic number of the atom.
**Electrons in the Atom:**
Electrons are subatomic particles that carry a negative charge. They orbit around the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons, ensuring that the atom has a balanced charge overall.
**Neutrons in the Nucleus:**
Neutrons are subatomic particles that have no charge (they are electrically neutral). They are also located in the nucleus along with protons. The number of neutrons in an atom can vary, resulting in different isotopes of the same element. Isotopes have the same atomic number (same number of protons) but different mass numbers (different number of neutrons).
**Significance of Atomic Number:**
The atomic number is a crucial characteristic of an atom because it determines the element's identity. Elements are organized in increasing order of their atomic numbers on the periodic table. For example, hydrogen has an atomic number of 1, helium has an atomic number of 2, and so on.
The atomic number defines the unique properties and behavior of an element. It determines the number of electrons in the atom, which influences the atom's chemical reactivity and bonding. It also provides information about the element's position in the periodic table, its atomic mass, and its isotopes.
Therefore, the correct answer to the given question is option 'D' – the atomic number (Z) of the nucleus represents the number of protons in it.
The distance of closest approach when a 15.0 MeV proton approaches gold nucleus (Z = 79) is- a)758 fm
- b)7.58 fm
- c)75.8 fm
- d)0.758 fm
Correct answer is option 'B'. Can you explain this answer?
The distance of closest approach when a 15.0 MeV proton approaches gold nucleus (Z = 79) is
a)
758 fm
b)
7.58 fm
c)
75.8 fm
d)
0.758 fm
|
|
Riya Banerjee answered |
Correct Answer :- b
Explanation : E = 15.0MeV
= 15 * 106 eV
= 15 * 106 * 1.6 * 10-19 J
= 15 * 1.6 * 10-13 J
E = (1/4πεo)*(ze2/r02)
r0 = (1/4πεo)*(ze2/E)
r0 = (9*109*79*(1.6*10-19)2)/(15*1.6*10-13)
= 75.84 * 10-16 m
= 7.58 fm
In hydrogen atom the angular momentum of the electron in the lowest energy state is- a)2h
- b)h/2π
- c)2π/h
- d)h/π
Correct answer is option 'B'. Can you explain this answer?
In hydrogen atom the angular momentum of the electron in the lowest energy state is
a)
2h
b)
h/2π
c)
2π/h
d)
h/π
|
|
Kiran Khanna answered |
C)h/π
d)h
The correct answer is d) h.
The angular momentum of an electron in the hydrogen atom is given by the formula L = nħ, where n is the principal quantum number and ħ is the reduced Planck constant.
In the lowest energy state, the electron is in the ground state with n = 1. Therefore, the angular momentum is L = 1ħ = h.
d)h
The correct answer is d) h.
The angular momentum of an electron in the hydrogen atom is given by the formula L = nħ, where n is the principal quantum number and ħ is the reduced Planck constant.
In the lowest energy state, the electron is in the ground state with n = 1. Therefore, the angular momentum is L = 1ħ = h.
The average number of neutrons released by the fission of one uranium atom is
a)3.0b)2c)2.5d)1Correct answer is option 'C'. Can you explain this answer?
|
|
Bhanu Saini answered |
Fission result in the production of typically 2 or 3 neutron so on the average about 2.5 neutron released per unit. so correct answer is option a
for option c one uranium atom split into one barium and one krypton atom releasing 3 neutron.
but in this question average is asking so according to me and books 2.5 is correct
for option c one uranium atom split into one barium and one krypton atom releasing 3 neutron.
but in this question average is asking so according to me and books 2.5 is correct
Nuclear mass M is found to be- a)always greater than total mass of its individual protons and neutrons
- b)always equal to the total mass of its individual neutrons
- c)always equal to the total mass of its individual protons and neutrons
- d)always less than total mass of its individual protons and neutrons
Correct answer is option 'D'. Can you explain this answer?
Nuclear mass M is found to be
a)
always greater than total mass of its individual protons and neutrons
b)
always equal to the total mass of its individual neutrons
c)
always equal to the total mass of its individual protons and neutrons
d)
always less than total mass of its individual protons and neutrons
|
|
Ritu Singh answered |
The actual mass is always less than the sum of the individual masses of the constituent protons and neutrons because energy is removed when the nucleus is formed. This energy has mass, which is removed from the total mass of the original particles.
What percentage of the mass of an atom is concentrated in the nucleus?- a)79.9%
- b)99.9%
- c)66.9%
- d)50.9%
Correct answer is option 'B'. Can you explain this answer?
What percentage of the mass of an atom is concentrated in the nucleus?
a)
79.9%
b)
99.9%
c)
66.9%
d)
50.9%
|
|
Jyoti Kapoor answered |
More than 99.99% of the mass of any atom is concentrated in its nucleus. If the mass of protons and neutrons (which are in the nucleus of every atom) is approximately one (1) atomic mass unit, then the relative mass of an electron is 0.0005 atomic mass units.
In Geiger-Marsden experiment very small deflection of the beam was expected because- a)there are no electrical forces at work
- b)positive charge and the negative electrons are distributed through the whole atom reducing electric field inside the atom
- c)particles are collimated by lead screens
- d)most particles pass through
Correct answer is option 'B'. Can you explain this answer?
In Geiger-Marsden experiment very small deflection of the beam was expected because
a)
there are no electrical forces at work
b)
positive charge and the negative electrons are distributed through the whole atom reducing electric field inside the atom
c)
particles are collimated by lead screens
d)
most particles pass through
|
|
Shraddha Choudhury answered |
Explanation:
The Geiger-Marsden experiment was conducted to study the structure of an atom. In this experiment, a beam of alpha particles was directed towards a thin gold foil. The alpha particles were expected to pass through the gold foil with little or no deflection, as it was believed that the positive charge and the negative electrons in an atom are distributed uniformly, reducing the electric field inside the atom. However, the results of the experiment were surprising, as some of the alpha particles were deflected at large angles, and some even bounced back.
Reasons for very small deflection of the beam:
- Electrical forces: According to Coulomb's law, any two charged particles exert a force on each other. In an atom, the positively charged nucleus and the negatively charged electrons are attracted to each other by electrical forces. However, the electrons are in constant motion, creating a cloud of negative charge around the nucleus. This cloud of negative charge reduces the electric field inside the atom, making it difficult for the alpha particles to be deflected.
- Distribution of charge: The positive charge in an atom is concentrated in the nucleus, while the negative charge is distributed throughout the atom. This distribution of charge makes the electric field inside the atom more uniform, reducing the chances of the alpha particles being deflected.
- Collimation of particles: The alpha particles were collimated by lead screens before they were directed towards the gold foil. This was done to ensure that the particles were traveling in a straight line and were not scattered by other particles or objects in the environment.
- Most particles pass through: Despite the above factors, it was still expected that some of the alpha particles would be deflected at small angles due to the random nature of the collisions between the particles and the atoms in the gold foil. However, it was not expected that some of the particles would be deflected at large angles or bounce back, as this implied that the positive charge in an atom was not uniformly distributed.
Conclusion:
In conclusion, the very small deflection of the beam was expected in the Geiger-Marsden experiment due to the distribution of charge in an atom and the reduction of electric field inside the atom. However, the unexpected results of the experiment led to the discovery of the nucleus and the development of the modern atomic model.
The Geiger-Marsden experiment was conducted to study the structure of an atom. In this experiment, a beam of alpha particles was directed towards a thin gold foil. The alpha particles were expected to pass through the gold foil with little or no deflection, as it was believed that the positive charge and the negative electrons in an atom are distributed uniformly, reducing the electric field inside the atom. However, the results of the experiment were surprising, as some of the alpha particles were deflected at large angles, and some even bounced back.
Reasons for very small deflection of the beam:
- Electrical forces: According to Coulomb's law, any two charged particles exert a force on each other. In an atom, the positively charged nucleus and the negatively charged electrons are attracted to each other by electrical forces. However, the electrons are in constant motion, creating a cloud of negative charge around the nucleus. This cloud of negative charge reduces the electric field inside the atom, making it difficult for the alpha particles to be deflected.
- Distribution of charge: The positive charge in an atom is concentrated in the nucleus, while the negative charge is distributed throughout the atom. This distribution of charge makes the electric field inside the atom more uniform, reducing the chances of the alpha particles being deflected.
- Collimation of particles: The alpha particles were collimated by lead screens before they were directed towards the gold foil. This was done to ensure that the particles were traveling in a straight line and were not scattered by other particles or objects in the environment.
- Most particles pass through: Despite the above factors, it was still expected that some of the alpha particles would be deflected at small angles due to the random nature of the collisions between the particles and the atoms in the gold foil. However, it was not expected that some of the particles would be deflected at large angles or bounce back, as this implied that the positive charge in an atom was not uniformly distributed.
Conclusion:
In conclusion, the very small deflection of the beam was expected in the Geiger-Marsden experiment due to the distribution of charge in an atom and the reduction of electric field inside the atom. However, the unexpected results of the experiment led to the discovery of the nucleus and the development of the modern atomic model.
What amount of energy is released in the fission of 95U235 ?- a)200 keV
- b)20 eV
- c)200 eV
- d)200 MeV
Correct answer is option 'D'. Can you explain this answer?
What amount of energy is released in the fission of 95U235 ?
a)
200 keV
b)
20 eV
c)
200 eV
d)
200 MeV

|
Divey Sethi answered |
The fission process represented by the equation, 92U235+0n1→56Ba144+36Kr89+30n1
Masses of reactants =234.39+1.01=235.4amu
Masses of products =143.28+88.89+3(1.01) =235.2amu
Energy released = mass difference =235.4−235.2=0.2amu=0.2×931∼200MeV
Masses of reactants =234.39+1.01=235.4amu
Masses of products =143.28+88.89+3(1.01) =235.2amu
Energy released = mass difference =235.4−235.2=0.2amu=0.2×931∼200MeV
Plutonium decays with a half-life of 24000 years. If the plutonium is stored for 72000 years, then the fraction of plutonium that remains is - a)1 /3
- b)1 /2
- c)1/8
- d)1 /4
Correct answer is option 'C'. Can you explain this answer?
Plutonium decays with a half-life of 24000 years. If the plutonium is stored for 72000 years, then the fraction of plutonium that remains is
a)
1 /3
b)
1 /2
c)
1/8
d)
1 /4
|
|
Mira Sharma answered |
The amount of plotinium after a time period of 72000 if the half life is 24000 will be
the initial amount x would be reduced to x/2 , in 24000 yrs
then it would lessen to x/4 in the next 24000yrs
and then to x/8 in the next 24000 yrs
that is it will reduce to x/8 in the next 72000yrs starting from x .
In the mass number range A = 30 to 170, the binding energy per nucleon is- a)decreases with increasing A
- b)increases linearly with A
- c)decreases linearly with A
- d)nearly constant
Correct answer is option 'D'. Can you explain this answer?
In the mass number range A = 30 to 170, the binding energy per nucleon is
a)
decreases with increasing A
b)
increases linearly with A
c)
decreases linearly with A
d)
nearly constant
|
|
Shraddha Choudhury answered |
Binding energy per nucleon in the mass number range A = 30 to 170
The binding energy per nucleon is the energy required to separate a nucleus into its constituent nucleons. It is a measure of the stability of the nucleus, and it depends on the mass number of the nucleus. In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
Explanation:
The binding energy per nucleon is given by the formula:
BE/A = (ZmH + NmN - M)/A
where BE is the binding energy, Z is the atomic number, N is the number of neutrons, mH is the mass of a hydrogen atom, mN is the mass of a neutron, and M is the mass of the nucleus.
In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant because the nuclear force between nucleons is nearly constant. This means that the energy required to separate a nucleon from the nucleus is nearly constant in this range.
The nuclear force between nucleons is a strong force that holds the nucleus together. It is a short-range force that depends on the distance between nucleons. In the mass number range A = 30 to 170, the distance between nucleons is nearly constant, and so the nuclear force is nearly constant.
Therefore, the binding energy per nucleon is nearly constant in this range because the nuclear force is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
The binding energy per nucleon is the energy required to separate a nucleus into its constituent nucleons. It is a measure of the stability of the nucleus, and it depends on the mass number of the nucleus. In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
Explanation:
The binding energy per nucleon is given by the formula:
BE/A = (ZmH + NmN - M)/A
where BE is the binding energy, Z is the atomic number, N is the number of neutrons, mH is the mass of a hydrogen atom, mN is the mass of a neutron, and M is the mass of the nucleus.
In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant because the nuclear force between nucleons is nearly constant. This means that the energy required to separate a nucleon from the nucleus is nearly constant in this range.
The nuclear force between nucleons is a strong force that holds the nucleus together. It is a short-range force that depends on the distance between nucleons. In the mass number range A = 30 to 170, the distance between nucleons is nearly constant, and so the nuclear force is nearly constant.
Therefore, the binding energy per nucleon is nearly constant in this range because the nuclear force is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
The ratio of volume of atom to volume of nucleus is- a)1/1000
- b)10
- c)1015
- d)1010
Correct answer is option 'C'. Can you explain this answer?
The ratio of volume of atom to volume of nucleus is
a)
1/1000
b)
10
c)
1015
d)
1010
|
|
Anaya Patel answered |
The ratio of the volume of the atom and the volume of the nucleus is 1015
The radius of an atomic nucleus is of the order of 10−13cm or 10−15m or one Fermi unit.
On the other hand, the radius of an atom is of the order of 10−8cm or 10−10m or one angstrom unit.
Note:
The radius of nucleus is much smaller than atomic radius.
The ratio of atomic radius to radius of nucleus is 10−10m /10−15m =105
Volume is proportional to cube of radius.
The ratio of atomic radius to radius of nucleus is (105)3=1015
The radius of an atomic nucleus is of the order of 10−13cm or 10−15m or one Fermi unit.
On the other hand, the radius of an atom is of the order of 10−8cm or 10−10m or one angstrom unit.
Note:
The radius of nucleus is much smaller than atomic radius.
The ratio of atomic radius to radius of nucleus is 10−10m /10−15m =105
Volume is proportional to cube of radius.
The ratio of atomic radius to radius of nucleus is (105)3=1015
Absorption line spectrum is obtained- a)If we pass off-white (discrete-spectrum) light through a cool gas
- b)If we pass white (continuous-spectrum) light through a hot gas
- c)If we pass off-white (discrete-spectrum) light through a hot gas
- d)If we pass white (continuous-spectrum) light through a cool gas
Correct answer is option 'D'. Can you explain this answer?
Absorption line spectrum is obtained
a)
If we pass off-white (discrete-spectrum) light through a cool gas
b)
If we pass white (continuous-spectrum) light through a hot gas
c)
If we pass off-white (discrete-spectrum) light through a hot gas
d)
If we pass white (continuous-spectrum) light through a cool gas
|
|
Nishant Sharma answered |
Answer is D
Fluorescence is- a)it consists of accelerated atoms/molecules striking suitable material
- b)it consists only of atoms going into stable excited states
- c)what happens in a fluorescent lamp
- d)it consists of a molecule, atom or nanostructure relaxing to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy
Correct answer is option 'D'. Can you explain this answer?
Fluorescence is
a)
it consists of accelerated atoms/molecules striking suitable material
b)
it consists only of atoms going into stable excited states
c)
what happens in a fluorescent lamp
d)
it consists of a molecule, atom or nanostructure relaxing to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy
|
|
Nikita Singh answered |
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. The most striking example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, while the emitted light is in the visible region, which gives the fluorescent substance a distinct color that can be seen only when exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after.
The nuclei of isotopes of a given element contain the same number of- a)neutrinos
- b)protons
- c)neutrons
- d)positrons
Correct answer is option 'B'. Can you explain this answer?
The nuclei of isotopes of a given element contain the same number of
a)
neutrinos
b)
protons
c)
neutrons
d)
positrons
|
|
Varanasi Sai Srinivasa K answered |
Atom of same element, contain same number of protons, they differ in number of neutrons .
This is known as isotope .
Therefore we can conclude that answer is [ B ]
This is known as isotope .
Therefore we can conclude that answer is [ B ]
The targets used in the alpha particle atomic experiments in the early 1900’s was:- a)metal foils
- b)alpha particles
- c)radioactive sources
- d)gold foil
Correct answer is option 'D'. Can you explain this answer?
The targets used in the alpha particle atomic experiments in the early 1900’s was:
a)
metal foils
b)
alpha particles
c)
radioactive sources
d)
gold foil
|
|
Shreya Singh answered |
It's Rutherford alpha Ray scattering experiment.....in this experiment gold foil was used...
In Bohr model of hydrogen atom, radiation is emitted when the electron- a)jumps from higher orbit into the lower orbit
- b)the electron escapes from the orbit
- c)jumps from lower orbit into the higher orbit
- d)revolves in an orbit
Correct answer is option 'A'. Can you explain this answer?
In Bohr model of hydrogen atom, radiation is emitted when the electron
a)
jumps from higher orbit into the lower orbit
b)
the electron escapes from the orbit
c)
jumps from lower orbit into the higher orbit
d)
revolves in an orbit
|
|
Yamuna Mani answered |
Answer is A
E2-E1=hv
E2-E1=hv
γ (Gamma) rays are- a)Singly ionized gas atoms
- b)Electromagnetic waves
- c)Helium nuclei
- d)Fast moving electrons
Correct answer is option 'B'. Can you explain this answer?
γ (Gamma) rays are
a)
Singly ionized gas atoms
b)
Electromagnetic waves
c)
Helium nuclei
d)
Fast moving electrons
|
|
Prakhar Inani answered |
Since gamma rays carry energy that's why they are electromagnetic waves.
The average binding energy of nucleus is- a)8 BeV
- b)8 Mev
- c)8 eV
- d)8 KeV
Correct answer is option 'B'. Can you explain this answer?
The average binding energy of nucleus is
a)
8 BeV
b)
8 Mev
c)
8 eV
d)
8 KeV
|
|
Rahul Bansal answered |
Excluding the lighter nuclei, the average binding energy per nucleon is about 8 MeV. The maximum binding energy per nucleon occurs at around mass number A = 50, and corresponds to the most stable nuclei.
Which of these statements about Bohr model hypothesis is correct?- a)velocity of electron is quantized
- b)mass of electron is quantized
- c)radius of electron is quantized
- d)angular momentum of electron is quantized
Correct answer is option 'D'. Can you explain this answer?
Which of these statements about Bohr model hypothesis is correct?
a)
velocity of electron is quantized
b)
mass of electron is quantized
c)
radius of electron is quantized
d)
angular momentum of electron is quantized
|
|
Krishna Iyer answered |
Bohr never assumed stable electron orbits with the electronic angular momentum quantized as l=mvr=(nh/2π) Quantization of angular momentum means that the radius of the orbit and the energy will be quantized as well. Bohr assumed that the discrete lines seen in the spectrum of the hydrogen atom were due to transitions of an electron from one allowed orbit/energy to another.
In Geiger-Marsden experiment, at the point of closest approach- a)the kinetic energy is not zero and the electrical potential is less than the initial kinetic energy supplied
- b)the kinetic energy is not zero and the electrical potential equals the initial kinetic energy supplied
- c)the kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
- d)the kinetic energy is not zero and the electrical potential is greater than the initial kinetic energy supplied
Correct answer is option 'C'. Can you explain this answer?
In Geiger-Marsden experiment, at the point of closest approach
a)
the kinetic energy is not zero and the electrical potential is less than the initial kinetic energy supplied
b)
the kinetic energy is not zero and the electrical potential equals the initial kinetic energy supplied
c)
the kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
d)
the kinetic energy is not zero and the electrical potential is greater than the initial kinetic energy supplied
|
|
Dino James answered |
The kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
Chapter doubts & questions for Nuclear Physics - Physics for Grade 12 2025 is part of Grade 12 exam preparation. The chapters have been prepared according to the Grade 12 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 12 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Nuclear Physics - Physics for Grade 12 in English & Hindi are available as part of Grade 12 exam.
Download more important topics, notes, lectures and mock test series for Grade 12 Exam by signing up for free.
Physics for Grade 12
142 videos|312 docs|132 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup