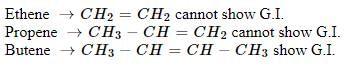All Exams >
EmSAT Achieve >
Physics for EmSAT Achieve >
All Questions
All questions of First and Second Law of Thermodynamics for EmSAT Achieve Exam
Which wall would allow the flow of thermal energy between systems A and B to achieve thermal equilibrium?
- a)Diathermic wall
- b)Adiabatic wall
- c)Diadiabatic wall
- d)Thermal wall
Correct answer is option 'A'. Can you explain this answer?
Which wall would allow the flow of thermal energy between systems A and B to achieve thermal equilibrium?

a)
Diathermic wall
b)
Adiabatic wall
c)
Diadiabatic wall
d)
Thermal wall
|
|
Rajesh Gupta answered |
Wall that permits *heat" to flow through them,such as engine block is called diathermic wall.
wall Perfectly insulating ball that doesn't allow the flow heat to them are called adiabatic walls.
wall Perfectly insulating ball that doesn't allow the flow heat to them are called adiabatic walls.
What is not true for a cyclic process?a) System returns to its initial state
b) ΔU = 0
c) ΔW= 0
d) ΔQ = -ΔW
Correct answer is option 'C'. Can you explain this answer?
b) ΔU = 0
c) ΔW= 0
d) ΔQ = -ΔW
Correct answer is option 'C'. Can you explain this answer?
|
|
Neha Joshi answered |
As work is a path function rather than a state function, we can easily say that work can often be graphically represented as the area under the PV graph. And as cyclic processes are represented as closed shapes on PV graph it is obvious that they have non zero area and thus work done is non zero.
The R/S configuration of these compounds are respectively.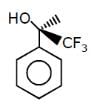
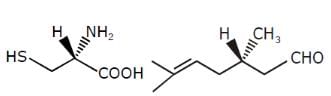
- a)R,R,S
- b)R,S,R
- c)R,S,S
- d)S,S,S
Correct answer is option 'A'. Can you explain this answer?
The R/S configuration of these compounds are respectively.


a)
R,R,S
b)
R,S,R
c)
R,S,S
d)
S,S,S

|
Mohit Rajpoot answered |
Applying the previously stated results we get A as the answer
The number of cis-trans isomer possible for the following compound 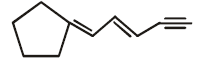
- a)2
- b)4
- c)6
- d)8
Correct answer is option 'A'. Can you explain this answer?
The number of cis-trans isomer possible for the following compound
a)
2
b)
4
c)
6
d)
8
|
|
Krishna Iyer answered |
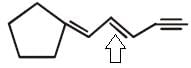
Since only this is a double bond with different groups attached so only it will show cis-trans isomerism
Which of the following will exhibit geometrical isomerism ?- a)1-phenyl-2-butene
- b)3-phenyl-1-butene
- c)2-phenyl-1-butene
- d)1, 1-diphenyl-1-propene
Correct answer is option 'A'. Can you explain this answer?
Which of the following will exhibit geometrical isomerism ?
a)
1-phenyl-2-butene
b)
3-phenyl-1-butene
c)
2-phenyl-1-butene
d)
1, 1-diphenyl-1-propene
|
|
Geetika Shah answered |
1-phenyl-2-butene shows geometrical isomerism in the form of cis and trans isomers.
However, the geometrical isomerism is not possible for 3-phenyl-1-butene, 2-phenyl-1-butene and 1,1-diphenyl-1-propane.
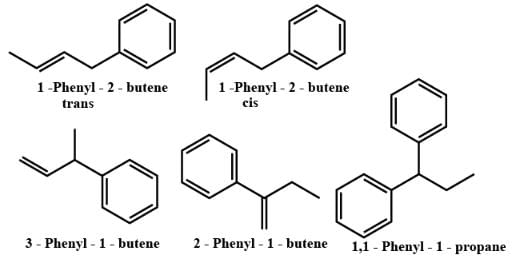
However, the geometrical isomerism is not possible for 3-phenyl-1-butene, 2-phenyl-1-butene and 1,1-diphenyl-1-propane.

The temperature inside a refrigerator is 4°C and the room temperature is 27°C. How many joules of heat will be delivered to the room for each joule of electricity consumed by the refrigerator?( Treat the refrigerator as ideal).- a)1 J
- b)12 J
- c)8.3 J
- d)13 J
Correct answer is option 'D'. Can you explain this answer?
The temperature inside a refrigerator is 4°C and the room temperature is 27°C. How many joules of heat will be delivered to the room for each joule of electricity consumed by the refrigerator?( Treat the refrigerator as ideal).
a)
1 J
b)
12 J
c)
8.3 J
d)
13 J
|
|
Krishna Iyer answered |
Coefficient of performance(cop) of a refrigerator,
β= Q2/Q1-Q2=T2/T1-T2=277/300-277=277/23≈12
OR, Q2/W=12. Therefore, W=1J.Q2=12J
Also, Q1=Q2+W
=(12+1) J
=13J
β= Q2/Q1-Q2=T2/T1-T2=277/300-277=277/23≈12
OR, Q2/W=12. Therefore, W=1J.Q2=12J
Also, Q1=Q2+W
=(12+1) J
=13J
In a Carnot engine 800 J of heat is absorbed from a source at 400 K and 640 J of heat is rejected to the sink. The temperature of the sink is:- a)273 K
- b)100 K
- c)320 K
- d)240 K
Correct answer is option 'C'. Can you explain this answer?
In a Carnot engine 800 J of heat is absorbed from a source at 400 K and 640 J of heat is rejected to the sink. The temperature of the sink is:
a)
273 K
b)
100 K
c)
320 K
d)
240 K
|
|
Nandini Patel answered |
In a Carnot engine, Q1/T1 = Q2/T2
so that the temperature of the sink,
T2 = T1Q2/Q1 = 400x640/800 = 320 K.
The internal energy and the work done by a system decreases by same amount then- a)The temperature must decrease
- b)The process must be adiabatic
- c)The process must be isothermal
- d)both a and b
Correct answer is option 'D'. Can you explain this answer?
The internal energy and the work done by a system decreases by same amount then
a)
The temperature must decrease
b)
The process must be adiabatic
c)
The process must be isothermal
d)
both a and b
|
|
Gaurav Kumar answered |
The internal energy of a system decreases by the same amount as the work done by the system.
Change in internal energy=work done+heat exchange
change in internal energy=work done if process has no heat exchange, i.e. it's adiabatic and the temperature must decrease
Change in internal energy=work done+heat exchange
change in internal energy=work done if process has no heat exchange, i.e. it's adiabatic and the temperature must decrease
Two neighbours of homologous series differ by - a)_CH
- b)_CH3
- c)_CH4
- d)_CH2
Correct answer is option 'D'. Can you explain this answer?
Two neighbours of homologous series differ by
a)
_CH
b)
_CH3
c)
_CH4
d)
_CH2
|
|
Rajesh Gupta answered |
A homologous series is a series of compounds in which each member differs from the next/previous by CH2 or 14 mass units. Physical properties change in a homologous series but chemical properties remain almost the same because the functional group does not change.
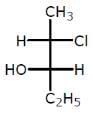 The compound with the above configuration is called.
The compound with the above configuration is called.- a)(2S, 3S)-2-chloro-3-hydroxypentane
- b)(2S, 3R)-2-chloro-3-hydroxypentane
- c)(2R, 3R)-2-chloro-3-hydroxypentane
- d)(2R, 3S)-2-chloro-3-hydroxypentane
Correct answer is option 'A'. Can you explain this answer?

The compound with the above configuration is called.
a)
(2S, 3S)-2-chloro-3-hydroxypentane
b)
(2S, 3R)-2-chloro-3-hydroxypentane
c)
(2R, 3R)-2-chloro-3-hydroxypentane
d)
(2R, 3S)-2-chloro-3-hydroxypentane
|
|
Krishna Iyer answered |
4th priority group always lie in vertical
Clockwise: R
Anticlockwise: S
At 2nd carbon 1 → 2 → 3 configuration is r but 4 groups are horizontal so conf. change from R to s
At 3rd carbon a → b → c conf. is r but d group is horizontal so conf. change from R to S
Hence,
2s,3s)-c-chloro-3 hydropentane
Clockwise: R
Anticlockwise: S
At 2nd carbon 1 → 2 → 3 configuration is r but 4 groups are horizontal so conf. change from R to s
At 3rd carbon a → b → c conf. is r but d group is horizontal so conf. change from R to S
Hence,
2s,3s)-c-chloro-3 hydropentane
The compounds C2H5OC2H5 and CH3OCH2CH2CH3 are- a)Chain isomers
- b)Geometrical isomers
- c)Metamers
- d)Conformational isomers
Correct answer is option 'C'. Can you explain this answer?
The compounds C2H5OC2H5 and CH3OCH2CH2CH3 are
a)
Chain isomers
b)
Geometrical isomers
c)
Metamers
d)
Conformational isomers
|
|
Raghav Bansal answered |
Here, ether is functional group and whenever there is change around same functional group they are metamers to each other.
These are chain as well as metamers but priority is given to metamerism even if we have to choose between metamer and position then, again metamer is to be opted first.
These are chain as well as metamers but priority is given to metamerism even if we have to choose between metamer and position then, again metamer is to be opted first.
What would be the horse power of a steam engine with average pressure of steam 9x 104 Nm-2 , the area of cross section of the piston is 0.2 m2, length of stroke is 0.6 m and piston makes 5 revolutions per second?- a)150 h.p.
- b)140.7 h.p.
- c)104.7 h.p.
- d)144.77 h.p.
Correct answer is option 'D'. Can you explain this answer?
What would be the horse power of a steam engine with average pressure of steam 9x 104 Nm-2 , the area of cross section of the piston is 0.2 m2, length of stroke is 0.6 m and piston makes 5 revolutions per second?
a)
150 h.p.
b)
140.7 h.p.
c)
104.7 h.p.
d)
144.77 h.p.
|
|
Pooja Shah answered |
Energy in 1 revolution=2 x P x a x l=2 x 9 x104 x 0.2 x0.6 j=21600J
Energy in n revolutions= 21600nJ
Power=E/t=21600n/t=21600 x 5=18000 J/s= 108000/746 h.p=144.77 h.p.
Energy in n revolutions= 21600nJ
Power=E/t=21600n/t=21600 x 5=18000 J/s= 108000/746 h.p=144.77 h.p.
An ideal gas undergoes four different processes from the same initial state (Fig.). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic.
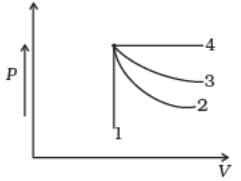
- a)4
- b)3
- c)1
- d)2
Correct answer is option 'D'. Can you explain this answer?
An ideal gas undergoes four different processes from the same initial state (Fig.). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic.


a)
4
b)
3
c)
1
d)
2
|
|
Raghav Bansal answered |
4 is isobaric process, 1 is isochoric. Out of 3 and 2, 3 has the smaller slope (magnitude) hence is isothermal. Remaining process 2 is adiabatic.
First law of thermodynamics tells us:- a)the nature of the process taking place
- b)the direction in which a given process can take place
- c)to what extent the process takes place
- d)that heat supplied is used to carry out the process
Correct answer is option 'D'. Can you explain this answer?
First law of thermodynamics tells us:
a)
the nature of the process taking place
b)
the direction in which a given process can take place
c)
to what extent the process takes place
d)
that heat supplied is used to carry out the process
|
|
Hansa Sharma answered |
First law of thermodynamics is based on law of conservation of energy i.e. energy supplied to a system has to be used in raising the internal energy of the system. So heat supplied is used to carry out the process.
∆U = Q + W.
∆U = Q + W.
Which conformer of cyclohexane is chiral- a)Chair
- b)Boat
- c)Twisted boat
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Which conformer of cyclohexane is chiral
a)
Chair
b)
Boat
c)
Twisted boat
d)
None of these
|
|
Gaurav Kumar answered |
We know that if a compound is symmetrical then then it is not chiral. hence chair conformation and boat conformations. Both are achiral whereas twist boat is chiral.
Which of the following are the extensive variables?- a)Internal energy, pressure and volume
- b)Pressure, temperature and density
- c)Internal energy, volume, total mass
- d)Pressure, temperature and volume
Correct answer is option 'C'. Can you explain this answer?
Which of the following are the extensive variables?
a)
Internal energy, pressure and volume
b)
Pressure, temperature and density
c)
Internal energy, volume, total mass
d)
Pressure, temperature and volume
|
|
Anjali Iyer answered |
An extensive variable is one which depends on system size (like mass or volume). ... An intensive variable is one which does not depend on system size (like temperature, pressure, or density).
The Zeroth law leads to the concept of- a)temprature
- b)heat
- c)internal energy
- d)work
Correct answer is option 'A'. Can you explain this answer?
The Zeroth law leads to the concept of
a)
temprature
b)
heat
c)
internal energy
d)
work
|
|
Gaurav Kumar answered |
Zeroth law states that if two thermodynamic systems are each in thermal equilibrium with a third one, then they are in thermal equilibrium with each other. Thus as it deals with thermal equilibrium it is very clear that it is a concept of temperature.
Phenol and benzyl alcohol are- a)functional isomers
- b)Homologous
- c)Position isomers
- d)None of these
Correct answer is option 'D'. Can you explain this answer?
Phenol and benzyl alcohol are
a)
functional isomers
b)
Homologous
c)
Position isomers
d)
None of these
|
|
Geetika Shah answered |
Phenol is C6H5(OH) while benzyl alcohol is C6H5(CH2OH).
Here there is a difference of −HC2 between their molecular formula.
According to the definition of homologous series, the adjacent members of the series differ by −CH2 group.
Thus, Phenol and benzyl alcohol are homologous of each other.
Here there is a difference of −HC2 between their molecular formula.
According to the definition of homologous series, the adjacent members of the series differ by −CH2 group.
Thus, Phenol and benzyl alcohol are homologous of each other.
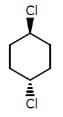
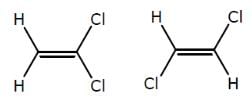 The above compounds differ in
The above compounds differ in- a)Configuration
- b)Conformation
- c)Structure
- d)Chirality
Correct answer is option 'C'. Can you explain this answer?

The above compounds differ in
a)
Configuration
b)
Conformation
c)
Structure
d)
Chirality
|
|
Hansa Sharma answered |
The first compound is in cis phase and the other one is trans phase. they differ in their structure.
The First Law of Thermodynamics states that:- a)ΔQ - W = ΔU
- b)ΔQ - ΔW = U
- c)ΔQ - ΔU = ΔW
- d)Q - W = U
Correct answer is option 'C'. Can you explain this answer?
The First Law of Thermodynamics states that:
a)
ΔQ - W = ΔU
b)
ΔQ - ΔW = U
c)
ΔQ - ΔU = ΔW
d)
Q - W = U
|
|
Preeti Iyer answered |
The first law of thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.
According to this law, some of the heat given to system is used to change the internal energy while the rest in doing work by the system. Mathematically,
ΔQ=ΔU+ΔW
where,
ΔQ = Heat supplied to the system
ΔW= Work done by the system.
ΔU = Change in the internal energy of the system.
If Q is positive, then there is a net heat transfer into the system, if W is positive, then there is work done by the system. So positive Q adds energy to the system and positive W takes energy from the system.
It can also be represented as ΔU=ΔQ-ΔW
We can say that internal energy tends to increase when heat is given to the system and vice versa.
If the door of refrigerator is left open inside a closed room, what would happen to the temperature of the room?- a)Room temperature would decrease
- b)Room temperature would increase
- c)Room temperature would be same as the temperature inside the refrigerator
- d)Room temperature would not be effected
Correct answer is option 'B'. Can you explain this answer?
If the door of refrigerator is left open inside a closed room, what would happen to the temperature of the room?
a)
Room temperature would decrease
b)
Room temperature would increase
c)
Room temperature would be same as the temperature inside the refrigerator
d)
Room temperature would not be effected
|
|
Riya Banerjee answered |
If you leave the door open, heat is merely recycled from the room into therefrigerator, then back into the room. A net room temperature increase wouldresult from the heat of the motor that would be constantly running to move energy around in a circle.
Find the final temperature of one mole of an ideal gas at an initial temperature to t K.The gas does 9 R joules of work adiabatically. The ratio of specific heats of this gas at constant pressure and at constant volume is 4/3.- a)(t-9)K
- b)(t - 4/3)K
- c)t + 3K
- d)(t - 3)K
Correct answer is option 'D'. Can you explain this answer?
Find the final temperature of one mole of an ideal gas at an initial temperature to t K.The gas does 9 R joules of work adiabatically. The ratio of specific heats of this gas at constant pressure and at constant volume is 4/3.
a)
(t-9)K
b)
(t - 4/3)K
c)
t + 3K
d)
(t - 3)K
|
|
Gaurav Kumar answered |
TInitial = t K
Work, W = 9R
Ratio of specific heats, γ = Cp / Cv = 4/3
In an adiabatic process, we have
W = R(TFinal – Tinitial) / (1-γ)
9R = R (TFinal – t) / (1 – 4/3)
TFinal – t = 9 (-1/3) = -3
TFinal = (t-3) K
Work, W = 9R
Ratio of specific heats, γ = Cp / Cv = 4/3
In an adiabatic process, we have
W = R(TFinal – Tinitial) / (1-γ)
9R = R (TFinal – t) / (1 – 4/3)
TFinal – t = 9 (-1/3) = -3
TFinal = (t-3) K
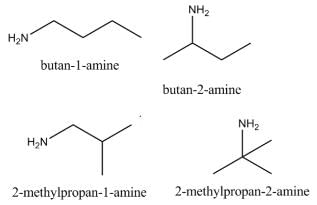
How many primary amines are possible for the formula C4H11N?- a)2
- b)3
- c)4
- d)5
Correct answer is option 'C'. Can you explain this answer?
How many primary amines are possible for the formula C4H11N?
a)
2
b)
3
c)
4
d)
5
|
|
Pooja Shah answered |
The following 4 structures are possible

Which of the following statement is true for a thermodynamical system where ∆U is the increase in internal energy and ∆W work done respectively?- a)∆U = ∆W in isothermal process
- b)∆U = ∆W in a adiabatic process
- c)∆U= -∆W in case of ideal gas
- d)∆U= -∆W in an adiabatic process
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is true for a thermodynamical system where ∆U is the increase in internal energy and ∆W work done respectively?
a)
∆U = ∆W in isothermal process
b)
∆U = ∆W in a adiabatic process
c)
∆U= -∆W in case of ideal gas
d)
∆U= -∆W in an adiabatic process
|
|
Hansa Sharma answered |
∆ U = ∆ Q - ∆ W
In an adiabatic process ∆Q is zero, therefore
∆ U = - ∆ W
In an adiabatic process ∆Q is zero, therefore
∆ U = - ∆ W
How many minimum no. of C-atoms are required for position & geometrical isomerism in alkene?- a)4,3
- b)4,4
- c)3,4
- d)3,3
Correct answer is option 'B'. Can you explain this answer?
How many minimum no. of C-atoms are required for position & geometrical isomerism in alkene?
a)
4,3
b)
4,4
c)
3,4
d)
3,3
|
|
Shail Majumdar answered |
This question is incomplete. Please provide more information.
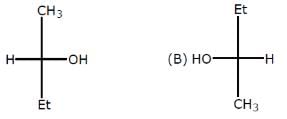 Relation between given pair is
Relation between given pair is- a)Enantiomer
- b)Diastereomer
- c)Identical
- d)Structural isomer
Correct answer is option 'C'. Can you explain this answer?

Relation between given pair is
a)
Enantiomer
b)
Diastereomer
c)
Identical
d)
Structural isomer
|
|
Rajesh Gupta answered |
The above pairs are known as identical pair. An isomer of a molecule has the number of atoms of each element but has a different arrangement of the atoms.It has the same molecular formula as the other molecule , with a different chemical structure.
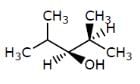
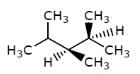
Which of the following structures represents a chiral compound ?- a)
- b)
- c)
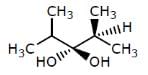
- d)
Correct answer is option 'D'. Can you explain this answer?
Which of the following structures represents a chiral compound ?
a)

b)

c)

d)

|
|
Gaurav Kumar answered |
This compound is chiral because its molecule has two chiral carbon atoms (C−3 and C-4).
ether molecules are chiral due to the presence of a plane of symmetry.
ether molecules are chiral due to the presence of a plane of symmetry.
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the- a)coefficient of performance
- b)principle of heat engine
- c)efficiency of heat engine
- d)efficiency of refrigerator
Correct answer is option 'A'. Can you explain this answer?
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the
a)
coefficient of performance
b)
principle of heat engine
c)
efficiency of heat engine
d)
efficiency of refrigerator
|
|
Lavanya Menon answered |
The ratio of quantity of heat removed per cycle from the contents of the refrigerator to the energy spent per cycle to remove this heat is called the coefficient of performance. It is the definition of coefficient of performance.
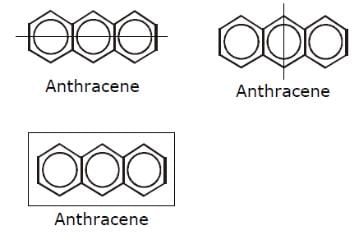
How many structural formula are possible when one of the hydrogen is replaced by a chlorine atom in anthracene?- a)3
- b)7
- c)4
- d)6
Correct answer is option 'A'. Can you explain this answer?
How many structural formula are possible when one of the hydrogen is replaced by a chlorine atom in anthracene?
a)
3
b)
7
c)
4
d)
6
|
|
Geetika Shah answered |
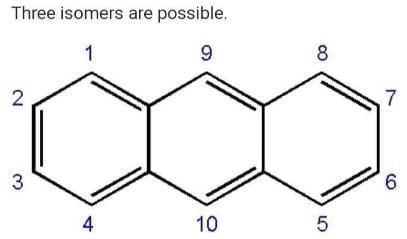
Position 1,4,5,8 are equal.
Similarly 2,3,6,7 are equal.
Position 9 and 10 are equal.
So we will get three possible isomers
Which of the following connot be written in an isomeric form?- a)CH3 – CH(OH) – CH2 – CH3
- b)CH3 – CHO
- c)CH2 = CH – Cl
- d)Cl – CH2CH2 – Cl
Correct answer is option 'C'. Can you explain this answer?
Which of the following connot be written in an isomeric form?
a)
CH3 – CH(OH) – CH2 – CH3
b)
CH3 – CHO
c)
CH2 = CH – Cl
d)
Cl – CH2CH2 – Cl
|
|
Gaurav Kumar answered |
CH3−CH(OH)−CH2−CH3 can have position isomer.
CH3−CHO can have functional isomers (enol).
Cl−CH2CH2−Cl can have position isomer.
But CH2=CH−Cl has one isomer but it is not preferred IUPAC name.
CH3−CHO can have functional isomers (enol).
Cl−CH2CH2−Cl can have position isomer.
But CH2=CH−Cl has one isomer but it is not preferred IUPAC name.
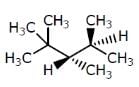
Which of the following compounds has two stereogenic centers (asymmetric carbons) ?- a)
- b)
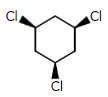
- c)
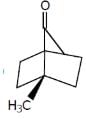
- d)
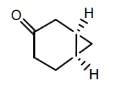
Correct answer is option 'D'. Can you explain this answer?
Which of the following compounds has two stereogenic centers (asymmetric carbons) ?
a)

b)

c)

d)

|
|
Raghav Bansal answered |
Asymmetric carbon is the carbon which has all the four attached groups different.And these carbons are also called chiral carbons.
Hence option A has asymmetric or chiral carbon.
Hence option A is correct.
Hence option A has asymmetric or chiral carbon.
Hence option A is correct.
Stereoisomers differ from each other in what respect ?- a)Composition
- b)Constitution
- c)Configuration
- d)Steric hindrance
Correct answer is option 'C'. Can you explain this answer?
Stereoisomers differ from each other in what respect ?
a)
Composition
b)
Constitution
c)
Configuration
d)
Steric hindrance
|
|
Riya Banerjee answered |
Stereoisomers. Generally defined, stereoisomers are isomers that have the same composition (that is, the same parts) but that differ in the orientation of those parts in space.
Isothermal process can be represented by which law?- a)Charle’s law
- b)Boyle’s law
- c)Gay-Lussac’s law
- d)2nd law of thermodynamics
Correct answer is option 'B'. Can you explain this answer?
Isothermal process can be represented by which law?
a)
Charle’s law
b)
Boyle’s law
c)
Gay-Lussac’s law
d)
2nd law of thermodynamics
|
|
Janhavi Choudhury answered |
's law
b)Boyle's law
c)Gay-Lussac's law
d)Joule's law
d) Joule's law.
b)Boyle's law
c)Gay-Lussac's law
d)Joule's law
d) Joule's law.
Refrigerators X and Y are removing 1000 J of heat from the freezer. Refrigerator X is working between -5° C and 25° C and refrigerator Y is working between -20° C and 20 °C. Find efficiency of refrigerator X and Y?- a)20,2
- b)7.9,6.5
- c)9.8,7.3
- d)8.9,7.3
Correct answer is option 'D'. Can you explain this answer?
Refrigerators X and Y are removing 1000 J of heat from the freezer. Refrigerator X is working between -5° C and 25° C and refrigerator Y is working between -20° C and 20 °C. Find efficiency of refrigerator X and Y?
a)
20,2
b)
7.9,6.5
c)
9.8,7.3
d)
8.9,7.3
|
|
Neha Joshi answered |
We know that the efficiency of refrigeration for a refrigerator is T2 / T1 + T2
Where T1 is source temperature and T2 is sink temperature
For refrigerator X we have T1 = 298K and T2 = 268K
Hence the efficiency of refrigeration = 268 / 298 - 268
= 268 / 30
= 8.93
For refrigerator Y we have T1 = 293K and T2 = 253K
Hence the efficiency of refrigeration = 253 / 293 - 253
= 253 / 40
= 6.35
Where T1 is source temperature and T2 is sink temperature
For refrigerator X we have T1 = 298K and T2 = 268K
Hence the efficiency of refrigeration = 268 / 298 - 268
= 268 / 30
= 8.93
For refrigerator Y we have T1 = 293K and T2 = 253K
Hence the efficiency of refrigeration = 253 / 293 - 253
= 253 / 40
= 6.35
The second law of thermodynamics says- a)Coefficient of performance can never be infinite for refrigerator
- b)Heat released to the cold reservoir can be zero
- c)Ideal gas can expand infinitely
- d)Efficiency of a heat engine can be 100%.
Correct answer is option 'A'. Can you explain this answer?
The second law of thermodynamics says
a)
Coefficient of performance can never be infinite for refrigerator
b)
Heat released to the cold reservoir can be zero
c)
Ideal gas can expand infinitely
d)
Efficiency of a heat engine can be 100%.
|
|
Raghav Bansal answered |
The second law of thermodynamics gives a fundamental limitation to the efficiency of a heat engine and the coefficient of performance of a refrigerator. It says that the efficiency of a heat engine can never be unity or 100%, this implies that the heat released to the cold reservoir can never be made zero.
For a refrigerator the second law says that the coefficient through performance can never be infinite, this implies that the external work can never be zero.
For a refrigerator the second law says that the coefficient through performance can never be infinite, this implies that the external work can never be zero.
Which word is defined by this statement: A measure of this disorder, or randomness?- a)energy
- b)enthalpy
- c)mass
- d)entropy
Correct answer is option 'D'. Can you explain this answer?
Which word is defined by this statement: A measure of this disorder, or randomness?
a)
energy
b)
enthalpy
c)
mass
d)
entropy
|
|
Janhavi Rane answered |
Understanding Entropy
Entropy is a fundamental concept in thermodynamics and statistical mechanics that quantifies the level of disorder or randomness in a system. Let's explore this in detail.
Definition of Entropy
- Measure of Disorder: Entropy can be understood as a measure of how spread out or dispersed the energy in a system is.
- Randomness: A higher entropy value indicates greater randomness and less predictability in the arrangement of particles in a system.
Importance of Entropy
- Second Law of Thermodynamics: This law states that in any energy transfer or transformation, the total entropy of a closed system will either increase or remain constant. This is a reflection of natural processes moving towards a state of maximum disorder.
- Spontaneous Processes: Entropy helps to explain why certain processes occur spontaneously. For example, ice melting in a warm room increases the entropy of the system, as the structured ice molecules become more disordered in liquid form.
Comparison with Other Options
- Energy: While energy is related to the ability to do work, it does not directly measure disorder.
- Enthalpy: This is a measure of total heat content in a system, not specifically disorder.
- Mass: Mass refers to the amount of matter in a system and does not provide information about the arrangement or randomness of that matter.
Conclusion
In summary, the correct answer is option 'D' – Entropy – because it specifically defines a measure of disorder or randomness within a system, making it essential for understanding the behavior of thermodynamic processes.
Entropy is a fundamental concept in thermodynamics and statistical mechanics that quantifies the level of disorder or randomness in a system. Let's explore this in detail.
Definition of Entropy
- Measure of Disorder: Entropy can be understood as a measure of how spread out or dispersed the energy in a system is.
- Randomness: A higher entropy value indicates greater randomness and less predictability in the arrangement of particles in a system.
Importance of Entropy
- Second Law of Thermodynamics: This law states that in any energy transfer or transformation, the total entropy of a closed system will either increase or remain constant. This is a reflection of natural processes moving towards a state of maximum disorder.
- Spontaneous Processes: Entropy helps to explain why certain processes occur spontaneously. For example, ice melting in a warm room increases the entropy of the system, as the structured ice molecules become more disordered in liquid form.
Comparison with Other Options
- Energy: While energy is related to the ability to do work, it does not directly measure disorder.
- Enthalpy: This is a measure of total heat content in a system, not specifically disorder.
- Mass: Mass refers to the amount of matter in a system and does not provide information about the arrangement or randomness of that matter.
Conclusion
In summary, the correct answer is option 'D' – Entropy – because it specifically defines a measure of disorder or randomness within a system, making it essential for understanding the behavior of thermodynamic processes.
For proper utilization of exergy, it is desirable to make first law efficiency ____ and the source and use temperatures should ____- a)as close to unity, be different
- b)as close to unity, match
- c)as close to zero, match
- d)as close to zero, be different
Correct answer is option 'B'. Can you explain this answer?
For proper utilization of exergy, it is desirable to make first law efficiency ____ and the source and use temperatures should ____
a)
as close to unity, be different
b)
as close to unity, match
c)
as close to zero, match
d)
as close to zero, be different
|
|
Raghav Bansal answered |
If first law efficiency is close to unity, the all the energy carried in by heat transfer is used and no heat is lost to the surroundings.
Geometrical isomers can be- a)Diastereomers or Enantiomers
- b)Structural isomers
- c)Inter convertable at room temprature.
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Geometrical isomers can be
a)
Diastereomers or Enantiomers
b)
Structural isomers
c)
Inter convertable at room temprature.
d)
None of these
|
|
Om Desai answered |
Diastereomers are isomers which are not mirror images of each other, while enantiomers are those which are mirror images of each other. A geometric isomer without any chiral centre is a mirror image of itself. Also, 2 geometric isomers, which are not mirror images of each other will be diastereomers.
Hence, geometrical isomers can be diastereomers or enantiomers.
Hence, geometrical isomers can be diastereomers or enantiomers.
Suppose we have a box filled with gas and a piston is also attached at the top of the box.What are the ways of changing the state of gas (and hence its internal energy)? Answer could be more than one choice.- a)Bring box in contact with a body with higher temperature
- b)Move the box so that it has kinetic energy
- c)Pushing the piston down so as to do work on the system
- d)both a and c
Correct answer is option 'D'. Can you explain this answer?
Suppose we have a box filled with gas and a piston is also attached at the top of the box.What are the ways of changing the state of gas (and hence its internal energy)? Answer could be more than one choice.
a)
Bring box in contact with a body with higher temperature
b)
Move the box so that it has kinetic energy
c)
Pushing the piston down so as to do work on the system
d)
both a and c
|
|
Shail Majumdar answered |
**Explanation:**
To understand why the correct answer is option 'D', let's analyze each option one by one:
**a) Bring box in contact with a body with higher temperature:**
When a box filled with gas is brought in contact with a body at a higher temperature, heat flows from the higher temperature body to the gas inside the box. This increases the temperature and hence the internal energy of the gas. Therefore, this option is valid for changing the state of the gas.
**b) Move the box so that it has kinetic energy:**
Moving the box so that it has kinetic energy does not directly change the state of the gas. It only changes the position and motion of the box. However, if the box is connected to the piston, and the piston is not fixed, the kinetic energy of the box can be transferred to the gas by pushing the piston down. This will do work on the system and change the state of the gas. Therefore, this option indirectly allows for changing the state of the gas.
**c) Pushing the piston down so as to do work on the system:**
Pushing the piston down compresses the gas inside the box, reducing its volume. This work is done on the system, and as a result, the internal energy of the gas increases. Therefore, this option is valid for changing the state of the gas.
**d) Both a and c:**
From the explanations above, it is clear that both options a and c allow for changing the state of the gas. Bringing the box in contact with a body at a higher temperature increases the internal energy of the gas, and pushing the piston down to do work on the system also increases the internal energy of the gas. Therefore, the correct answer is option 'D' - both a and c.
By using both options a and c, we can effectively change the state of the gas by increasing its internal energy through heat transfer and work done on the system.
To understand why the correct answer is option 'D', let's analyze each option one by one:
**a) Bring box in contact with a body with higher temperature:**
When a box filled with gas is brought in contact with a body at a higher temperature, heat flows from the higher temperature body to the gas inside the box. This increases the temperature and hence the internal energy of the gas. Therefore, this option is valid for changing the state of the gas.
**b) Move the box so that it has kinetic energy:**
Moving the box so that it has kinetic energy does not directly change the state of the gas. It only changes the position and motion of the box. However, if the box is connected to the piston, and the piston is not fixed, the kinetic energy of the box can be transferred to the gas by pushing the piston down. This will do work on the system and change the state of the gas. Therefore, this option indirectly allows for changing the state of the gas.
**c) Pushing the piston down so as to do work on the system:**
Pushing the piston down compresses the gas inside the box, reducing its volume. This work is done on the system, and as a result, the internal energy of the gas increases. Therefore, this option is valid for changing the state of the gas.
**d) Both a and c:**
From the explanations above, it is clear that both options a and c allow for changing the state of the gas. Bringing the box in contact with a body at a higher temperature increases the internal energy of the gas, and pushing the piston down to do work on the system also increases the internal energy of the gas. Therefore, the correct answer is option 'D' - both a and c.
By using both options a and c, we can effectively change the state of the gas by increasing its internal energy through heat transfer and work done on the system.
Refrigerator transfers heat from the cold cooling coils to warm surroundings, which law of thermodynamics favour this process- a)Zeroth law of thermodynamics
- b)Third law of thermodynamics
- c)First law of thermodynamics
- d)Second law of thermodynamics
Correct answer is option 'D'. Can you explain this answer?
Refrigerator transfers heat from the cold cooling coils to warm surroundings, which law of thermodynamics favour this process
a)
Zeroth law of thermodynamics
b)
Third law of thermodynamics
c)
First law of thermodynamics
d)
Second law of thermodynamics
|
|
Rajat Patel answered |
Refrigerator follows the principle of clausius statement of second law of thermodynamics. It does not violate second law of thermodynamics because it takes energy to transfer heat from low temperature body to high temperature body. Electrical work is given to refrigerator to extract heat from low temperature body and to transfer it to higher temperature body. If any refrigerator is transferring heat from low temperature body to higher temperature body without any external energy then we can say that it violates second law of thermodynamics.But in actual it takes energy to do.
Which of the following is an example of heat pump?- a)Internal combustion engine
- b)Blower heater
- c)Refrigerator
- d)Carnot engine
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of heat pump?
a)
Internal combustion engine
b)
Blower heater
c)
Refrigerator
d)
Carnot engine
|
|
Om Desai answered |
A heat pump is an electrical device that heats a building by pumping heat in from the cold outside. In other words, it’s the same as a refrigerator, but its purpose is to warm the hot reservoir rather than to cool the cold reservoir (even though it does both).
The number of isomers of C5H10 is- a)10
- b)11
- c)12
- d)13
Correct answer is option 'D'. Can you explain this answer?
The number of isomers of C5H10 is
a)
10
b)
11
c)
12
d)
13
|
|
Raghav Bansal answered |
Correct Answer :- d
Explanation : Given molecular formula is C5H10.
⇒ There are 6 isomeric alkenes by including Cis/trans isomers of pent−2−ene.
⇒ 7 Cycloalkanes also possible.
Totally it have 13 isomers.
Hot coffee in a thermos flask is shaken vigorously, considering it as a system which of the statement is not true?- a)Temperature of the system rises
- b)Internal energy of the coffee increased
- c)Heat energy has been added to coffee
- d)Work is done on the system
Correct answer is option 'C'. Can you explain this answer?
Hot coffee in a thermos flask is shaken vigorously, considering it as a system which of the statement is not true?
a)
Temperature of the system rises
b)
Internal energy of the coffee increased
c)
Heat energy has been added to coffee
d)
Work is done on the system
|
|
Preeti Iyer answered |
No, heat is not transferred as the flask is insulated from the surroundings ∴dQ=0
Internal energy of a system increases by 60 J when 140 Jof heat is added to the gaseous system. The amount of work done would be:- a)80 J
- b)100 J
- c)200 J
- d)140 J
Correct answer is option 'A'. Can you explain this answer?
Internal energy of a system increases by 60 J when 140 Jof heat is added to the gaseous system. The amount of work done would be:
a)
80 J
b)
100 J
c)
200 J
d)
140 J
|
|
Lavanya Menon answered |
We know that dq = dU + dW
And as dU = 60 and dq = 140J
We get dW = 140 - 60 = 80J
And as dU = 60 and dq = 140J
We get dW = 140 - 60 = 80J
C3H8 belongs to the homologous series of - a)Alkynes
- b)Alkanes
- c)Alkenes
- d)Cyclo alkanes
Correct answer is option 'B'. Can you explain this answer?
C3H8 belongs to the homologous series of
a)
Alkynes
b)
Alkanes
c)
Alkenes
d)
Cyclo alkanes
|
|
Rajesh Gupta answered |
C3H6 and C4H8 belongs to same homologous series as they both belongs to alkene series whose formula is CnH2n, here n = no. of carbon atoms.
Chapter doubts & questions for First and Second Law of Thermodynamics - Physics for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of First and Second Law of Thermodynamics - Physics for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Physics for EmSAT Achieve
208 videos|329 docs|212 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup