All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Electrolysis for EmSAT Achieve Exam
When Al2O3 is electrolysed ,cation and anions are discharged. For a given quantity of electricity,ratio of number of moles of Al and O2 gas is - a)1 : 1
- b)2 : 1
- c)2 : 3
- d)4 : 3
Correct answer is option 'D'. Can you explain this answer?
When Al2O3 is electrolysed ,cation and anions are discharged. For a given quantity of electricity,ratio of number of moles of Al and O2 gas is
a)
1 : 1
b)
2 : 1
c)
2 : 3
d)
4 : 3
|
|
Niti Mishra answered |
When same quantity of electricity is passed, elements/gases are formed in the ratio of their equivalents
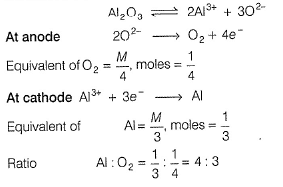 .
.
Only One Option Correct TypeThis section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correctQ. During the electrolysis of aqueous Zn(NO3)2 solution- a)Zn plates out at the cathode
- b)Zn plates out at the anode
- c)H2 gas is evolved at the anode
- d)O2 gas is evolved at the anode
Correct answer is option 'D'. Can you explain this answer?
Only One Option Correct Type
This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
During the electrolysis of aqueous Zn(NO3)2 solution
a)
Zn plates out at the cathode
b)
Zn plates out at the anode
c)
H2 gas is evolved at the anode
d)
O2 gas is evolved at the anode

|
Gauri Khanna answered |
In preference to reduction of Zn2+ at cathode, O2- (aq) is oxidised to O2 at anode.
In the electrorefining of metals, impure metal is- a)cathode and oxidation taken place
- b)anode and oxidation takes place
- c)anode and reduction takes place
- d)cathode and reduction takes place
Correct answer is option 'B'. Can you explain this answer?
In the electrorefining of metals, impure metal is
a)
cathode and oxidation taken place
b)
anode and oxidation takes place
c)
anode and reduction takes place
d)
cathode and reduction takes place

|
Ameya Basu answered |
Electrorefining of Metals:
In the electrorefining process, impure metal is purified through electrolysis.
Impure Metal as Anode:
- In electrorefining, the impure metal is attached to the anode.
- The anode undergoes oxidation, where the impurities present in the metal are oxidized and dissolve into the electrolyte.
- This leaves behind a more pure form of the metal at the cathode.
Therefore, the correct statement is:
Option B: Anode and oxidation takes place
In the electrorefining process, impure metal is purified through electrolysis.
Impure Metal as Anode:
- In electrorefining, the impure metal is attached to the anode.
- The anode undergoes oxidation, where the impurities present in the metal are oxidized and dissolve into the electrolyte.
- This leaves behind a more pure form of the metal at the cathode.
Therefore, the correct statement is:
Option B: Anode and oxidation takes place
A solution of copper(II) sulphate (VI) is electrolysed between copper electrodes by a currrent of 10.0 A for exactly 9650 s.Which remains unchanged?- a)Molar concentration of solution
- b)Cathodic plate
- c)Anodic plate
- d)All of these
Correct answer is option 'A'. Can you explain this answer?
A solution of copper(II) sulphate (VI) is electrolysed between copper electrodes by a currrent of 10.0 A for exactly 9650 s.Which remains unchanged?
a)
Molar concentration of solution
b)
Cathodic plate
c)
Anodic plate
d)
All of these

|
Ishani Patel answered |
0.5 mole of copper is dissolved from the anode. Thus, its mass decreases. 0.5 mole of copper from the anode is deposited at the cathode. Thus, its mass increases.
Thus, molar concentration of aqueous solution of CuSO4 remains unchanged.
100 mL of a buffer of 1 M NH3(aq) and 1 M NH4+(aq) are placed in two volatic cells separately.A current of 1.5 A is passed through both cells for 20 min. If electrolysis of water only takes place2H2O +O2 + 4e- → 4OH- (RHS2H2O → 4H+. + O2 + 4e- (LHS)then pH of the - a)LHS half-cell will increase
- b)RHS half-cell will increase
- c)both half-cell will increase
- d)both half-cell will decrease
Correct answer is option 'B'. Can you explain this answer?
100 mL of a buffer of 1 M NH3(aq) and 1 M NH4+(aq) are placed in two volatic cells separately.A current of 1.5 A is passed through both cells for 20 min. If electrolysis of water only takes place
2H2O +O2 + 4e- → 4OH- (RHS
2H2O → 4H+. + O2 + 4e- (LHS)
then pH of the
a)
LHS half-cell will increase
b)
RHS half-cell will increase
c)
both half-cell will increase
d)
both half-cell will decrease

|
Sinjini Tiwari answered |
In RHS, [NH3] increases hence, pOH decreases
pH = 14 - pOH
Hence, pH of RHS increases.
In LHS, [NH4+] increases, hence pOH increases and pH decreases.
In a fuel cell, following reactions takes place and electricity is produced. AnodicH2+2OH- → 2H2O + 2e-CathodicO2+2H2O + 4e- → 4OH-If 100.8 L of H2 at STP reacts in 96500 s,what is the average current produced (in ampere)? - a)1.0 mol
- b)2.0 mol
- c)3.0 mol
- d)4.0 mol
Correct answer is '9'. Can you explain this answer?
In a fuel cell, following reactions takes place and electricity is produced.
Anodic
H2+2OH- → 2H2O + 2e-
Cathodic
O2+2H2O + 4e- → 4OH-
If 100.8 L of H2 at STP reacts in 96500 s,what is the average current produced (in ampere)?
a)
1.0 mol
b)
2.0 mol
c)
3.0 mol
d)
4.0 mol

|
Anoushka Chopra answered |
Select the correct observation about electrolysis.- a)Electric current is used to drive a non-spontaneous reaction
- b)Ecell is positive and ΔG is negative
- c)Cations and anions are discharged at the cathode and anode respectively
- d)Overvoltage is responsible that a particular reaction takes place
Correct answer is option 'A,C,D'. Can you explain this answer?
Select the correct observation about electrolysis.
a)
Electric current is used to drive a non-spontaneous reaction
b)
Ecell is positive and ΔG is negative
c)
Cations and anions are discharged at the cathode and anode respectively
d)
Overvoltage is responsible that a particular reaction takes place
|
|
Shalini Choudhary answered |
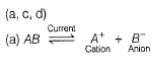
Thus, electric current is used to carry out a non-spontaneous reaction.
Thus. (a) is correct.
(b) Since reactions are reverse of electrochemical cell, hence
Thus. (a) is correct.
(b) Since reactions are reverse of electrochemical cell, hence
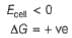
thus, (b) is incorrect
(c) cation goes to cathode and anion goes to anode and are discharged. Thus, correct
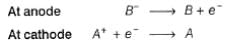
(d) some additional voltage called overvoltage is set up and thus other reactions also take place. thus correct
Passage IIIn hydrogen economy fuel-cell,anodic and cathodic reactions are AnodicH2+2OH- → 2H2O + 2e-CathodicO2+2H2O + 2e- → 4OH-67.2 L H2 at STP react in 15 min.Entire current is used for electro deposition of copper from copper(II) sulphate solution. Average current produced in fuel cell is - a)610.00 A
- b)620.00 A
- c)617.8 A
- d)643.3 A
Correct answer is option 'D'. Can you explain this answer?
Passage II
In hydrogen economy fuel-cell,anodic and cathodic reactions are
Anodic
H2+2OH- → 2H2O + 2e-
Cathodic
O2+2H2O + 2e- → 4OH-
67.2 L H2 at STP react in 15 min.Entire current is used for electro deposition of copper from copper(II) sulphate solution. Average current produced in fuel cell is
a)
610.00 A
b)
620.00 A
c)
617.8 A
d)
643.3 A

|
Shraddha Gupta answered |
1 Faraday of electricity is passed through the solution containing 1 mole each of AgNO3, CuSO4, AlCl3 and SiCl4. Elements are discharged at the cathode.Number of moles of Ag, Cu,Al and Si formed will be in the ratio of - a)1 : 1 : 1 : 1
- b)1 : 2 : 3 : 4
- c)6 : 4 : 3 : 1
- d)12 : 6 : 4 : 3
Correct answer is option 'D'. Can you explain this answer?
1 Faraday of electricity is passed through the solution containing 1 mole each of AgNO3, CuSO4, AlCl3 and SiCl4. Elements are discharged at the cathode.Number of moles of Ag, Cu,Al and Si formed will be in the ratio of
a)
1 : 1 : 1 : 1
b)
1 : 2 : 3 : 4
c)
6 : 4 : 3 : 1
d)
12 : 6 : 4 : 3
|
|
Aarya Khanna answered |
Explanation:
To solve this problem, we need to determine the moles of each element formed at the cathode when 1 Faraday of electricity is passed through the solution containing 1 mole each of AgNO3, CuSO4, AlCl3, and SiCl4.
Step 1: Determine the number of moles of each compound
Given that there is 1 mole of each compound in the solution, the number of moles of AgNO3, CuSO4, AlCl3, and SiCl4 is 1 mole each.
Step 2: Calculate the number of moles of each element
We need to determine the moles of Ag, Cu, Al, and Si formed at the cathode.
Since AgNO3 contains 1 mole of Ag, CuSO4 contains 1 mole of Cu, AlCl3 contains 1 mole of Al, and SiCl4 contains 1 mole of Si, the number of moles of Ag, Cu, Al, and Si formed will be the same as the number of moles of the corresponding compounds.
Therefore, the ratio of moles of Ag, Cu, Al, and Si formed will be 1:1:1:1.
Step 3: Simplify the ratio
The given options are in different ratios, so we need to simplify the ratio of 1:1:1:1.
The simplified ratio of 1:1:1:1 is 12:6:4:3.
Therefore, the correct answer is option D) 12:6:4:3.
The metal that can not be obtained by electrolysis of an aqueous solution of its salt is[JEE Main 2014]- a)Ag
- b)Ca
- c)Cu
- d)Cr
Correct answer is option 'B'. Can you explain this answer?
The metal that can not be obtained by electrolysis of an aqueous solution of its salt is
[JEE Main 2014]
a)
Ag
b)
Ca
c)
Cu
d)
Cr

|
Juhi Deshpande answered |
Higher the position of element in the electrochemical series more difficult is the reduction of its cations.
If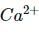 (aq) is electrolysed, water is reduced in preference to it. Hence, it cannot be reduced electrolytically from their aqueous solution.
(aq) is electrolysed, water is reduced in preference to it. Hence, it cannot be reduced electrolytically from their aqueous solution.
If
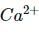 (aq) is electrolysed, water is reduced in preference to it. Hence, it cannot be reduced electrolytically from their aqueous solution.
(aq) is electrolysed, water is reduced in preference to it. Hence, it cannot be reduced electrolytically from their aqueous solution.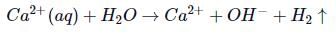
Passage IIIn hydrogen economy fuel-cell,anodic and cathodic reactions are AnodicH2+2OH- → 2H2O + 2e-CathodicO2+2H2O + 2e- → 4OH-67.2 L H2 at STP react in 15 min.Entire current is used for electro deposition of copper from copper(II) sulphate solution. Copper deposited would be - a)1.0 mol
- b)2.0 mol
- c)3.0 mol
- d)4.0 mol
Correct answer is option 'C'. Can you explain this answer?
Passage II
In hydrogen economy fuel-cell,anodic and cathodic reactions are
Anodic
H2+2OH- → 2H2O + 2e-
Cathodic
O2+2H2O + 2e- → 4OH-
67.2 L H2 at STP react in 15 min.Entire current is used for electro deposition of copper from copper(II) sulphate solution. Copper deposited would be
a)
1.0 mol
b)
2.0 mol
c)
3.0 mol
d)
4.0 mol
|
|
Mihir Joshi answered |
-> 2H2O + 4e-
CathodicO2 + 4e- -> 2O2-
CathodicO2 + 4e- -> 2O2-
In the electrolysis of aqueous sodium chloride solution, two types of reactions can take place at anode :I. 2Cl- (aq) → Cl2(g) +2e-II. 2H2O(l)g → O2 (g) + 4H+(aq) + 4e-Select the correct statement(s) about these.- a)Electrode potentials of I and II are quite similar
- b)I is favoured in higher (NaCl) and II is favoured in higher pH.
- c)Because of overvoltage ,Cl2 and not O2 is formed
- d)Electrode potentials of I and II are different
Correct answer is option 'A,B,C'. Can you explain this answer?
In the electrolysis of aqueous sodium chloride solution, two types of reactions can take place at anode :
I. 2Cl- (aq) → Cl2(g) +2e-
II. 2H2O(l)g → O2 (g) + 4H+(aq) + 4e-
Select the correct statement(s) about these.
a)
Electrode potentials of I and II are quite similar
b)
I is favoured in higher (NaCl) and II is favoured in higher pH.
c)
Because of overvoltage ,Cl2 and not O2 is formed
d)
Electrode potentials of I and II are different

|
Janhavi Kaur answered |
(c) Experrnent indicates that the applied voltage required is always greater than the voltage calculated from the standard E0ox or E0red. The additional voltage required is called overvoltage. Due to this, Cl2 and not O2 s formed. This, (c) 6 correct.
Passage IA constant current of 30 A is passed through an aqueous solution of NaCl for a time of 1.00 h.Thus Cl2 formed under STP condition is - a)12.54 L
- b)11.22 L
- c)22.40 L
- d)5.60 L
Correct answer is option 'A'. Can you explain this answer?
Passage I
A constant current of 30 A is passed through an aqueous solution of NaCl for a time of 1.00 h.
Thus Cl2 formed under STP condition is
a)
12.54 L
b)
11.22 L
c)
22.40 L
d)
5.60 L

|
Ameya Basu answered |
- **Given Data**
- Current (I) = 30 A
- Time (t) = 1.00 h
- Molar mass of Cl2 = 71 g/mol
- **Calculating the Amount of Charge Passed**
- Q = I * t
- Q = 30 A * 3600 s (1 hour = 3600 seconds)
- Q = 108000 C
- **Calculating the Moles of Cl2 Formed**
- 1 Faraday (F) of charge = 96500 C
- 1 mole of electrons = 2 moles of Cl2
- Moles of Cl2 = Q / (2F)
- Moles of Cl2 = 108000 C / (2 * 96500 C)
- Moles of Cl2 = 0.561 moles
- **Calculating the Volume of Cl2 at STP**
- Volume of 1 mole of gas at STP = 22.4 L
- Volume of Cl2 formed = Moles of Cl2 * 22.4 L
- Volume of Cl2 formed = 0.561 moles * 22.4 L
- Volume of Cl2 formed = 12.57 L
Therefore, the volume of Cl2 formed under STP conditions is approximately 12.57 L. Since the closest option is 12.54 L (option A), the correct answer is A.
- Current (I) = 30 A
- Time (t) = 1.00 h
- Molar mass of Cl2 = 71 g/mol
- **Calculating the Amount of Charge Passed**
- Q = I * t
- Q = 30 A * 3600 s (1 hour = 3600 seconds)
- Q = 108000 C
- **Calculating the Moles of Cl2 Formed**
- 1 Faraday (F) of charge = 96500 C
- 1 mole of electrons = 2 moles of Cl2
- Moles of Cl2 = Q / (2F)
- Moles of Cl2 = 108000 C / (2 * 96500 C)
- Moles of Cl2 = 0.561 moles
- **Calculating the Volume of Cl2 at STP**
- Volume of 1 mole of gas at STP = 22.4 L
- Volume of Cl2 formed = Moles of Cl2 * 22.4 L
- Volume of Cl2 formed = 0.561 moles * 22.4 L
- Volume of Cl2 formed = 12.57 L
Therefore, the volume of Cl2 formed under STP conditions is approximately 12.57 L. Since the closest option is 12.54 L (option A), the correct answer is A.
Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.- a)E°cell of the net reaction is zero
- b)metal to be refined is dissolved in the solution and deposited at the cathode.
- c)Both (a) and (b)
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.
a)
E°cell of the net reaction is zero
b)
metal to be refined is dissolved in the solution and deposited at the cathode.
c)
Both (a) and (b)
d)
None of the above
|
|
Mihir Joshi answered |
Refining of impure metal is done by electrolysis using the impure metal as the anode. This process is known as electrorefining or electrolytic refining. It is commonly used to purify metals such as copper, zinc, and nickel.
Ecell of the net reaction is zero:
- The Ecell (cell potential) of the net reaction during the electrorefining process is not zero. In fact, it is a positive value. This means that the reaction is spontaneous and can proceed without the need for an external energy source. The positive Ecell indicates that the impure metal at the anode is oxidized and ions are being reduced at the cathode.
Metal to be refined is dissolved in the solution and deposited at the cathode:
- The metal to be refined is indeed dissolved in the electrolyte solution during the electrorefining process. When an electric current is passed through the electrolyte, metal cations from the impure metal at the anode are transferred into the electrolyte as metal ions. These metal ions then migrate towards the cathode, where they are reduced and deposited as pure metal. This deposition of pure metal at the cathode helps in the purification process.
Both (a) and (b):
- The correct statement about the refining of impure metal by electrolysis is that both statement (a) and (b) are correct. The Ecell of the net reaction is not zero, but a positive value indicating the spontaneity of the process. Additionally, the metal to be refined is dissolved in the electrolyte solution and deposited at the cathode, leading to the purification of the metal.
None of the above:
- This option is incorrect as both statement (a) and (b) are correct. The Ecell is not zero, and the metal is dissolved in the solution and deposited at the cathode during the electrorefining process.
In conclusion, the correct statement about the refining of impure metal by electrolysis is that both statement (a) and (b) are correct.
Ecell of the net reaction is zero:
- The Ecell (cell potential) of the net reaction during the electrorefining process is not zero. In fact, it is a positive value. This means that the reaction is spontaneous and can proceed without the need for an external energy source. The positive Ecell indicates that the impure metal at the anode is oxidized and ions are being reduced at the cathode.
Metal to be refined is dissolved in the solution and deposited at the cathode:
- The metal to be refined is indeed dissolved in the electrolyte solution during the electrorefining process. When an electric current is passed through the electrolyte, metal cations from the impure metal at the anode are transferred into the electrolyte as metal ions. These metal ions then migrate towards the cathode, where they are reduced and deposited as pure metal. This deposition of pure metal at the cathode helps in the purification process.
Both (a) and (b):
- The correct statement about the refining of impure metal by electrolysis is that both statement (a) and (b) are correct. The Ecell of the net reaction is not zero, but a positive value indicating the spontaneity of the process. Additionally, the metal to be refined is dissolved in the electrolyte solution and deposited at the cathode, leading to the purification of the metal.
None of the above:
- This option is incorrect as both statement (a) and (b) are correct. The Ecell is not zero, and the metal is dissolved in the solution and deposited at the cathode during the electrorefining process.
In conclusion, the correct statement about the refining of impure metal by electrolysis is that both statement (a) and (b) are correct.
In the refining of silver by electrolytic method, current of 5A is passed for 2 h using 100 g of impure anode of silver (of 95% purity). Weight of silver anode after electrolysis is- a)40.29 g
- b)59.71 g
- c)57.59 g
- d)42.41 g
Correct answer is option 'C'. Can you explain this answer?
In the refining of silver by electrolytic method, current of 5A is passed for 2 h using 100 g of impure anode of silver (of 95% purity). Weight of silver anode after electrolysis is
a)
40.29 g
b)
59.71 g
c)
57.59 g
d)
42.41 g

|
Janhavi Kaur answered |
By Faraday 's first law of electrolysis
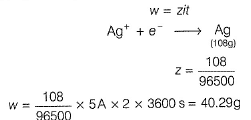
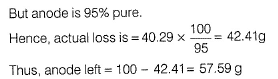
What product are formed during the electrolysis of a concentrated aqueous solution of sodium chloride using an electrolytic cell in which electrodes are separated by a porous pot?I. Cl2(g)
II. NaOH(aq)
III. H2(g)
IV. NaClO(aq)
V. NaClO3(aq) Select the correct choice. - a)I and II
- b)I , II and V
- c)I , IiI and V
- d)I , II and III
Correct answer is option 'D'. Can you explain this answer?
What product are formed during the electrolysis of a concentrated aqueous solution of sodium chloride using an electrolytic cell in which electrodes are separated by a porous pot?
I. Cl2(g)
II. NaOH(aq)
III. H2(g)
IV. NaClO(aq)
V. NaClO3(aq)
II. NaOH(aq)
III. H2(g)
IV. NaClO(aq)
V. NaClO3(aq)
Select the correct choice.
a)
I and II
b)
I , II and V
c)
I , IiI and V
d)
I , II and III

|
Anoushka Chopra answered |
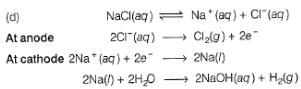
Cl2(g) is formed at anode and NaOH and H2 are formed at cathode. Since, cathode and anode are separated. Hence. there is no reaction between the products formed at the cathode and anode.
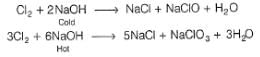
Note: but these reaction do not occur
Note: NaCl is neutral pH = 7 initially. After electrolysis , NaOH is formed
Hence, solution become, basic pH >7
Which pair of electrolysis could be distinguished by the products of electrolysis using inert electrodes?- a)1 M CuSO4 ,1 M CuCl2
- b)1 M KCl ,1 M KI
- c)1 M AgNO3 ,1 M CuSO4
- d)1 M CuBr2 , 1 M NiBr2
Correct answer is option 'B,C'. Can you explain this answer?
Which pair of electrolysis could be distinguished by the products of electrolysis using inert electrodes?
a)
1 M CuSO4 ,1 M CuCl2
b)
1 M KCl ,1 M KI
c)
1 M AgNO3 ,1 M CuSO4
d)
1 M CuBr2 , 1 M NiBr2

|
Asha Nair answered |
a(Cu So4 and CuCl2 changes to Cu and solution becomes colourless
(b) KCl changes to Cl2 (yellow)
KI changes to I2 (voilet)
KI changes to I2 (voilet)
Thus, distinguished.
(c) AgNo3 No change
CuSo4 (blue) changes to colourless
(d) CuBr2 (blue) both changes to colourles.
NiBr2 (Blue)
Comprehension TypeThis section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d) Passage IA constant current of 30 A is passed through an aqueous solution of NaCl for a time of 1.00 h.Thus NaOH formed due to electrolysis is- a)40.2 g
- b)44.8 g
- c)31.1 g
- d)22.4 g
Correct answer is option 'B'. Can you explain this answer?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage I
A constant current of 30 A is passed through an aqueous solution of NaCl for a time of 1.00 h.
Thus NaOH formed due to electrolysis is
a)
40.2 g
b)
44.8 g
c)
31.1 g
d)
22.4 g
|
|
Tanvi Bose answered |
Electrolysis of NaCl Solution
Calculation of NaOH formed
In summary, passing a constant current of 30 A through an aqueous solution of NaCl for 1 hour will result in the production of NaOH and HCl due to electrolysis. To calculate the amount of NaOH formed, we use the formula mentioned above and plug in the given values. The correct answer is option 'B', which is 44.8 g of NaOH formed.
- A constant current of 30 A is passed through an aqueous solution of NaCl for 1 hour
- NaCl undergoes electrolysis and produces NaOH and HCl
- We need to calculate the amount of NaOH formed due to electrolysis
Calculation of NaOH formed
- The formula for calculating the amount of NaOH formed is:
- Amount of NaOH = (Current × Time × Atomic weight of Na × 1 mol of NaOH)/ (2 × Faraday's constant)
- Plugging in the values, we get:
- Amount of NaOH = (30 × 3600 × 23 × 1)/ (2 × 96500) = 44.8 g
- Therefore, the correct answer is option 'B'
In summary, passing a constant current of 30 A through an aqueous solution of NaCl for 1 hour will result in the production of NaOH and HCl due to electrolysis. To calculate the amount of NaOH formed, we use the formula mentioned above and plug in the given values. The correct answer is option 'B', which is 44.8 g of NaOH formed.
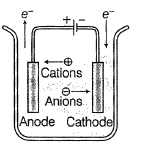
Schematic diagram of an electrolytic cell is- a)
- b)
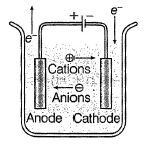
- c)
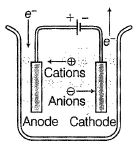
- d)None of these is the correct presentation
Correct answer is option 'B'. Can you explain this answer?
Schematic diagram of an electrolytic cell is
a)
b)
c)
d)
None of these is the correct presentation

|
Baishali Chakraborty answered |
In electrolytic cell,
Cation moves to cathode and anion moves to anode.
Electrons move anode to cathode.
An electrochemical cell was based on the following reaction:Mn(OH)2(s) + H2O2(aq) → MnO2(s) + 2H2O (e)During the opeartion of this for 1 min, 0.135 g of MnO2 was produced. What is the average electric current (in ampere ) produced by the cell?
Correct answer is '5'. Can you explain this answer?
An electrochemical cell was based on the following reaction:
Mn(OH)2(s) + H2O2(aq) → MnO2(s) + 2H2O (e)
During the opeartion of this for 1 min, 0.135 g of MnO2 was produced. What is the average electric current (in ampere ) produced by the cell?

|
Gauri Khanna answered |
What is the key role of hydrogen in the manufacturing process of margarine?- a)It acts as a preservative.
- b)It provides a creamy texture.
- c)It enhances the flavor.
- d)It facilitates hydrogenation of oils.
Correct answer is option 'D'. Can you explain this answer?
a)
It acts as a preservative.
b)
It provides a creamy texture.
c)
It enhances the flavor.
d)
It facilitates hydrogenation of oils.

|
Arizona Academy answered |
Hydrogen plays a crucial role in the manufacturing of margarine by facilitating the hydrogenation of oils. This process involves adding hydrogen to unsaturated fats to convert them into saturated fats, resulting in a semi-solid consistency ideal for margarine production.
What is one of the main reasons for using electroplating with nickel?- a) To create a thicker layer of metal
- b) To enhance the aesthetic appearance of objects
- c) To increase the electrical resistance of objects
- d) To reduce the weight of objects
Correct answer is option 'B'. Can you explain this answer?
What is one of the main reasons for using electroplating with nickel?
a)
To create a thicker layer of metal
b)
To enhance the aesthetic appearance of objects
c)
To increase the electrical resistance of objects
d)
To reduce the weight of objects

|
Arien Instructors answered |
One of the main reasons for using electroplating with nickel is to enhance the aesthetic appearance of objects. Nickel plating not only provides a shiny finish but also improves corrosion resistance, making it popular in jewelry and decorative items.
What ions are present in the electrolysis of acidified water?- a) Na+ and Cl-
- b) Cu2+ and SO42-
- c) K+ and I-
- d) H+ and OH-
Correct answer is option 'D'. Can you explain this answer?
What ions are present in the electrolysis of acidified water?
a)
Na+ and Cl-
b)
Cu2+ and SO42-
c)
K+ and I-
d)
H+ and OH-

|
Arien Instructors answered |
In the electrolysis of acidified water, the ions present include hydrogen ions (H+) and hydroxide ions (OH-). These ions play crucial roles during the electrolysis process, leading to the production of hydrogen and oxygen gases.
Which of the following is not an application of electrolysis?- a)Production of aluminum
- b)Electroplating of jewelry
- c)Electrolysis of water to produce hydrogen fuel
- d)Production of plastics
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not an application of electrolysis?
a)
Production of aluminum
b)
Electroplating of jewelry
c)
Electrolysis of water to produce hydrogen fuel
d)
Production of plastics
|
|
Catalyst Learningacademy answered |
The production of plastics is not an application of electrolysis. However, electrolysis is involved in the production of aluminum, electroplating of jewelry, and electrolysis of water for hydrogen fuel production.
What is the process of electrorefining?- a)Purifying a metal using electrolysis
- b)Refining a metal using heat
- c)Combining two metals using electrolysis
- d)Breaking down a metal using heat
Correct answer is option 'A'. Can you explain this answer?
What is the process of electrorefining?
a)
Purifying a metal using electrolysis
b)
Refining a metal using heat
c)
Combining two metals using electrolysis
d)
Breaking down a metal using heat
|
|
Paradigm Institute answered |
Electrorefining is the process of purifying a metal using electrolysis. It allows impurities to be selectively removed from a metal to obtain a higher purity product.
Which of the following is not a product of the electrolysis of water?- a)Oxygen gas
- b)Hydrogen gas
- c)Sodium hydroxide
- d)Oxygen gas and hydrogen gas are both products
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not a product of the electrolysis of water?
a)
Oxygen gas
b)
Hydrogen gas
c)
Sodium hydroxide
d)
Oxygen gas and hydrogen gas are both products
|
|
Paradigm Institute answered |
Sodium hydroxide is not a product of the electrolysis of water. The products of electrolyzing water are hydrogen gas and oxygen gas.
Which of the following gases is produced at the anode during the electrolysis of brine?- a)Oxygen gas
- b)Hydrogen gas
- c)Chlorine gas
- d)Carbon dioxide gas
Correct answer is option 'C'. Can you explain this answer?
Which of the following gases is produced at the anode during the electrolysis of brine?
a)
Oxygen gas
b)
Hydrogen gas
c)
Chlorine gas
d)
Carbon dioxide gas
|
|
Catalyst Learningacademy answered |
Chlorine gas is produced at the anode during the electrolysis of brine (sodium chloride solution). Oxygen gas is produced at the anode during the electrolysis of water.
What happens at the cathode during electrolysis?- a)Reduction occurs
- b)Oxidation occurs
- c)The electrolyte breaks down
- d)No reaction occurs
Correct answer is option 'A'. Can you explain this answer?
What happens at the cathode during electrolysis?
a)
Reduction occurs
b)
Oxidation occurs
c)
The electrolyte breaks down
d)
No reaction occurs
|
|
Catalyst Learningacademy answered |
At the cathode during electrolysis, reduction occurs. Reduction involves the gain of electrons, leading to the formation of new substances.
What happens at the anode during electrolysis?- a)Oxidation occurs
- b)Reduction occurs
- c)The electrolyte breaks down
- d)No reaction occurs
Correct answer is option 'A'. Can you explain this answer?
What happens at the anode during electrolysis?
a)
Oxidation occurs
b)
Reduction occurs
c)
The electrolyte breaks down
d)
No reaction occurs
|
|
Catalyst Learningacademy answered |
At the anode during electrolysis, oxidation occurs. Oxidation involves the loss of electrons, leading to the formation of new substances.
Which of the following metals can be extracted from their ores using electrolysis?- a)Copper
- b)Silver
- c)Iron
- d)Aluminum
Correct answer is option 'D'. Can you explain this answer?
Which of the following metals can be extracted from their ores using electrolysis?
a)
Copper
b)
Silver
c)
Iron
d)
Aluminum
|
|
Paradigm Institute answered |
Aluminum can be extracted from its ore (bauxite) using electrolysis. Copper, silver, and iron are typically extracted using other methods.
Which of the following best describes electrolysis?- a)The process of breaking down compounds using electricity
- b)The process of breaking down compounds using heat
- c)The process of combining elements using electricity
- d)The process of combining elements using heat
Correct answer is option 'A'. Can you explain this answer?
Which of the following best describes electrolysis?
a)
The process of breaking down compounds using electricity
b)
The process of breaking down compounds using heat
c)
The process of combining elements using electricity
d)
The process of combining elements using heat
|
|
Catalyst Learningacademy answered |
The process of breaking down compounds using electricity is known as electrolysis. It involves the passage of an electric current through an electrolyte to induce a chemical reaction.
In the electrosynthesis,potassium manganate (VII) is converted to manganese(IV) dioxide. By passage of 1F of electrolysis ,one mole of potassium manganate(VII) will form manganese dioxide.- a)1.00 mol
- b)0.50 mol
- c)0.66 mol
- d)0.33 mol
Correct answer is option 'D'. Can you explain this answer?
In the electrosynthesis,potassium manganate (VII) is converted to manganese(IV) dioxide. By passage of 1F of electrolysis ,one mole of potassium manganate(VII) will form manganese dioxide.
a)
1.00 mol
b)
0.50 mol
c)
0.66 mol
d)
0.33 mol

|
Sinjini Tiwari answered |
Manganate (VII) is MnO4- with ON of Mn = + 7
1 mole required 3F of electricity thus, 1 F will reduce only (1/3) mole MnO4- t o MnO2.
Thus, MnO2 formed = 0.33 mol.
1 mole required 3F of electricity thus, 1 F will reduce only (1/3) mole MnO4- t o MnO2.
Thus, MnO2 formed = 0.33 mol.
Matching List TypeChoices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correctFour electrolytic cells containing solution of ZnSO4, AgNO3, CuSO4 and dil. H2SO4 are connected in series. A steady current of 1.5 A was passed through them until 77 mL of O2 gas at STP is formed in anode compartment of H2SO4 solution. Match the different elements/gas formed In Column I with their values in Column II and select the answer from the codes given below. (Cu = 63.5, Zn = 65, Ag = 108, S = 32, H = 1, 0 = 16)
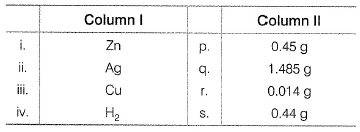 Codes i ii iii iv
Codes i ii iii iv - a)

- b)

- c)
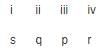
- d)

Correct answer is option 'A'. Can you explain this answer?
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
Four electrolytic cells containing solution of ZnSO4, AgNO3, CuSO4 and dil. H2SO4 are connected in series. A steady current of 1.5 A was passed through them until 77 mL of O2 gas at STP is formed in anode compartment of H2SO4 solution. Match the different elements/gas formed In Column I with their values in Column II and select the answer from the codes given below. (Cu = 63.5, Zn = 65, Ag = 108, S = 32, H = 1, 0 = 16)
Codes i ii iii iv
a)

b)

c)
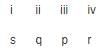
d)


|
Sinjini Tiwari answered |
What type of compounds can undergo electrolysis when an electric current flows through them?- a)Covalent compounds
- b)Ionic compounds
- c)Metallic compounds
- d)Organic compounds
Correct answer is option 'B'. Can you explain this answer?
a)
Covalent compounds
b)
Ionic compounds
c)
Metallic compounds
d)
Organic compounds

|
Arizona Academy answered |
Ionic compounds are capable of undergoing electrolysis when an electric current passes through them. This is because ionic compounds can dissociate into ions in molten form or when dissolved in a solution, allowing the movement of ions to carry the electrical charge. This process leads to the decomposition of the compound into its constituent elements.
Where do negative ions, or anions, move within the electrolyte during electrolysis?- a)Cathode
- b)Anode
- c)Power source
- d)Solution
Correct answer is option 'B'. Can you explain this answer?
a)
Cathode
b)
Anode
c)
Power source
d)
Solution

|
Arizona Academy answered |
Negative ions, or anions, within the electrolyte move towards the anode during electrolysis. At the anode, these anions release electrons as part of oxidation reactions. This movement of anions towards the anode is a key aspect of electrolysis, contributing to the overall flow of charge and the transformation of chemical species in the electrolyte. Understanding the behavior of anions is essential for comprehending the processes that occur during electrolysis.
What is the function of the cathode in an electrolytic cell?- a)Attract anions
- b)Attract cations
- c)Release electrons
- d)Produce positively charged ions
Correct answer is option 'B'. Can you explain this answer?
a)
Attract anions
b)
Attract cations
c)
Release electrons
d)
Produce positively charged ions

|
Arizona Academy answered |
The cathode in an electrolytic cell is the electrode that attracts positively charged ions (cations). When the cations reach the cathode, they can gain electrons and undergo reduction reactions, leading to the formation of new substances or the deposition of metals, depending on the specific electrolysis process.
Which of the following is an example of an electrolyte?- a)Pure water
- b)Vinegar (acetic acid)
- c)Cooking oil
- d)Mercury
Correct answer is option 'B'. Can you explain this answer?
Which of the following is an example of an electrolyte?
a)
Pure water
b)
Vinegar (acetic acid)
c)
Cooking oil
d)
Mercury
|
|
Catalyst Learningacademy answered |
Vinegar (acetic acid) is an example of an electrolyte as it can conduct electricity when dissolved in water. Pure water, cooking oil, and mercury are not electrolytes.
What is the chemical formula for sodium chloride?- a)NaCl2
- b)NaCl
- c)Na2Cl
- d)Na2Cl2
Correct answer is option 'B'. Can you explain this answer?
What is the chemical formula for sodium chloride?
a)
NaCl2
b)
NaCl
c)
Na2Cl
d)
Na2Cl2
|
|
Catalyst Learningacademy answered |
The chemical formula for sodium chloride is NaCl. It is composed of one sodium ion (Na+) and one chloride ion (Cl-).
What is the process of electroplating?- a)Coating a metal object with a layer of gold using electrolysis
- b)Coating a metal object with a layer of plastic using electrolysis
- c)Coating a metal object with a layer of paint using electrolysis
- d)Coating a metal object with a layer of oil using electrolysis
Correct answer is option 'A'. Can you explain this answer?
What is the process of electroplating?
a)
Coating a metal object with a layer of gold using electrolysis
b)
Coating a metal object with a layer of plastic using electrolysis
c)
Coating a metal object with a layer of paint using electrolysis
d)
Coating a metal object with a layer of oil using electrolysis
|
|
Catalyst Learningacademy answered |
Electroplating is the process of coating a metal object with a layer of gold (or other metals) using electrolysis. It is commonly used to enhance the appearance and protect against corrosion.
What is the primary purpose of electroplating?- a)To enhance the appearance of an object
- b)To increase the weight of an object
- c)To reduce the melting point of an object
- d)To decrease the conductivity of an object
Correct answer is option 'A'. Can you explain this answer?
What is the primary purpose of electroplating?
a)
To enhance the appearance of an object
b)
To increase the weight of an object
c)
To reduce the melting point of an object
d)
To decrease the conductivity of an object
|
|
Paradigm Institute answered |
The primary purpose of electroplating is to enhance the appearance of an object by coating it with a layer of a different metal. It can also provide protection against corrosion.
What is the primary role of the strong electrolyte during electrolysis?- a) To facilitate the movement of ions
- b) To decrease the conductivity of the solution
- c) To provide a barrier to ion movement
- d) To increase the temperature of the solution
Correct answer is option 'A'. Can you explain this answer?
What is the primary role of the strong electrolyte during electrolysis?
a)
To facilitate the movement of ions
b)
To decrease the conductivity of the solution
c)
To provide a barrier to ion movement
d)
To increase the temperature of the solution

|
Arien Instructors answered |
The primary role of a strong electrolyte during electrolysis is to facilitate the movement of ions. Strong electrolytes dissociate fully into ions, which enhances the conductivity of the solution and allows for efficient electrochemical reactions to occur.
What is the process of electroplating used for?- a) To add a layer of metal onto an object
- b) To purify metals
- c) To create alloys
- d) To increase the reactivity of metals
Correct answer is option 'A'. Can you explain this answer?
What is the process of electroplating used for?
a)
To add a layer of metal onto an object
b)
To purify metals
c)
To create alloys
d)
To increase the reactivity of metals

|
Arien Instructors answered |
Electroplating is a process used to deposit a layer of metal onto an object. This technique is commonly employed to improve the appearance, corrosion resistance, and durability of the object being plated, making it a valuable method in many industrial applications.
Which of the following ions is preferentially discharged at the anode during the electrolysis of acidified water?- a) O2
- b) OH-
- c) SO42-
- d) H+
Correct answer is option 'B'. Can you explain this answer?
Which of the following ions is preferentially discharged at the anode during the electrolysis of acidified water?
a)
O2
b)
OH-
c)
SO42-
d)
H+

|
Arien Instructors answered |
During the electrolysis of acidified water, hydroxide ions (OH-) are preferentially discharged at the anode. They are oxidized to produce oxygen gas, while hydrogen ions (H+) are discharged at the cathode to produce hydrogen gas.
In the context of electrolysis, what is meant by the term "oxidizing agent"?- a) A substance that reduces other substances
- b) A substance that does not participate in reactions
- c) A substance that accepts electrons
- d) A substance that donates electrons
Correct answer is option 'C'. Can you explain this answer?
In the context of electrolysis, what is meant by the term "oxidizing agent"?
a)
A substance that reduces other substances
b)
A substance that does not participate in reactions
c)
A substance that accepts electrons
d)
A substance that donates electrons

|
Arien Instructors answered |
An oxidizing agent is a substance that accepts electrons during a chemical reaction, leading to the oxidation of another substance. In electrolysis, the species being oxidized at the anode is often the one losing electrons to the oxidizing agent.
Chapter doubts & questions for Electrolysis - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Electrolysis - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup










