All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Thermochemical Equations for EmSAT Achieve Exam
From the following data, the heat of formation of Ca(OH)2(s) at 18°C is ………..kcal: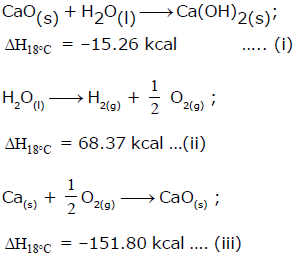
- a)-98.69
- b)-235.43
- c)194.91
- d) 98.69
Correct answer is option 'B'. Can you explain this answer?
From the following data, the heat of formation of Ca(OH)2(s) at 18°C is ………..kcal:
a)
-98.69
b)
-235.43
c)
194.91
d)
98.69

|
Knowledge Hub answered |
The correct answer is Option B.
Ca(s) + O2(g) + H2(g) → Ca(OH)2 , ΔHf = ?
Desired equation = eq (iii) + eq(i) - eq (ii)
ΔHf = (−151.80)+(−15.26)−(−68.37)
ΔHf = (-151.80)+(-15.26)-(-68.37)
ΔHf = −235.43KCalmol−1
ΔHf = (-151.80)+(-15.26)-(-68.37)
ΔHf = −235.43KCalmol−1
The reaction CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g) has ΔH = -25 kCal.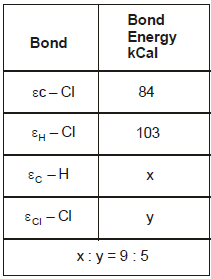 From the given data, what is the bond energy of Cl - Cl bond
From the given data, what is the bond energy of Cl - Cl bond- a)70 kCal
- b)80 kCal
- c) 67.75 kCal
- d) 57.75 kCal
Correct answer is option 'D'. Can you explain this answer?
The reaction CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g) has ΔH = -25 kCal.
From the given data, what is the bond energy of Cl - Cl bond
a)
70 kCal
b)
80 kCal
c)
67.75 kCal
d)
57.75 kCal

|
Kamna Science Academy answered |
During bond breakage energy is absorbed and during bond formation it is released. From the reaction we can say that 1 C-H bond is broken 1 Cl-Cl bond is broken 1 c-cl bond is formed and 1 h-cl bond is formed. so using the sign conventions the equation becomes
x+y-84-103= -25 (∆H = -25)
5x=9y ..putting x=9/5y we get y = 57.75 kCal
x+y-84-103= -25 (∆H = -25)
5x=9y ..putting x=9/5y we get y = 57.75 kCal
NH3(g) + 3Cl2(g)  NCl3(g) + 3HCl(g) ; -ΔH1N2(g) + 3H2(g)
NCl3(g) + 3HCl(g) ; -ΔH1N2(g) + 3H2(g)  2NH3(g) ; ΔH2H2(g) + Cl2(g)
2NH3(g) ; ΔH2H2(g) + Cl2(g)  2HCl(g) ; ΔH3The heat of formation of NCl3 (g) in the terms of ΔH1, ΔH2 and ΔH3 is
2HCl(g) ; ΔH3The heat of formation of NCl3 (g) in the terms of ΔH1, ΔH2 and ΔH3 is- a)
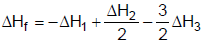
- b)
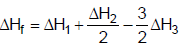
- c)
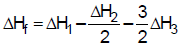
- d) None
Correct answer is option 'A'. Can you explain this answer?
NH3(g) + 3Cl2(g) 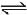 NCl3(g) + 3HCl(g) ; -ΔH1
NCl3(g) + 3HCl(g) ; -ΔH1
N2(g) + 3H2(g) 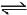 2NH3(g) ; ΔH2
2NH3(g) ; ΔH2
H2(g) + Cl2(g) 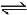 2HCl(g) ; ΔH3
2HCl(g) ; ΔH3
The heat of formation of NCl3 (g) in the terms of ΔH1, ΔH2 and ΔH3 is
a)
b)
c)
d)
None
|
|
Naina Sharma answered |
The formation of NCl3 be like
½ N2 + 3/2Cl2 ⇋ NCl3
We can see that for this setup, we need to have eqn (ii) divided by 2, reversing eqn (iii) and multiplying it by 3/2 and then adding all these to equation (i).
So option a is correct.
½ N2 + 3/2Cl2 ⇋ NCl3
We can see that for this setup, we need to have eqn (ii) divided by 2, reversing eqn (iii) and multiplying it by 3/2 and then adding all these to equation (i).
So option a is correct.
How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -- a)13.7 kcal
- b)27.4 kcal
- c)6.85 kcal
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -
a)
13.7 kcal
b)
27.4 kcal
c)
6.85 kcal
d)
None of the above
|
|
Om Desai answered |
For the reaction of 1 mole of H+ and OH-,we have 13.6 kcal energy released. In H2SO4, we have 2 moles of H+. So for its complete neutralisation, we need 2 moles of NaOH.
So in the end, 2 moles of H+ reacts with2 moles of OH- and 13.6 2 = 27.4 kcal energy is released.
So in the end, 2 moles of H+ reacts with2 moles of OH- and 13.6 2 = 27.4 kcal energy is released.
In the combustion of 4g. of CH4, 2.5 K cal of heat is liberated. The heat of combustion of CH4 is -- a) 20 K. cals
- b) 10 K. cals
- c) 2.5 K. cals
- d)5 K. cals
Correct answer is option 'B'. Can you explain this answer?
In the combustion of 4g. of CH4, 2.5 K cal of heat is liberated. The heat of combustion of CH4 is -
a)
20 K. cals
b)
10 K. cals
c)
2.5 K. cals
d)
5 K. cals
|
|
Riya Banerjee answered |
Mol. wt. of methane =16gm
Heat liberated during the combustion of 4gm methane = 2.5kcal
Heat liberated during the combustion of 16gm methane = 2.5/4×16=10kcal
Hence the heat of combustion of methane is 10 kcal.
Heat liberated during the combustion of 4gm methane = 2.5kcal
Heat liberated during the combustion of 16gm methane = 2.5/4×16=10kcal
Hence the heat of combustion of methane is 10 kcal.
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -- a)T1 = T2
- b)T1 = 2T2
- c)T1 = 4T2
- d)T2 = 9T1
Correct answer is option 'A'. Can you explain this answer?
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -
a)
T1 = T2
b)
T1 = 2T2
c)
T1 = 4T2
d)
T2 = 9T1
|
|
Shreya Gupta answered |
For which of the following change ΔH ≠ ΔE ?
a) H2(g) + I2(g) → 2HI (g)
b) HCl (aq) + NaOH (aq) → NaCl(aq) + H2O (l)
c) C(s) + O2(g) → CO2(g)
d) N2(g) + 3H2(g) → 2NH3(g)
Correct answer is option 'D'. Can you explain this answer?
|
|
Lavanya Menon answered |
∆H = ∆U+∆ngRT
For ∆H not equal ∆U, ∆ng should not equal zero.
This happens only in option d, where ∆ng = -2
For ∆H not equal ∆U, ∆ng should not equal zero.
This happens only in option d, where ∆ng = -2
50.0 mL of 0.10 M HCl is mixed with 50.0 mL of 0.10 M NaOH. The solution temperature rises by 3.0°C Calculate the enthalpy of neutralization per mole of HCl. [take proper assumptions]- a)-2.5 × 102 kJ
- b)-1.3 × 102 kJ
- c)-8.4 × 101 kJ
- d)-6.3 × 101 kJ
Correct answer is option 'A'. Can you explain this answer?
50.0 mL of 0.10 M HCl is mixed with 50.0 mL of 0.10 M NaOH. The solution temperature rises by 3.0°C Calculate the enthalpy of neutralization per mole of HCl. [take proper assumptions]
a)
-2.5 × 102 kJ
b)
-1.3 × 102 kJ
c)
-8.4 × 101 kJ
d)
-6.3 × 101 kJ
|
|
Hansa Sharma answered |
No. of moles of HCl = 5 millimoles
No. of moles of NaOH = 5 millimoles
Mass of solution mixed = 50 gm+50 gm=100 gm
ΔH=−cmΔT(c=4.18 kJKg−1)
⇒ ΔH=−4.18×0.1×3
⇒ ΔH=−1.254 kJ (For 5 millimoles of water formed)
For 1 mole water = −1.254/5×10−3
=−2.5×102kJ
No. of moles of NaOH = 5 millimoles
Mass of solution mixed = 50 gm+50 gm=100 gm
ΔH=−cmΔT(c=4.18 kJKg−1)
⇒ ΔH=−4.18×0.1×3
⇒ ΔH=−1.254 kJ (For 5 millimoles of water formed)
For 1 mole water = −1.254/5×10−3
=−2.5×102kJ
(i) Cis - 2 - butene → trans - 2 - butene, ΔH1 (ii) Cis - 2 - butene → 1 - butene, ΔH2(iii) Trans - 2 - butene is more stable than cis - 2 - butene.(iv) Enthalpy of combustion of 1 - butene, ΔH = -649.8 kcal/mol(v) 9ΔH1 + 5 ΔH2 = 0(vi) Enthalpy of combustion of trans 2 - butene, ΔH = -647.0 kcal/molThe value of ΔH1 & ΔH2 in Kcal/mole are- a)-1.0, 1.8
- b)1.8, -1.0
- c)-5,9
- d)-2, 3, 6
Correct answer is option 'A'. Can you explain this answer?
(i) Cis - 2 - butene → trans - 2 - butene, ΔH1
(ii) Cis - 2 - butene → 1 - butene, ΔH2
(iii) Trans - 2 - butene is more stable than cis - 2 - butene.
(iv) Enthalpy of combustion of 1 - butene, ΔH = -649.8 kcal/mol
(v) 9ΔH1 + 5 ΔH2 = 0
(vi) Enthalpy of combustion of trans 2 - butene, ΔH = -647.0 kcal/mol
The value of ΔH1 & ΔH2 in Kcal/mole are
a)
-1.0, 1.8
b)
1.8, -1.0
c)
-5,9
d)
-2, 3, 6
|
|
Raghav Bansal answered |
Trans-2-butene + 6O2(g) → 4CO2(g) + 4H2O(l)
∆H = -647.0 kcal/mol ---(I)
1 - butene + 6O2(g) → 4CO2(g) + 4H2O(l)
ΔH = -649.8 kcal/mol ---(II)
On (I)-(II)
Trans-2-butene → 1 - butene
ΔH = 2.7 kcal/mol
ΔH = (Hf)1-Butene - (Hf)trans-2-butene
= H2 - H1
H2 - H1 = 2.7 -----(A)
And 9ΔH1 + 5ΔH2 = 0 -----(B)
On solving eqn (A) and (B), we get
H1 = -1.0 and H2 = 1.8
∆H = -647.0 kcal/mol ---(I)
1 - butene + 6O2(g) → 4CO2(g) + 4H2O(l)
ΔH = -649.8 kcal/mol ---(II)
On (I)-(II)
Trans-2-butene → 1 - butene
ΔH = 2.7 kcal/mol
ΔH = (Hf)1-Butene - (Hf)trans-2-butene
= H2 - H1
H2 - H1 = 2.7 -----(A)
And 9ΔH1 + 5ΔH2 = 0 -----(B)
On solving eqn (A) and (B), we get
H1 = -1.0 and H2 = 1.8
If x1, x2 and x3 are enthalpies of H - H, O = O and O - H bonds respectively, and x4 is the enthalpy of vaporisation of water, estimate the standard enthalpy of combustion of hydrogen- a)
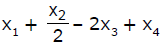
- b)
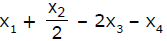
- c)
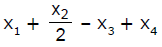
- d)
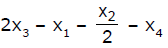
Correct answer is option 'B'. Can you explain this answer?
If x1, x2 and x3 are enthalpies of H - H, O = O and O - H bonds respectively, and x4 is the enthalpy of vaporisation of water, estimate the standard enthalpy of combustion of hydrogen
a)
b)
c)
d)
|
|
Neha Sharma answered |
Combustion of hydrogen:
H2 + ½ O2→H2O (H−O−H)
As water contains two O−H bonds.
So, combustion enthalpy of hydrogen is:
ΔH= Bond energy of reactant − Bond energy of the product − Enthalpy of vaporization.
ΔH=x1+22−2x3−x4
H2 + ½ O2→H2O (H−O−H)
As water contains two O−H bonds.
So, combustion enthalpy of hydrogen is:
ΔH= Bond energy of reactant − Bond energy of the product − Enthalpy of vaporization.
ΔH=x1+22−2x3−x4
. Select the correct order in the following :- a) 1 erg > 1 joule > 1 cal
- b)1 cal > 1 joule > 1 erg
- c) 1 erg > 1 cal > 1 joule
- d)1 joule > 1 cal > 1 erg
Correct answer is option 'B'. Can you explain this answer?
. Select the correct order in the following :
a)
1 erg > 1 joule > 1 cal
b)
1 cal > 1 joule > 1 erg
c)
1 erg > 1 cal > 1 joule
d)
1 joule > 1 cal > 1 erg
|
|
Lavanya Menon answered |
107 erg = 1 joule and 4.2 joules = 1 cal
Therefore 1 cal > 1 joule > 1 erg
Therefore 1 cal > 1 joule > 1 erg
The enthalpy of combustion of a substance -- a)is always positive
- b) is always negative
- c) can be either zero or greater than zero
- d)is unpredictable till calculations are done.
Correct answer is option 'B'. Can you explain this answer?
The enthalpy of combustion of a substance -
a)
is always positive
b)
is always negative
c)
can be either zero or greater than zero
d)
is unpredictable till calculations are done.

|
Mohit Rajpoot answered |
The reason behind is that combustion reactions are always exothermic. So the enthalpy of combustion is always less than zero.
For the allotropic change represented by the equation C (graphite) → C (diamond), ΔH = 1.9 kJ. If 6 g of diamond and 6 g of graphite are separately burnt to yield CO2, the heat liberated in first case is- a) less than in the second case by 1.9 kJ
- b) more than in the second case by 11.4 kJ
- c) more than in the second case by 0.95 kJ
- d) less than in the second case by 11.4 kJ
Correct answer is option 'C'. Can you explain this answer?
For the allotropic change represented by the equation C (graphite) → C (diamond), ΔH = 1.9 kJ. If 6 g of diamond and 6 g of graphite are separately burnt to yield CO2, the heat liberated in first case is
a)
less than in the second case by 1.9 kJ
b)
more than in the second case by 11.4 kJ
c)
more than in the second case by 0.95 kJ
d)
less than in the second case by 11.4 kJ
|
|
Riya Banerjee answered |
∆H given in the question is for one mole of C (g). If 6 gm of diamond and graphite are burnt in oxygen then the C (diamond) will first convert to graphite and then it will form CO2. While C (graphite) will directly form CO2. So due to the conversion of diamond into graphite, we will get extra heat. And since we have 6gm (0.5 mol) of diamond, so the heat released will be 0.5×1.9 kJ or 0.95 kJ more than the second case.
The enthalpy of neutralisation of HCl and NaOH is -57 kJ mol-1. The heat evolved at constant pressure (in kJ) when 0.5 mole of H2SO4 react with 0.75 mole of NaOH is equal to- a)57 ×
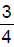
- b) 57 × 0.5
- c)57
- d) 57 × 0.25
Correct answer is option 'A'. Can you explain this answer?
The enthalpy of neutralisation of HCl and NaOH is -57 kJ mol-1. The heat evolved at constant pressure (in kJ) when 0.5 mole of H2SO4 react with 0.75 mole of NaOH is equal to
a)
57 ×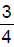
b)
57 × 0.5
c)
57
d)
57 × 0.25

|
Knowledge Hub answered |
-57 kJ of heat evolved when 1 mole of NaOH reacted with an acid.
We have 1 mole of H+ and 0.75 moles of OH-. So OH- iis limiting reagent. Or only 0.75 moles of OH- will be used. So for 1 mole of OH-, we have -57 kJ heat released. Therefore for 0.75 moles, we have ¾× -57 kJ heat released.
We have 1 mole of H+ and 0.75 moles of OH-. So OH- iis limiting reagent. Or only 0.75 moles of OH- will be used. So for 1 mole of OH-, we have -57 kJ heat released. Therefore for 0.75 moles, we have ¾× -57 kJ heat released.
HA + OH- → H2O + A- + q1 kJH+ + OH- → H2O + q2 kJThe enthalpy of dissociation of HA is- a) (q1+ q2)
- b) (q1 - q2 )
- c) (q2 - q1)
- d)- (q1+ q2)
Correct answer is option 'C'. Can you explain this answer?
HA + OH- → H2O + A- + q1 kJ
H+ + OH- → H2O + q2 kJ
The enthalpy of dissociation of HA is
a)
(q1+ q2)
b)
(q1 - q2 )
c)
(q2 - q1)
d)
- (q1+ q2)

|
Tanvi Roy answered |
HA + OH⁻ → H₂O + A⁻ + q1…..(1)
H₂O + q2 → H ⁺ + OH⁻…..(2)
Adding eqn 1 and 2,
HA + q2 → H⁺ + A⁻ + q1
hence , bond dissociation energy of HA is (q2 - q1)
How much heat will be required at constant pressure to form 1.28 kg of CaC2 from CaO(s) & C(s) ?Given :ΔfH°(CaO, s) = -152 kcal/molΔfH°(CaC2, s) = -14 kcal/molΔfH°(CO, g) = -26 kcal/mol- a)+112 kcal
- b) 224 kcal
- c) 3840 kcal
- d) 2240 kcal
Correct answer is option 'D'. Can you explain this answer?
How much heat will be required at constant pressure to form 1.28 kg of CaC2 from CaO(s) & C(s) ?
Given :
ΔfH°(CaO, s) = -152 kcal/mol
ΔfH°(CaC2, s) = -14 kcal/mol
ΔfH°(CO, g) = -26 kcal/mol
a)
+112 kcal
b)
224 kcal
c)
3840 kcal
d)
2240 kcal
|
|
Suresh Iyer answered |
Reaction is CaO + C → CaC2+CO
1.28 kg of CaC2 means 20 mole CaC2 is needed
For 1 mole dH=-26-14-(-152)=112
For 20 Mole 112×20=2240
1.28 kg of CaC2 means 20 mole CaC2 is needed
For 1 mole dH=-26-14-(-152)=112
For 20 Mole 112×20=2240
ΔH for CaCO3(s) → CaO(s) + CO2(g) is 176 kJ mol-1 at 1240 K. The ΔU for the change is equal to :- a)160 kj
- b)165.6 kj
- c)186.3 kj
- d)180.0 kj
Correct answer is option 'B'. Can you explain this answer?
ΔH for CaCO3(s) → CaO(s) + CO2(g) is 176 kJ mol-1 at 1240 K. The ΔU for the change is equal to :
a)
160 kj
b)
165.6 kj
c)
186.3 kj
d)
180.0 kj
|
|
Suresh Reddy answered |
∆H = ∆E + ∆ngRT
176 = ∆E + 1×8.314×1240/1000
∆E = 176 - 10.30 = 165.69 kJ
176 = ∆E + 1×8.314×1240/1000
∆E = 176 - 10.30 = 165.69 kJ
The value of ΔHsol. of BaCl2(s) and BaCl2. 2H2O (s) are – a kJ and b kJ respectively. The value of ΔH Hydration of BaCl2 (s) is-- a)b – a
- b)a + b
- c)– a – b
- d) a – b
Correct answer is option 'C'. Can you explain this answer?
The value of ΔHsol. of BaCl2(s) and BaCl2. 2H2O (s) are – a kJ and b kJ respectively. The value of ΔH Hydration of BaCl2 (s) is-
a)
b – a
b)
a + b
c)
– a – b
d)
a – b
|
|
Divyansh Saha answered |
BaCl2(s) + aq. ⟶ BaCl2 aq.
∆H = -a kJ ------(i)
BaCl2 2H2O(s) + aq. ⟶ BaCl2 aq. + 2H2O
∆H = b kJ -----(ii)
Enthalpy of hydration means that the molecule is just hydeedratt
The reaction should be
(i) - (ii) or
BaCl2(s) + 2H2O ⟶ BaCl2.2H2O ∆H = -a - b kJ
∆H = -a kJ ------(i)
BaCl2 2H2O(s) + aq. ⟶ BaCl2 aq. + 2H2O
∆H = b kJ -----(ii)
Enthalpy of hydration means that the molecule is just hydeedratt
The reaction should be
(i) - (ii) or
BaCl2(s) + 2H2O ⟶ BaCl2.2H2O ∆H = -a - b kJ
Ammonium nitrate can decompose with explosion by the following reaction.NH4NO3 (s) → N2O (g) + 2H2O ;ΔH = -37.0 KJ/molCalculate the heat produced when 2.50g of NH4NO3 decomposes -- a) 1.06 KJ
- b)0.96 KJ
- c)1.16 KJ
- d) 1.26 KJ
Correct answer is option 'C'. Can you explain this answer?
Ammonium nitrate can decompose with explosion by the following reaction.
NH4NO3 (s) → N2O (g) + 2H2O ;
ΔH = -37.0 KJ/mol
Calculate the heat produced when 2.50g of NH4NO3 decomposes -
a)
1.06 KJ
b)
0.96 KJ
c)
1.16 KJ
d)
1.26 KJ
|
|
Sandeep Chawla answered |
NH4NO3 (s) → N2O (g) + 2H2O ; ΔH = -37.0 KJ/mol from the above data, we can show that ΔH is for 1 mole of NH4NO3.
For 2.5 g of NH4NO3, we have 2.5/80 = 0.03125 moles
Therefore, heat released = (-37)×(0.03125) = -1.16 kJ
For 2.5 g of NH4NO3, we have 2.5/80 = 0.03125 moles
Therefore, heat released = (-37)×(0.03125) = -1.16 kJ
The standard molar enthalpies for formation of cyclohexane (l) & benzene (l) at 25°C are -156 & + 49 kJ/mol respectively. The standard enthalpy of hydrogenation of cyclohexane (l) at 25° is -119 kJ mol-1. Use these data to estimate the magnitude of the resonance energy of benzene.- a) 252 kJ mol-1
- b) 240 kJ mol-1
- c)152 kJ mol-1
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
The standard molar enthalpies for formation of cyclohexane (l) & benzene (l) at 25°C are -156 & + 49 kJ/mol respectively. The standard enthalpy of hydrogenation of cyclohexane (l) at 25° is -119 kJ mol-1. Use these data to estimate the magnitude of the resonance energy of benzene.
a)
252 kJ mol-1
b)
240 kJ mol-1
c)
152 kJ mol-1
d)
None of these
|
|
Ananya Sharma answered |
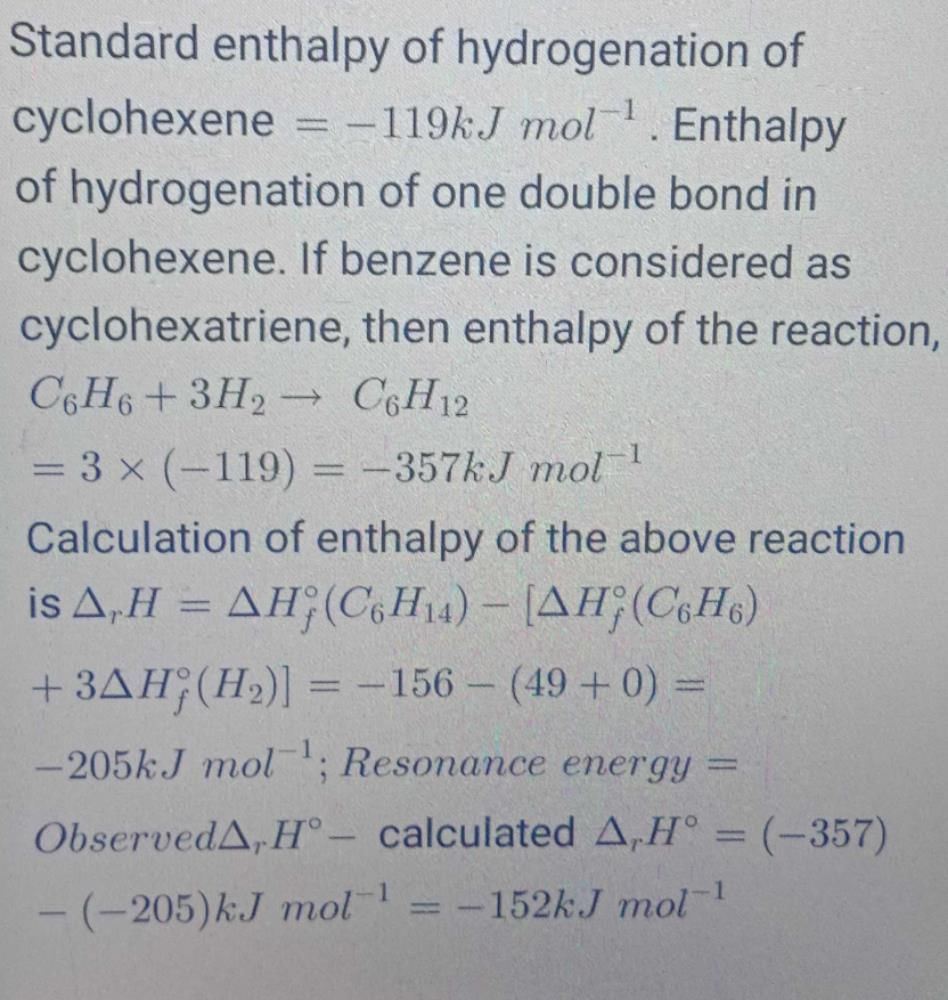
The net heat change in a chemical reaction is same whether it is brought about in two or more different ways in one or several steps. It is known as -- a)Hess's law
- b) Law of conservation of energy
- c)Henry's law
- d)Joule's principle
Correct answer is option 'A'. Can you explain this answer?
The net heat change in a chemical reaction is same whether it is brought about in two or more different ways in one or several steps. It is known as -
a)
Hess's law
b)
Law of conservation of energy
c)
Henry's law
d)
Joule's principle
|
|
Sinjini Pillai answered |
According to Hess law, the thermal effects of a reaction depends upon the initial and final conditions of the reacting substances. It does not depend upon the immediate steps.
For the process, melting of ice at 260 K the ΔH is -- a) Negative
- b) Positive
- c) Zero
- d)Cannot be predicted
Correct answer is option 'B'. Can you explain this answer?
For the process, melting of ice at 260 K the ΔH is -
a)
Negative
b)
Positive
c)
Zero
d)
Cannot be predicted
|
|
Pranavi Banerjee answered |
Enthalpy of fusion (ΔHfus) is 6.01 kJ/mol. This means that for every mole of ice that melts, 6.01 kJ of energy is required to break the bonds between the water molecules and convert the solid into a liquid at a constant temperature of 260 K. This process is endothermic, meaning it absorbs heat from the surroundings as energy is required to break the intermolecular forces holding the ice together. Once all the ice has melted, the temperature of the water will remain constant until all of the ice has completely melted.
The enthalpy of neutralisation of a weak acid in 1 M solution with a strong base is -56.1 kJ mol-1. If the enthalpy of ionization of the acid is 1.5 kJ mol-1 and enthalpy of neutralization of the strong acid with a strong base is -57.3 kJ equiv-1, what is % ionization of the weak acid in molar solution (assume the acid to be monobasic) ?- a)10
- b)15
- c)20
- d)80
Correct answer is option 'C'. Can you explain this answer?
The enthalpy of neutralisation of a weak acid in 1 M solution with a strong base is -56.1 kJ mol-1. If the enthalpy of ionization of the acid is 1.5 kJ mol-1 and enthalpy of neutralization of the strong acid with a strong base is -57.3 kJ equiv-1, what is % ionization of the weak acid in molar solution (assume the acid to be monobasic) ?
a)
10
b)
15
c)
20
d)
80
|
|
Chirag Mehta answered |
The correct answer is option C
Ideally, the enthalpy of neutralization should be - 57.3 K.J + 1.5 K.J = - 55.8 KJ.
But it is - 56.1 KJ.
∴ Energy used for neutralization
= 57.3 - 56.1 = 1.2 KJ
∴ Percent ionization of weak acid
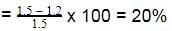
∴ % of weak acid in solution = 20%
Ideally, the enthalpy of neutralization should be - 57.3 K.J + 1.5 K.J = - 55.8 KJ.
But it is - 56.1 KJ.
∴ Energy used for neutralization
= 57.3 - 56.1 = 1.2 KJ
∴ Percent ionization of weak acid
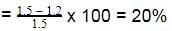
∴ % of weak acid in solution = 20%
Enthalpy of formation of compound is -- a) always positive
- b) always negative
- c) can be either negative or zero
- d)can be positive or negative
Correct answer is option 'D'. Can you explain this answer?
Enthalpy of formation of compound is -
a)
always positive
b)
always negative
c)
can be either negative or zero
d)
can be positive or negative
|
|
Rajat Patel answered |
More the enthalpy of formation less will be the stability. Heat of formation may be positive or negative. Stability of exothermic compound is more than that of endothermic compound, therefore greater the liberated energy greater is the stability.
ΔrH of which of the following reactions is zero ?- a) H2(g) → 2H+(g) + 2e-
- b)2H(g) + aq → 2H+(aq) + 2e-
- c)2H(g) → 2H+(g) + 2e-
- d) H2(g) + aq → 2H+(aq) + 2e-
Correct answer is option 'D'. Can you explain this answer?
ΔrH of which of the following reactions is zero ?
a)
H2(g) → 2H+(g) + 2e-
b)
2H(g) + aq → 2H+(aq) + 2e-
c)
2H(g) → 2H+(g) + 2e-
d)
H2(g) + aq → 2H+(aq) + 2e-
|
|
Megha Basak answered |
In equation d, H+(aq) is formed from H2(g). Enthalpy of formation of both entity is considered to be zero. So, ∆rH (∆Hproduct - ∆Hreactant) is zero.
According to Hess's Law the thermal effect of a reaction depends on -- a)Initial concentration of reactants
- b)Final condition of the reacting substance
- c)Intermediate states of a reaction
- d) Initial and final conditions of the reacting substances
Correct answer is option 'D'. Can you explain this answer?
According to Hess's Law the thermal effect of a reaction depends on -
a)
Initial concentration of reactants
b)
Final condition of the reacting substance
c)
Intermediate states of a reaction
d)
Initial and final conditions of the reacting substances

|
Madhavan Patel answered |
Hess's law states that the change of enthalpy in a chemical reaction (i.e. the heat of reaction at constant pressure) is independent of the pathway between the initial and final states. Hess's law allows the enthalpy change (ΔH) for a reaction to be calculated even when it cannot be measured directly.
When enthalpy of reactants is higher than product then reaction will be- a)endothermic
- b)spontaneous
- c)non-spontaneous
- d)exothermic
Correct answer is option 'D'. Can you explain this answer?
When enthalpy of reactants is higher than product then reaction will be
a)
endothermic
b)
spontaneous
c)
non-spontaneous
d)
exothermic
|
|
Rajat Patel answered |
The enthalpies of these reactions are less than zero, and are therefore exothermic reactions. A system of reactants that absorbs heat from the surroundings in an endothermic reaction has a positive \(ΔH\), because the enthalpy of the products is higher than the enthalpy of the reactants of the system.
Reaction involving gold have been of particular interest to a chemist. Consider the following reactions.Au(OH)3 + 4 HCl → HAuCl4 + 3H2O, ΔH = -28 kcalAu(OH)3 + 4 HBr → HAuBr4 + 3 H2O, ΔH = -36.8 kcalIn an experiment there was an absorption of 0.44 kcal when one mole of HAuBr4 was mixed with 4 moles of HCl. What is the percentage conversion of HAuBr4 into HAuCl4 ?- a) 0.5 %
- b)0.6 %
- c)5 %
- d)50 %
Correct answer is option 'C'. Can you explain this answer?
Reaction involving gold have been of particular interest to a chemist. Consider the following reactions.
Au(OH)3 + 4 HCl → HAuCl4 + 3H2O, ΔH = -28 kcal
Au(OH)3 + 4 HBr → HAuBr4 + 3 H2O, ΔH = -36.8 kcal
In an experiment there was an absorption of 0.44 kcal when one mole of HAuBr4 was mixed with 4 moles of HCl. What is the percentage conversion of HAuBr4 into HAuCl4 ?
a)
0.5 %
b)
0.6 %
c)
5 %
d)
50 %

|
Nabanita Deshpande answered |
Au(OH)3 + 4HCl → HAuCl4+ 3H2O…(1) ∆H₁=-28kcal
Au(OH)3 + 4HBr → HAuBr4 + 3H2O ...(2) ∆H₂= -36.8kcal
To convert HAuBr4 to HAuCl4, the net reaction is
HAuBr4 + 4HCl→ HAuCl4 + 4HBr ...∆H=?
For the above reaction:
∆H =∆H₁ - ∆H₂ = -28 - (-36.8) = 8.8 kcal
Thus to convert one mole of HAuBr4 to HAuCl4 we require 8.8 kcal energy but since the energy absorbed is 0.44 kcal.
hence %conversion =[(0.44)/(8.8)] x 100
= 5%
Hence the percentage conversion is 5%.
From the following data at 25°C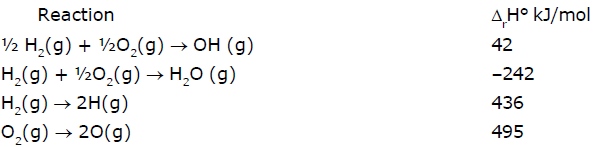 Which of the following statement(s) is/are correct :
Which of the following statement(s) is/are correct :
Statement(a) : ΔrH° for the reaction H2O(g) → 2H(g) + O(g) is 925 kJ/mol
Statement(b) : ΔrH° for the reaction OH(g) → H(g) + O(g) is 502 kJ/mol
Statement(c) : Enthalpy of formation of H(g) is-–218 kJ/mol
Statement(d) : Enthalpy of formation of OH(g) is 42 kJ/mol- a)Statement c
- b)Statement a, b, d
- c)Statement b,c
- d)Statement a, d
Correct answer is option 'D'. Can you explain this answer?
From the following data at 25°C
Which of the following statement(s) is/are correct :
Statement(a) : ΔrH° for the reaction H2O(g) → 2H(g) + O(g) is 925 kJ/mol
Statement(b) : ΔrH° for the reaction OH(g) → H(g) + O(g) is 502 kJ/mol
Statement(c) : Enthalpy of formation of H(g) is-–218 kJ/mol
Statement(d) : Enthalpy of formation of OH(g) is 42 kJ/mol
Statement(a) : ΔrH° for the reaction H2O(g) → 2H(g) + O(g) is 925 kJ/mol
Statement(b) : ΔrH° for the reaction OH(g) → H(g) + O(g) is 502 kJ/mol
Statement(c) : Enthalpy of formation of H(g) is-–218 kJ/mol
Statement(d) : Enthalpy of formation of OH(g) is 42 kJ/mol
a)
Statement c
b)
Statement a, b, d
c)
Statement b,c
d)
Statement a, d
|
|
Arya Dasgupta answered |

Let's check statement (a)
Statement(a) : ΔrH° for the reaction H2O(g) → 2H(g) + O(g) is 925 kJ/mol
For this, we need - (II) + (III) + ½ (IV)
We get, H2O(g) → 2H(g) + O(g) - (-242)+436+½ 495 = 925.5 kJ mol-1
So it is true.
Let's check statement (b)
Statement(b) : ΔrH° for the reaction OH(g) → H(g) + O(g) is 502 kJ/mol
For this we need -(I)+½ (III)+½ (IV)
We get OH(g) → H(g) + O(g) -(42) + ½ (436) + ½ (495) = 423.5 kJ mol-1
So statement (b) is wrong.
Let's check statement (c)
Statement(c) : Enthalpy of formation of H(g) is -218 kJ/mol
We can see that for enthalpy of formation, we need to divide eqn (III) by 2
So, it would become :-
½ H2(g) → H(g)
436/2 = 218 kJ
So, statement (c) is wrong.
Let's check statement (d)
Statement(d) : Enthalpy of formation of OH(g) is 42 kJ/mol
For that, we have eqn (I) as it is. So, statement (d) is correct.
For hypothetical reaction -A(g) + B (g) → C (g) + D (g)Which of the following statements is correct -- a)ΔH = ΔE
- b)ΔH > ΔE
- c)ΔH < ΔE
- d) unpredictable
Correct answer is option 'A'. Can you explain this answer?
For hypothetical reaction -
A(g) + B (g) → C (g) + D (g)
Which of the following statements is correct -
a)
ΔH = ΔE
b)
ΔH > ΔE
c)
ΔH < ΔE
d)
unpredictable
|
|
Pragati Singh answered |
Explanation:
Definition of ΔH and ΔE:
- ΔH represents the change in enthalpy of a system during a reaction, which is the heat transfer at constant pressure.
- ΔE represents the change in internal energy of a system during a reaction, which is the heat transfer at constant volume.
Relationship between ΔH and ΔE:
- In the given reaction, since the reactants and products are all in the gaseous phase, there is no change in volume during the reaction. Therefore, the reaction can be assumed to occur at constant volume.
- At constant volume, the change in enthalpy (ΔH) is equal to the change in internal energy (ΔE) plus the product of pressure and change in volume (ΔV), but as there is no change in volume, ΔH is equal to ΔE.
- Hence, the correct statement is ΔH = ΔE.
Therefore, option 'a' is correct, and the relationship between ΔH and ΔE in this hypothetical reaction is that they are equal due to the constant volume assumption.
Definition of ΔH and ΔE:
- ΔH represents the change in enthalpy of a system during a reaction, which is the heat transfer at constant pressure.
- ΔE represents the change in internal energy of a system during a reaction, which is the heat transfer at constant volume.
Relationship between ΔH and ΔE:
- In the given reaction, since the reactants and products are all in the gaseous phase, there is no change in volume during the reaction. Therefore, the reaction can be assumed to occur at constant volume.
- At constant volume, the change in enthalpy (ΔH) is equal to the change in internal energy (ΔE) plus the product of pressure and change in volume (ΔV), but as there is no change in volume, ΔH is equal to ΔE.
- Hence, the correct statement is ΔH = ΔE.
Therefore, option 'a' is correct, and the relationship between ΔH and ΔE in this hypothetical reaction is that they are equal due to the constant volume assumption.
Chapter doubts & questions for Thermochemical Equations - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermochemical Equations - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









