All Exams >
MCAT >
General Chemistry for MCAT >
All Questions
All questions of Covalent Bonds for MCAT Exam
What is the systematic name of the following amino acid, which is commonly known as asparagine?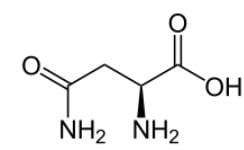
- a)(R)-2-amino-3-carboxylbutanamide
- b)(S)-2-amino-3-carbomylpropanoic acid
- c)(R)-3-amino-3-carboxylpropanamide
- d)(S)-2-amino-4-carbomylbutanoic acid
Correct answer is option 'B'. Can you explain this answer?
What is the systematic name of the following amino acid, which is commonly known as asparagine?

a)
(R)-2-amino-3-carboxylbutanamide
b)
(S)-2-amino-3-carbomylpropanoic acid
c)
(R)-3-amino-3-carboxylpropanamide
d)
(S)-2-amino-4-carbomylbutanoic acid
|
|
Ayesha Joshi answered |
In naming any compound systemically, we must determine the primary substituent group which will become the suffix. Here it is the carboxylic acid group. We are looking for some kind of -oic acid.
We can determine the stereochemistry of the compound, which is (S), and that is shown below:
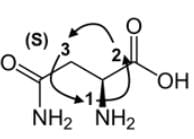
Technically, we should have the answer with those two pieces of information, but there are two answers with To be thorough, the next step in IUPAC nomenclature is to find the longest carbon chain starting at the carbon of the carboxylic acid. It would seem it would be 4 carbons, but it will be only 3 carbons because of the amide group as a prefix.
The amine group at C-2 will become 2-amino, and the amide group attached at C-3, the substituent including the carbon, will become 3-carbamoyl and not 4-carbamoy
The name of the compound is (S)-2-amino-3-carbomylpropanoic acid.
The parent compound, 2-butanol, undergoes a set of reactions to produce compounds A and B. Which of the following statements accurately describes the absolute and relative configurations of the compounds in the reaction series below?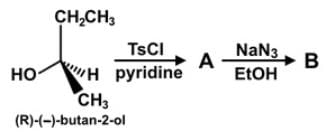
- a)The relative configurations of A and B can be determined to be (R) and (S), while the absolute and relative configurations of the parent is already known
- b)The relative configurations of the products are (-) and (+), while the absolute configuration of 2-butanol has to be determined.
- c)The relative configurations of A and B can be determined to be (S) and (R), and the absolute configurations cannot be determined without further experimentation.
- d)The absolute configurations of A and B can be determined to be (R) and (S), but the relative configurations cannot be determined without further experimentation.
Correct answer is option 'A'. Can you explain this answer?
The parent compound, 2-butanol, undergoes a set of reactions to produce compounds A and B. Which of the following statements accurately describes the absolute and relative configurations of the compounds in the reaction series below?

a)
The relative configurations of A and B can be determined to be (R) and (S), while the absolute and relative configurations of the parent is already known
b)
The relative configurations of the products are (-) and (+), while the absolute configuration of 2-butanol has to be determined.
c)
The relative configurations of A and B can be determined to be (S) and (R), and the absolute configurations cannot be determined without further experimentation.
d)
The absolute configurations of A and B can be determined to be (R) and (S), but the relative configurations cannot be determined without further experimentation.
|
|
Ayesha Joshi answered |
When 2-butanol is placed in pyridine with 4-toluenesulfonyl chloride, the alcohol is converted into the tosylate, which makes it a better leaving group. The reaction is known to occur with retention of configuration,here is no change in the stereochemistry.
When the tosylate is then placed into a sodium azide and ethanol solution, the azide acts as a nucleophile in a SN2 fashion to attack from the backside and substitute the tosylate with an azide group. There is an inversion of stereochemistry due to the backside attack.
2-butanol has a configuration of (R), so then compound A will have an (R) configuration and compound B an (S) configuration due to the inversion. These are our relative configurations of A and B.
Since we know the sign of rotation and the designation of R or S for the parent, we have its absolute configuration.
While for compounds A and B, without further experimentation, we do not ascertain whether R corresponds to (+) or (-). But, because you know the configuration of the starting alcohol, then the absolute configuration of the product is also known.
The answer is that the relative configurations of A and B can be determined to be (R) and (S), while the absolute and relative configurations of the parent is already known.
Which of the following statements most accurately describes the details of the hybridization of sulfur hexafluoride?- a)The sulfur atom undergoes excitation to promote two electrons from the 2s orbital to two empty 3d orbitals.
- b)Sulfur forms six σ hybridized bonds with fluorine atoms, and each fluorine atom makes use of a half-filled 3pz orbital for the bond formation.
- c)The orbitals are all oriented such that all bond angles are 90° and have an octahedral configuration.
- d)In the excited state, intermixing of one s, three p and three d orbitals gives 6 half-filled sp2d3 hybrid orbitals.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements most accurately describes the details of the hybridization of sulfur hexafluoride?
a)
The sulfur atom undergoes excitation to promote two electrons from the 2s orbital to two empty 3d orbitals.
b)
Sulfur forms six σ hybridized bonds with fluorine atoms, and each fluorine atom makes use of a half-filled 3pz orbital for the bond formation.
c)
The orbitals are all oriented such that all bond angles are 90° and have an octahedral configuration.
d)
In the excited state, intermixing of one s, three p and three d orbitals gives 6 half-filled sp2d3 hybrid orbitals.
|
|
Bella Perry answered |
Understanding Sulfur Hexafluoride Hybridization
Sulfur hexafluoride (SF6) is a molecular compound where sulfur is centrally located and bonded to six fluorine atoms. The hybridization and bonding details provide insight into its geometric structure.
Excitation and Hybridization
- In the formation of SF6, the sulfur atom undergoes excitation, where an electron from the 3s orbital is promoted to the 3d orbital. This is crucial for accommodating six bonding pairs.
- Sulfur utilizes one 3s orbital, three 3p orbitals, and two 3d orbitals to create six equivalent hybrid orbitals known as sp3d2 hybrid orbitals.
Bond Formation
- Each of the six sp3d2 hybrid orbitals forms a sigma bond with a fluorine atom. Each fluorine atom contributes a half-filled 2p orbital for bond formation.
Molecular Geometry
- The resulting molecular geometry of SF6 is octahedral. This configuration allows for optimal spatial arrangement of the bonded fluorine atoms.
- The bond angles in an octahedral structure are exactly 90 degrees, confirming that the statement regarding bond angles in option 'C' is accurate.
Conclusion
- The hybridization of sulfur in SF6 is a clear example of how electron promotion and orbital hybridization lead to specific molecular geometries, confirming that option 'C' accurately describes the details of sulfur hexafluoride hybridization.
Sulfur hexafluoride (SF6) is a molecular compound where sulfur is centrally located and bonded to six fluorine atoms. The hybridization and bonding details provide insight into its geometric structure.
Excitation and Hybridization
- In the formation of SF6, the sulfur atom undergoes excitation, where an electron from the 3s orbital is promoted to the 3d orbital. This is crucial for accommodating six bonding pairs.
- Sulfur utilizes one 3s orbital, three 3p orbitals, and two 3d orbitals to create six equivalent hybrid orbitals known as sp3d2 hybrid orbitals.
Bond Formation
- Each of the six sp3d2 hybrid orbitals forms a sigma bond with a fluorine atom. Each fluorine atom contributes a half-filled 2p orbital for bond formation.
Molecular Geometry
- The resulting molecular geometry of SF6 is octahedral. This configuration allows for optimal spatial arrangement of the bonded fluorine atoms.
- The bond angles in an octahedral structure are exactly 90 degrees, confirming that the statement regarding bond angles in option 'C' is accurate.
Conclusion
- The hybridization of sulfur in SF6 is a clear example of how electron promotion and orbital hybridization lead to specific molecular geometries, confirming that option 'C' accurately describes the details of sulfur hexafluoride hybridization.
Which of the following molecules does NOT have an atom that is sp-hybridized?- a)NO2
- b)
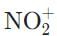
- c)HC≡C-
- d)CO2-
Correct answer is option 'A'. Can you explain this answer?
Which of the following molecules does NOT have an atom that is sp-hybridized?
a)
NO2
b)
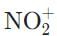
c)
HC≡C-
d)
CO2-
|
|
Ayesha Joshi answered |
The acetylide ion (HCC‾ ) has 2 sp-hybridized carbons due to the triple bond since two unhybridized p orbitals are needed to form the two π bonds. The C-H bond is a sigma bond formed from the overlap of a sp hybridized and 1s orbital.
The nitronium ion (NO2⁺) has an sp-hybridized nitrogen, and the cation is formed from removing the lone electron from paramagnetic NO. The nitrogen is double-bonded to each of the oxygens necessitating two unhybridized p orbitals to form the two π bonds.
Carbon dioxide should be familiar as a linear molecule, in which the carbon has no lone pairs. The central carbon is sp-hybridized since it is double-bonded to each of the oxygens.
The nitrite ion (NO2‾) will be sp2-hybridized since nitrogen forms a π bond with one of the oxygens through an unhybridized p orbital. The other orbitals become hybridized into sp2, and nitrogen forms a hybridized bond to each of the oxygens and has a lone pair in the other hybridized orbital. One oxygen is single-bonded and has a negative charge, and the other is double-bonded and neutral.
Sanglifehrin A (SFA) is a novel cyclophilin-binding compound showing immunosuppressive activity, but its role has not been entirely elucidated yet. How many stereogenic centers (R,S; E/Z) are there in the following compound sanglifehrin A?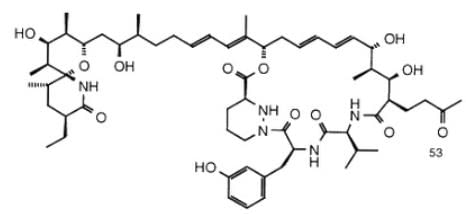
- a)21
- b)17
- c)26
- d)18
Correct answer is option 'A'. Can you explain this answer?
Sanglifehrin A (SFA) is a novel cyclophilin-binding compound showing immunosuppressive activity, but its role has not been entirely elucidated yet. How many stereogenic centers (R,S; E/Z) are there in the following compound sanglifehrin A?

a)
21
b)
17
c)
26
d)
18
|
|
Ayesha Joshi answered |
This is an exercise in counting the total number of stereogenic centers. Stereogenic centers include chiral carbons and double-bonded carbons in cis-trans alkenes.
On the left half of the molecule, there are 9 asymmetric carbons and 2 double bonds:
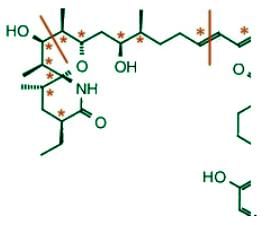
On the right half of the molecule, there are 8 asymmetric carbons and 2 double bonds:
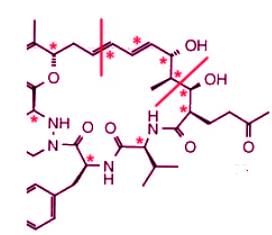
There are 21 stereogenic centers in total in this compound.
How many stereogenic centers are there in the following tetrasubstituted adamantane molecule?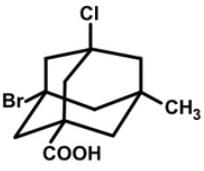
- a)0
- b)1
- c)4
- d)6
Correct answer is option 'B'. Can you explain this answer?
How many stereogenic centers are there in the following tetrasubstituted adamantane molecule?

a)
0
b)
1
c)
4
d)
6
|
|
Ayesha Joshi answered |
The adamantane derivative features four asymmetric substituted carbon atoms, and it only exists as two stereoisomers (enantiomers) rather than the sixteen predicted by the 2n rule. However, the number of observed stereoisomers is only 2 since once the first stereocenter is defined, the remaining three are determined by their relative configuration.
The configurations of the asymmetric bridgehead carbon units in this structure are (R), (S), (R) and (R) respectively, for the Br, Cl, CO2H and CH3 substituents.
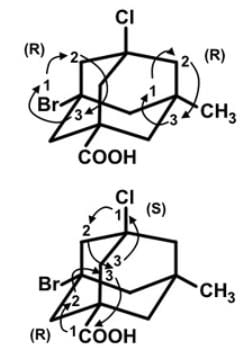
The central adamantyl residue may be regarded as an extended tetrahedron, and the stereogenic center is located in the center of the adamantyl cage, shown by the dotted lines below connecting at the center of the molecule, and the configuration shown here is (R).
Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?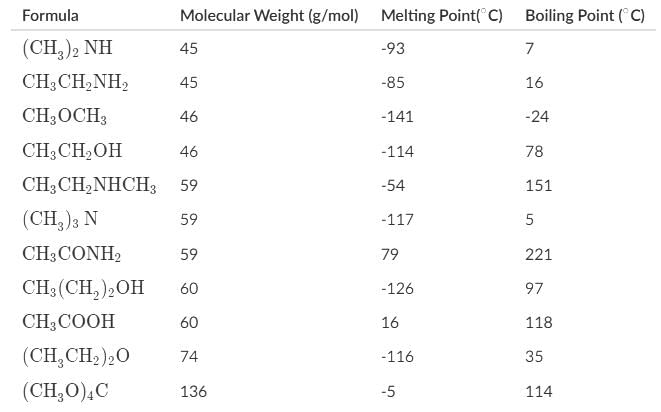
- a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.
- b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.
- c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.
- d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?

a)
The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.
b)
Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.
c)
Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.
d)
Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.
|
|
Ayesha Joshi answered |
Alcohols, carboxylic acids, and only 1° and 2° amines and amides may serve as H-bond donors and acceptors. Ethers, ketones, 3° amines and amides can serve as acceptors.
Carboxylic acids generally have high boiling points, but amides can technically have boiling points almost 100 degrees greater than carboxylic acids
The dimeric H-bonded structure appears to be a good representation of acetic acid in both the gaseous and liquid state. If acetic acid dimerized, it would have a molecular weight of 120 and a boiling point similar to a molecule of that weight.
Tetramethoxymethane is a molecule with a molecular weight of 136, which is sufficiently close to 120, and a boiling point of 114. The similar boiling point with acetic acid confirms our suspicions that it dimerized in liquid and gaseous phases.
Amides indeed have high melting points, which makes most of them solids at room temperatures. Amides have higher boiling points than carboxylic acids due to its highly polar carbonyl group as indicated by its resonance structure, leaving carbon with a negative charge and nitrogen with a positive charge, and the H-bonding between primary and secondary amides.
Which of the following pairs of atoms is likely to form a covalent bond?- a)Sodium and chlorine
- b)Lithium and fluorine
- c)Potassium and oxygen
- d)Carbon and hydrogen
Correct answer is option 'D'. Can you explain this answer?
Which of the following pairs of atoms is likely to form a covalent bond?
a)
Sodium and chlorine
b)
Lithium and fluorine
c)
Potassium and oxygen
d)
Carbon and hydrogen
|
|
Ayesha Joshi answered |
Carbon and hydrogen are both nonmetals and tend to form covalent bonds when they combine.
Which of the following statements about covalent bonds is true?- a)Covalent bonds involve the transfer of electrons.
- b)Covalent bonds are formed between a metal and a nonmetal.
- c)Covalent bonds result from the sharing of electrons.
- d)Covalent bonds are typically stronger than ionic bonds.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements about covalent bonds is true?
a)
Covalent bonds involve the transfer of electrons.
b)
Covalent bonds are formed between a metal and a nonmetal.
c)
Covalent bonds result from the sharing of electrons.
d)
Covalent bonds are typically stronger than ionic bonds.
|
|
Ayesha Joshi answered |
In covalent bonds, electrons are shared between atoms, allowing them to achieve a more stable electron configuration.
Which of the following elements typically forms covalent bonds?- a)Sodium
- b)Oxygen
- c)Calcium
- d)Potassium
Correct answer is option 'B'. Can you explain this answer?
Which of the following elements typically forms covalent bonds?
a)
Sodium
b)
Oxygen
c)
Calcium
d)
Potassium
|
|
Ayesha Joshi answered |
Covalent bonds are formed between nonmetallic elements. Oxygen is a nonmetal and therefore tends to form covalent bonds.
Chapter doubts & questions for Covalent Bonds - General Chemistry for MCAT 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Covalent Bonds - General Chemistry for MCAT in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
General Chemistry for MCAT
164 videos|11 docs|16 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









