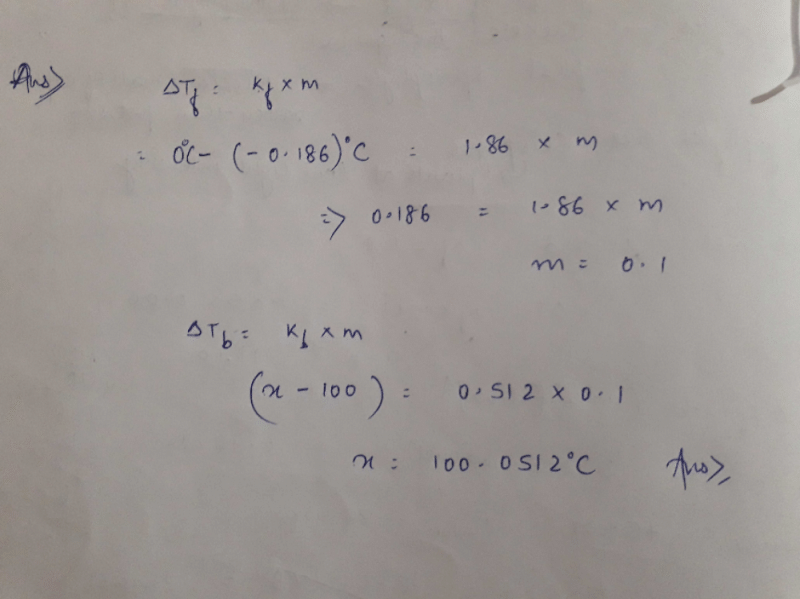All Exams >
JEE >
Chapter-wise Tests for JEE Main & Advanced >
All Questions
All questions of Solutions for JEE Exam
When the solvent is in solid state, solution is- a)Solid solution
- b)Gaseous solution
- c)Solution
- d)Liquid solution
Correct answer is option 'A'. Can you explain this answer?
When the solvent is in solid state, solution is
a)
Solid solution
b)
Gaseous solution
c)
Solution
d)
Liquid solution

|
Aditi answered |
When both the solute and solvent are in solid state ,then the solution is called as solid solution or solid sol .
Molality is expressed in- a)mol/kg
- b)mol/L
- c)L/mol
- d)g/L
Correct answer is option 'A'. Can you explain this answer?
Molality is expressed in
a)
mol/kg
b)
mol/L
c)
L/mol
d)
g/L

|
Siddharth Abimanyu answered |
Molality=moles of solute/kg of solvent... so option A
Equal volume of 1 M urea and 1 M glucose are mixed. the mixture will have- a)same osmotic pressure
- b)lower osmatic pressure
- c)higher osmotic pressure
- d)none of these
Correct answer is option 'A'. Can you explain this answer?
Equal volume of 1 M urea and 1 M glucose are mixed. the mixture will have
a)
same osmotic pressure
b)
lower osmatic pressure
c)
higher osmotic pressure
d)
none of these
|
|
Anaya Patel answered |
The correct answer is option A
The mixture will have the same osmotic pressure as the two individual values.
πurea=0.1×ST
πglucose=0.1×ST
πmix=(0.1ST+0.1ST) / 2
=0.1ST
The mixture will have the same osmotic pressure as the two individual values.
πurea=0.1×ST
πglucose=0.1×ST
πmix=(0.1ST+0.1ST) / 2
=0.1ST
The number of moles of KCl in 3 L of 3 M solution is- a)27 moles
- b)1 moles
- c)9 moles
- d)3 moles
Correct answer is option 'C'. Can you explain this answer?
The number of moles of KCl in 3 L of 3 M solution is
a)
27 moles
b)
1 moles
c)
9 moles
d)
3 moles
|
|
Om Desai answered |
In 3M there will be 3 moles per litre therefore in 3 litres there will be 9 moles.
the molarity ofa solution obtained by mixing 750 ml of 0.5(M) hcl with 250ml of 2(M) hcl will be?- a)1.25 M
- b)2.5 M
- c)1.00 M
- d)0.875 M
Correct answer is option 'D'. Can you explain this answer?
the molarity ofa solution obtained by mixing 750 ml of 0.5(M) hcl with 250ml of 2(M) hcl will be?
a)
1.25 M
b)
2.5 M
c)
1.00 M
d)
0.875 M
|
|
Vilas Kumar answered |
M = M1V1+ M2V2 / V1+V2. M = 750*0.5+250*2 / 750+250. M = 875 / 1000. M = 0.875
A solution in which no more solute can be dissolved at a given temperature and pressure is called a saturated solution. At saturated solution stage equilibrium gets established among which two processes?- a)Dissolution and crystallisation
- b)Dissolution and condensation
- c)Sublimation and crystallisation
- d)Evaporation and condensation
Correct answer is 'A'. Can you explain this answer?
A solution in which no more solute can be dissolved at a given temperature and pressure is called a saturated solution. At saturated solution stage equilibrium gets established among which two processes?
a)
Dissolution and crystallisation
b)
Dissolution and condensation
c)
Sublimation and crystallisation
d)
Evaporation and condensation
|
|
Gaurav Kumar answered |
At saturated solution stage equilibrium gets established among dissolution and crystallisation.
Can you explain the answer of this question below: Mole fraction of ethyl chloride and methanol in a ternary solution is 0.6 and 0.32 respectively. What is the mole fraction of third component. Also identify the solvent in this ternary solution.
- A:
0.03, Ethyl chloride
- B:
0.08, cannot be determined
- C:
0.03, Methanol
- D:
0.08, Ethyl chloride
The answer is d.
Mole fraction of ethyl chloride and methanol in a ternary solution is 0.6 and 0.32 respectively. What is the mole fraction of third component. Also identify the solvent in this ternary solution.
0.03, Ethyl chloride
0.08, cannot be determined
0.03, Methanol
0.08, Ethyl chloride

|
Infinity Academy answered |
Mole fraction of third component is 1 - (0.6 + 0.32) = 0.08.
Since ethyl chloride has highest mole fraction so it is the solvent.
Since ethyl chloride has highest mole fraction so it is the solvent.
A solution in which no more solute can be dissolved at the given temperature and pressure is called a- a)Unsaturated solution
- b)Dilute solution
- c)Solid solution
- d)Saturated solution
Correct answer is option 'D'. Can you explain this answer?
A solution in which no more solute can be dissolved at the given temperature and pressure is called a
a)
Unsaturated solution
b)
Dilute solution
c)
Solid solution
d)
Saturated solution
|
|
Om Desai answered |
The correct answer is option D
In a saturated solution, more solute cannot be dissolved at a given temperature.
This is because, the solute dissolves in a solvent because of space between particles of solvent but on continuous addition of solute, the space between the solvent particles gets fulfilled. Thus no more solute particle can dissolve in a solvent.
In a saturated solution, more solute cannot be dissolved at a given temperature.
This is because, the solute dissolves in a solvent because of space between particles of solvent but on continuous addition of solute, the space between the solvent particles gets fulfilled. Thus no more solute particle can dissolve in a solvent.
Which of the following components form an ideal solution?- a)Ethyl alcohol and benzene
- b)Acetone and aniline
- c)Water and nitric acid
- d)Benzene and toluene
Correct answer is option 'D'. Can you explain this answer?
Which of the following components form an ideal solution?
a)
Ethyl alcohol and benzene
b)
Acetone and aniline
c)
Water and nitric acid
d)
Benzene and toluene
|
|
Swati Verma answered |
The correct answer is option D
The solutions which obey Raoult's law over the entire range of concentration are known as ideal solutions.
For ideal solution, the enthalpy of mixing of the pure components to form the solution is zero and the volume of mixing is also zero, i.e.,
△mixH=0 and △mixV=0
Thus, this type of solution is a solution of benzene and toluene.
The solutions which obey Raoult's law over the entire range of concentration are known as ideal solutions.
For ideal solution, the enthalpy of mixing of the pure components to form the solution is zero and the volume of mixing is also zero, i.e.,
△mixH=0 and △mixV=0
Thus, this type of solution is a solution of benzene and toluene.
In a 0.2 molal aqueous solution of a weak acid HX the degree of ionization is 0.3 . Taking kƒ for water as 1.85, the freezing point of the solution will be nearest to _ [aieee-2003]- a) –0.260ºC
- b)+ 0.480ºC
- c)–0.480ºC
- d) –0.360ºC
Correct answer is option 'C'. Can you explain this answer?
In a 0.2 molal aqueous solution of a weak acid HX the degree of ionization is 0.3 . Taking kƒ for water as 1.85, the freezing point of the solution will be nearest to _ [aieee-2003]
a)
–0.260ºC
b)
+ 0.480ºC
c)
–0.480ºC
d)
–0.360ºC

|
Tamkeen Fatima answered |
ΔTf=i.Kf.m, where i=vant hoff's factor, Kf=const &m=molality. i=1+(n-1)α, where n=no. of ions furnished by one molecule. For a weak acid of HX type, n=2(H+ & X-). So, i=1+(2-1)0.3=1+0.3=1.3 So, ΔTf=1.3x1.85x0.2=0.481 freezing point=freezing point of water-ΔTf=0-0.481=-0.481
Which of the following gases will be least soluble in water?- a)Hydrogen chloride
- b)Sulphur dioxide
- c)Ammonia
- d)Nitrogen
Correct answer is option 'D'. Can you explain this answer?
Which of the following gases will be least soluble in water?
a)
Hydrogen chloride
b)
Sulphur dioxide
c)
Ammonia
d)
Nitrogen
|
|
Nikita Singh answered |
The correct answer is Option D.
The reason for poor solubility of N2 in water is that they just occupy the intermolecular spaces and stay there by interaction with its surrounding molecules by weak van der Waals forces.So it is least soluble while other gases form hydrogen bonds with water.
The reason for poor solubility of N2 in water is that they just occupy the intermolecular spaces and stay there by interaction with its surrounding molecules by weak van der Waals forces.So it is least soluble while other gases form hydrogen bonds with water.
The level of contamination of chloroform was found to be 15 ppm. It means 15 g of chloroform is present in how many grams of solution?- a)1000 g
- b)106 g
- c)100 g
- d)1.0 g
Correct answer is option 'B'. Can you explain this answer?
The level of contamination of chloroform was found to be 15 ppm. It means 15 g of chloroform is present in how many grams of solution?
a)
1000 g
b)
106 g
c)
100 g
d)
1.0 g
|
|
Lavanya Menon answered |
1 ppm is equivalent to 1 part out of 1 million (106) parts.
∴ Mass percent of 15 ppm chloroform in water
∴ Mass percent of 15 ppm chloroform in water

⇒ 1.5 x 10-3 g chloroform present in 100 g water
Thus, 15g chloroform will be present in water
Thus, 15g chloroform will be present in water

Mole fraction of glycerine, C3H5(OH)3 in a solution containing 36 gm of water and 46 gm of glycerine is:- a)0.40
- b)0.20
- c)0.46
- d)0.36
Correct answer is option 'B'. Can you explain this answer?
Mole fraction of glycerine, C3H5(OH)3 in a solution containing 36 gm of water and 46 gm of glycerine is:
a)
0.40
b)
0.20
c)
0.46
d)
0.36
|
|
Naina Bansal answered |
No. of moles of glycerine= 46/92 (where 92 is M.M. of glycerine)
= 0.5moles
no. of moles of water= 36/18(where 18 is M.M. of water)
= 2moles
so mole fraction oh glycerine = No. of moles of glycerine/No. of moles of glycerine + No. of moles of water
= 0.5/2 + 0.5
= 0.20
Which is correct about Henry's law- a)The gas in contact with the liquid should behave as an ideal gas
- b)There should not be any chemical interaction between the gas and liquid
- c)The pressure applied should be high
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
Which is correct about Henry's law
a)
The gas in contact with the liquid should behave as an ideal gas
b)
There should not be any chemical interaction between the gas and liquid
c)
The pressure applied should be high
d)
All of these
|
|
Sri Suhas answered |
Yes, because if the gases in the mixture or solution will react then there will be partly solution and partly compound due to which the solution concentration will change and we will not get a proper Henry constant to the solution.
Hope this helps you. If you find an answer to this never hesitate to put it in the answer box.
Which of the following concentration factor is affected by change in temperature ? [AIEEE-2002]- a)Molarity
- b)Molality
- c) Mol fraction
- d)Weight fraction
Correct answer is option 'A'. Can you explain this answer?
Which of the following concentration factor is affected by change in temperature ? [AIEEE-2002]
a)
Molarity
b)
Molality
c)
Mol fraction
d)
Weight fraction
|
|
Mira Sharma answered |
Molarity is temperature dependent. In the formula of molarity, you have L of solution. Volume is temperature-dependent. It varies with changing temperature. Because of this, molarity changes if the temp changes.
Can you explain the answer of this question below:The value for Henry’s constant for helium, hydrogen, nitrogen and oxygen at 293 K are 144.97 kbar, 69.16 kbar, 76.48 kbar and 34.86 kbar respectively. Which of the gas will be having maximum solubility?
- A:
Hydrogen
- B:
Oxygen
- C:
Helium
- D:
Nitrogen
The answer is b.
The value for Henry’s constant for helium, hydrogen, nitrogen and oxygen at 293 K are 144.97 kbar, 69.16 kbar, 76.48 kbar and 34.86 kbar respectively. Which of the gas will be having maximum solubility?
Hydrogen
Oxygen
Helium
Nitrogen
|
|
Rohit Shah answered |
Higher the value of Henry’s constant lower is the solubility of gas.
Benzoic acid dissolved in benzene will show a molecular weight of- a)366g
- b)122g
- c)61g
- d)244g
Correct answer is option 'D'. Can you explain this answer?
Benzoic acid dissolved in benzene will show a molecular weight of
a)
366g
b)
122g
c)
61g
d)
244g

|
Dr Manju Sen answered |
In benzene, benzoic acid exist as dimer. So the molecular weight of benzoic acid gets doubled.
(C6H5COOH)2=2×122=244 units .
The ratio of osmotic pressure of a 1:1 electrolyte AB to that of a non electrolyte solute of same concentration is- a)2:1
- b)1:1
- c)0.5:1
- d)0.1:3
Correct answer is option 'A'. Can you explain this answer?
The ratio of osmotic pressure of a 1:1 electrolyte AB to that of a non electrolyte solute of same concentration is
a)
2:1
b)
1:1
c)
0.5:1
d)
0.1:3
|
|
Anjali Iyer answered |
Van’t Hoff factor of 1:1 electrolyte is 2 since it dissociates into two ions. van’t Hoff factor of a non electrolyte is 1 since it does not dissociate into ions. Hence ratio of osmotic pressure will be 2:1
Mole fraction of ethyl chloride and methanol in a ternary solution is 0.6 and 0.32 respectively. What is the mole fraction of third component. Also identify the solvent in this ternary solution.- a)0.03, Ethyl chloride
- b)0.08, cannot be determined
- c)0.03, Methanol
- d)0.08, Ethyl chloride
Correct answer is option 'D'. Can you explain this answer?
Mole fraction of ethyl chloride and methanol in a ternary solution is 0.6 and 0.32 respectively. What is the mole fraction of third component. Also identify the solvent in this ternary solution.
a)
0.03, Ethyl chloride
b)
0.08, cannot be determined
c)
0.03, Methanol
d)
0.08, Ethyl chloride

|
Anu answered |
•sum of mole fractions are always 1.so mole fraction of 3rd component =1-(0.6+0.32)=0.8 • solvent is the component of a solution that is present in the greatest amount. so ethyl chloride is solvent
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be - [AIEEE 2007]- a)350
- b)300
- c)700
- d)360
Correct answer is option 'A'. Can you explain this answer?
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be - [AIEEE 2007]
a)
350
b)
300
c)
700
d)
360
|
|
Vivek Rana answered |
The correct answer is Option A.
Total pressure =290 mm
Vapour pressure of propyl alcohol =200 mm
Mole fraction of ethyl alcohol = 0.6
Mol fraction of propyl alcohol =1− 0.6 = 0.4
PT = P0A × χA + P0B × (1−χA)
290 = 200 × 0.4 + PoB × 0.6
0.6 × P0B = 290 − 80
P0B = 350 mm
Vapour pressure of propyl alcohol =200 mm
Mole fraction of ethyl alcohol = 0.6
Mol fraction of propyl alcohol =1− 0.6 = 0.4
PT = P0A × χA + P0B × (1−χA)
290 = 200 × 0.4 + PoB × 0.6
0.6 × P0B = 290 − 80
P0B = 350 mm
According to Henry’s Law at a constant temperature the solubility of gas in a liquid is directly proportional to the- a)Mass of gas
- b)Density of gas
- c)Volume of gas
- d)Pressure of gas
Correct answer is option 'D'. Can you explain this answer?
According to Henry’s Law at a constant temperature the solubility of gas in a liquid is directly proportional to the
a)
Mass of gas
b)
Density of gas
c)
Volume of gas
d)
Pressure of gas
|
|
Mira Sharma answered |
According to Henry’s law the solubility of gas in a liquid is directly proportional to the pressure of the gas
Mathematically mass of the dissolved gas α pressure of the gas
let mass of dissolved gas in unit volume = m
pressure of gas = p
to remove sign of proportional we us a constant here KH is Henry’s law constant
so we get
m = KH*p
we can measure mass of solute in term of molar fraction also
Hence our formula will
X = KH*p
Application:
1. In Packing of soda cans :- To increase the solubility of CO2 gas in soda water , bottles of soda water is always packed under higher pressure.
2. In Deep see diving: - As nitrogen is a more soluble gas in our blood and at deep see pressure increase so its solubility also increases, when scuba diver tries to come rapidly toward the surface of water, pressure decreased and Dissolved N2 gas comes back from the blood and make bubbles in his veins. It because bends .To avoid bends diver use oxygen diluted with helium because helium is less soluble in blood.
Henry's Law states that: The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution.
Only One Option Correct Type
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correctWhich of the following concentration factors is affected by change in temperature?- a)Molarity
- b)Molality
- c)Mole Fraction
- d)Weight Fraction
Correct answer is option 'A'. Can you explain this answer?
Only One Option Correct Type
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Which of the following concentration factors is affected by change in temperature?
a)
Molarity
b)
Molality
c)
Mole Fraction
d)
Weight Fraction
|
|
Poulomi Desai answered |
Explanation:
When a solute is dissolved in a solvent, the concentration of the solution can be expressed in different ways such as molarity, molality, mole fraction, and weight fraction. The concentration factors that depend on the number of moles of solutes and solvents are molarity, molality, and mole fraction. The concentration factor that depends on the mass of solute and solvent is weight fraction.
Effect of temperature on concentration factors:
The effect of temperature on the concentration factors is different for each factor. Let's see how temperature affects each concentration factor.
Molarity:
Molarity is defined as the number of moles of solute per liter of solution. It is affected by temperature because the volume of the solution changes with temperature due to thermal expansion or contraction. When the temperature increases, the volume of the solution increases, which decreases the molarity of the solution because the same number of moles of solute is distributed in a larger volume. Similarly, when the temperature decreases, the volume of the solution decreases, which increases the molarity of the solution because the same number of moles of solute is distributed in a smaller volume. Therefore, molarity is affected by temperature.
Molality:
Molality is defined as the number of moles of solute per kilogram of solvent. It is not affected by temperature because the mass of the solvent remains constant with temperature, and the number of moles of solute is also constant. Therefore, molality is independent of temperature.
Mole fraction:
Mole fraction is defined as the ratio of the number of moles of solute to the total number of moles of solute and solvent. It is not affected by temperature because the number of moles of solute and solvent changes with temperature, but the ratio remains constant. Therefore, mole fraction is independent of temperature.
Weight fraction:
Weight fraction is defined as the ratio of the mass of solute to the total mass of solute and solvent. It is not affected by temperature because the mass of the solute and solvent changes with temperature, but the ratio remains constant. Therefore, weight fraction is independent of temperature.
Conclusion:
From the above explanation, we can conclude that only molarity is affected by temperature, whereas molality, mole fraction, and weight fraction are independent of temperature.
When a solute is dissolved in a solvent, the concentration of the solution can be expressed in different ways such as molarity, molality, mole fraction, and weight fraction. The concentration factors that depend on the number of moles of solutes and solvents are molarity, molality, and mole fraction. The concentration factor that depends on the mass of solute and solvent is weight fraction.
Effect of temperature on concentration factors:
The effect of temperature on the concentration factors is different for each factor. Let's see how temperature affects each concentration factor.
Molarity:
Molarity is defined as the number of moles of solute per liter of solution. It is affected by temperature because the volume of the solution changes with temperature due to thermal expansion or contraction. When the temperature increases, the volume of the solution increases, which decreases the molarity of the solution because the same number of moles of solute is distributed in a larger volume. Similarly, when the temperature decreases, the volume of the solution decreases, which increases the molarity of the solution because the same number of moles of solute is distributed in a smaller volume. Therefore, molarity is affected by temperature.
Molality:
Molality is defined as the number of moles of solute per kilogram of solvent. It is not affected by temperature because the mass of the solvent remains constant with temperature, and the number of moles of solute is also constant. Therefore, molality is independent of temperature.
Mole fraction:
Mole fraction is defined as the ratio of the number of moles of solute to the total number of moles of solute and solvent. It is not affected by temperature because the number of moles of solute and solvent changes with temperature, but the ratio remains constant. Therefore, mole fraction is independent of temperature.
Weight fraction:
Weight fraction is defined as the ratio of the mass of solute to the total mass of solute and solvent. It is not affected by temperature because the mass of the solute and solvent changes with temperature, but the ratio remains constant. Therefore, weight fraction is independent of temperature.
Conclusion:
From the above explanation, we can conclude that only molarity is affected by temperature, whereas molality, mole fraction, and weight fraction are independent of temperature.
The value for Henry’s constant for argon, carbon dioxide, methane and vinyl chloride at 298 K are 40.3 kbar, 1.67 kbar, 0.413 kbar and 0.611 kbar respectively. Which of the gas will be having least solubility?- a)Argon
- b)Vinyl chloride
- c)Methane
- d)Carbon dioxide
Correct answer is option 'A'. Can you explain this answer?
The value for Henry’s constant for argon, carbon dioxide, methane and vinyl chloride at 298 K are 40.3 kbar, 1.67 kbar, 0.413 kbar and 0.611 kbar respectively. Which of the gas will be having least solubility?
a)
Argon
b)
Vinyl chloride
c)
Methane
d)
Carbon dioxide
|
|
Preeti Iyer answered |
Higher the value of Henry’s constant lower is the solubility of gas.
Can you explain the answer of this question below: Which of the following would be the correct unit of expressing Henry’s law constant?- A:K
- B:atm
- C:kg
- D:mol/L
The answer is b.
Which of the following would be the correct unit of expressing Henry’s law constant?
A:
K
B:
atm
C:
kg
D:
mol/L

|
Rutuja Pawar answered |
According to Henry's law, P=K × x Where...P=pressure, k=Henry's law constant, x=mole fraction .•.k=P/x x is unitless .•.unit of Henry's law constant is atm...Hope u get it...😄😄😄
When common salt is dissolved in water:- a)Melting point of solution increases
- b)Boiling point of solution increases
- c)Boiling point of solution decreases
- d)both melting .point and boiling point decreases
Correct answer is option 'B'. Can you explain this answer?
When common salt is dissolved in water:
a)
Melting point of solution increases
b)
Boiling point of solution increases
c)
Boiling point of solution decreases
d)
both melting .point and boiling point decreases
|
|
Rajat Patel answered |
So the boiling point of water will increase. When salt is added to water, then the intermolecular forces between water molecules gets altered due to dissociation of NaCl into sodium and chloride ions.
0.01 M solution of common salt (NaCl) and (CH3COOH ) is taken. If their osmotic pressures are p1and p2 resp., what will be the correct statement correlating their osmotic pressures?- a)p1 = p2
- b)p1 > p2
- c)p1< p2
- d)p1 = p2 =O
Correct answer is option 'B'. Can you explain this answer?
0.01 M solution of common salt (NaCl) and (CH3COOH ) is taken. If their osmotic pressures are p1and p2 resp., what will be the correct statement correlating their osmotic pressures?
a)
p1 = p2
b)
p1 > p2
c)
p1< p2
d)
p1 = p2 =O
|
|
Arka Das answered |
Correlating Osmotic Pressures of NaCl and CH3COOH Solution
Heading: Introduction
When two solutions are separated by a semipermeable membrane, the movement of solvent molecules occurs from a region of higher solvent concentration to a region of lower solvent concentration. This phenomenon is known as osmosis.
Heading: Osmotic Pressure
Osmotic pressure is defined as the pressure that must be applied to the solution to prevent the net flow of solvent molecules from the solution to the pure solvent across a semipermeable membrane. It is directly proportional to the concentration of solute particles in the solution.
Heading: Experimental Set-Up
A 0.01 M solution of NaCl and CH3COOH was taken. The osmotic pressures of the solutions were measured separately.
Heading: Correlation of Osmotic Pressures
The osmotic pressure of NaCl solution will be higher than the osmotic pressure of CH3COOH solution. This is because NaCl dissociates completely in water, producing two particles (Na+ and Cl-), whereas CH3COOH partially dissociates to produce fewer particles. Thus, the NaCl solution has a higher concentration of solute particles, leading to a higher osmotic pressure.
Heading: Correct Statement
Option B is the correct statement, which states that p1 > p2. The osmotic pressure of NaCl solution is greater than the osmotic pressure of CH3COOH solution.
Heading: Introduction
When two solutions are separated by a semipermeable membrane, the movement of solvent molecules occurs from a region of higher solvent concentration to a region of lower solvent concentration. This phenomenon is known as osmosis.
Heading: Osmotic Pressure
Osmotic pressure is defined as the pressure that must be applied to the solution to prevent the net flow of solvent molecules from the solution to the pure solvent across a semipermeable membrane. It is directly proportional to the concentration of solute particles in the solution.
Heading: Experimental Set-Up
A 0.01 M solution of NaCl and CH3COOH was taken. The osmotic pressures of the solutions were measured separately.
Heading: Correlation of Osmotic Pressures
The osmotic pressure of NaCl solution will be higher than the osmotic pressure of CH3COOH solution. This is because NaCl dissociates completely in water, producing two particles (Na+ and Cl-), whereas CH3COOH partially dissociates to produce fewer particles. Thus, the NaCl solution has a higher concentration of solute particles, leading to a higher osmotic pressure.
Heading: Correct Statement
Option B is the correct statement, which states that p1 > p2. The osmotic pressure of NaCl solution is greater than the osmotic pressure of CH3COOH solution.
Which of the two has lower freezing point, 2m NaCl or 5m NaCl aqueous solution?- a)2m NaCl solution
- b)Both have same freezing point
- c)5m NaCl solution
- d)The solutions cannot freeze
Correct answer is option 'C'. Can you explain this answer?
Which of the two has lower freezing point, 2m NaCl or 5m NaCl aqueous solution?
a)
2m NaCl solution
b)
Both have same freezing point
c)
5m NaCl solution
d)
The solutions cannot freeze
|
|
Lavanya Menon answered |
The correct answer is option C
ΔTf= Kf x m Since ΔTf for 5m NaCl will be higher than for 2m, 5m NaCl solution freezes at a lower temperature.
ΔTf= Kf x m Since ΔTf for 5m NaCl will be higher than for 2m, 5m NaCl solution freezes at a lower temperature.
Two solutions of a substance (non electrolyte) are mixed in the following manner. 480 ml of 1.5 M first solution + 520 mL of 1.2 M second solution. What is the molarity of the final mixture ? [AIEEE-2005]- a)1.50 M
- b)1.20 M
- c)2.70 M
- d)1.344 M
Correct answer is option 'D'. Can you explain this answer?
Two solutions of a substance (non electrolyte) are mixed in the following manner. 480 ml of 1.5 M first solution + 520 mL of 1.2 M second solution. What is the molarity of the final mixture ? [AIEEE-2005]
a)
1.50 M
b)
1.20 M
c)
2.70 M
d)
1.344 M
|
|
Nikita Singh answered |
The correct answer is option D
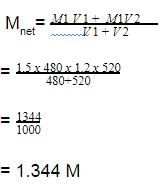
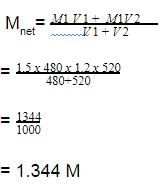
Some solute particles in solution collide with other solid solute particles present and get separated out of solution. This process is known as- a)Condensation
- b)Dissolution
- c)Sublimation
- d)Crystalisation
Correct answer is option 'D'. Can you explain this answer?
Some solute particles in solution collide with other solid solute particles present and get separated out of solution. This process is known as
a)
Condensation
b)
Dissolution
c)
Sublimation
d)
Crystalisation
|
|
Rajesh Gupta answered |
The correct answer is option D
Some solute particles in solution collide with the solid solute particles and get separated out of solution. This process is called crystallization. A stage is reached when the two processes take place at the same rate. Under such conditions, the number of solute particles going into solution will be equal to the solute particles separating out and a state of dynamic equilibrium is achieved.
Some solute particles in solution collide with the solid solute particles and get separated out of solution. This process is called crystallization. A stage is reached when the two processes take place at the same rate. Under such conditions, the number of solute particles going into solution will be equal to the solute particles separating out and a state of dynamic equilibrium is achieved.
What is the osmotic pressure of a solution containing 3.42 g of cane sugar in 1litre solution at 27°C .Molar mass of cane sugar is 342 g.- a)0.246 atm
- b)2.46 atm
- c)0.2 atm
- d)1 atm
Correct answer is option 'A'. Can you explain this answer?
What is the osmotic pressure of a solution containing 3.42 g of cane sugar in 1litre solution at 27°C .Molar mass of cane sugar is 342 g.
a)
0.246 atm
b)
2.46 atm
c)
0.2 atm
d)
1 atm
|
|
Rajesh Gupta answered |
The correct answer is Option A.
Π = (W / M * V) * R * T
Π = (3.42 / 345 * 1 L) * 300 K * 0.0821
= 0.02463 atm
Π = (W / M * V) * R * T
Π = (3.42 / 345 * 1 L) * 300 K * 0.0821
= 0.02463 atm
A binary liquid solution is prepared by mixing n-heptane and ethanol. Which one of the following statements is correct regarding the behaviour of the solution ? [AIEEE 2009]- a)The solution is non-ideal, showing +ve deviation form Raoult's Law
- b)The solution is non-ideal, showing –ve deviation from Raoult's Law
- c) N-heptane shows +ve deviation while ethanol shows -ve deviation from Raoult's Law
- d)The solution formed is an ideal solution
Correct answer is option 'A'. Can you explain this answer?
A binary liquid solution is prepared by mixing n-heptane and ethanol. Which one of the following statements is correct regarding the behaviour of the solution ?
[AIEEE 2009]
a)
The solution is non-ideal, showing +ve deviation form Raoult's Law
b)
The solution is non-ideal, showing –ve deviation from Raoult's Law
c)
N-heptane shows +ve deviation while ethanol shows -ve deviation from Raoult's Law
d)
The solution formed is an ideal solution
|
|
Rajesh Gupta answered |
The correct answer is Option A.
n-Heptane is non-polar and ethanol is polar. Hence the mixture formed by these two liquids is non-ideal. The stronger forces of attraction between n-heptane-n-heptane and ethanol-ethanol are replaced by weaker forces of attraction between n-heptane-ethanol in the solution. Therefore the escaping tendency of the liquid molecules into the vapour phase increases. This will increase the vapour pressure more than expected from Raoult's law. This is a positive deviation.
Hence, the correct option is B
n-Heptane is non-polar and ethanol is polar. Hence the mixture formed by these two liquids is non-ideal. The stronger forces of attraction between n-heptane-n-heptane and ethanol-ethanol are replaced by weaker forces of attraction between n-heptane-ethanol in the solution. Therefore the escaping tendency of the liquid molecules into the vapour phase increases. This will increase the vapour pressure more than expected from Raoult's law. This is a positive deviation.
Hence, the correct option is B
What is the effect of temperature on the osmotic pressure of solution?- a)It decreases with increase in temperature
- b)It decreases very very slowly with increase in temperature
- c)No effect of temperature
- d)It increases with increase in temperature
Correct answer is option 'D'. Can you explain this answer?
What is the effect of temperature on the osmotic pressure of solution?
a)
It decreases with increase in temperature
b)
It decreases very very slowly with increase in temperature
c)
No effect of temperature
d)
It increases with increase in temperature
|
|
Sreemoyee Choudhury answered |
Effect of Temperature on Osmotic Pressure
Osmotic pressure is the pressure required to prevent the flow of solvent molecules through a semipermeable membrane into a solution containing solute molecules. The osmotic pressure is directly proportional to the concentration of the solution and inversely proportional to the volume of the solution. However, temperature also affects the osmotic pressure of a solution.
Increase in Temperature Increases Osmotic Pressure
When the temperature of a solution is increased, the kinetic energy of the solvent and solute molecules increases. This results in an increase in the number of collisions between the solvent and solute molecules. As a result, more solvent molecules pass through the semipermeable membrane into the solution containing solute molecules, increasing the concentration of the solution and thus increasing the osmotic pressure.
This can be explained by the following equation:
Π = CRT
where Π is the osmotic pressure, C is the concentration of the solution, R is the gas constant, and T is the temperature in Kelvin. As the temperature increases, the value of T increases, and thus the osmotic pressure increases.
Therefore, the correct answer is option D - It increases with an increase in temperature.
Conclusion
In conclusion, the osmotic pressure of a solution increases with an increase in temperature due to an increase in the kinetic energy of the molecules, resulting in an increase in the number of collisions between the solvent and solute molecules.
Osmotic pressure is the pressure required to prevent the flow of solvent molecules through a semipermeable membrane into a solution containing solute molecules. The osmotic pressure is directly proportional to the concentration of the solution and inversely proportional to the volume of the solution. However, temperature also affects the osmotic pressure of a solution.
Increase in Temperature Increases Osmotic Pressure
When the temperature of a solution is increased, the kinetic energy of the solvent and solute molecules increases. This results in an increase in the number of collisions between the solvent and solute molecules. As a result, more solvent molecules pass through the semipermeable membrane into the solution containing solute molecules, increasing the concentration of the solution and thus increasing the osmotic pressure.
This can be explained by the following equation:
Π = CRT
where Π is the osmotic pressure, C is the concentration of the solution, R is the gas constant, and T is the temperature in Kelvin. As the temperature increases, the value of T increases, and thus the osmotic pressure increases.
Therefore, the correct answer is option D - It increases with an increase in temperature.
Conclusion
In conclusion, the osmotic pressure of a solution increases with an increase in temperature due to an increase in the kinetic energy of the molecules, resulting in an increase in the number of collisions between the solvent and solute molecules.
Which one of the following statement is false? [AIEEE-2004]- a) Raoult's law states that the vapour pressure of a component over a solution is proportional to its mole fraction
- b)The osmotic pressure (p) of a solution is given by the equation p = MRT where M is the molarity of the solution
- c)The correct order of osmotic pressure for 0.01 M aqueous solution of each compound is BaCl2 > KCl >CH3COOH > sucrose.
- d)Two sucrose solutions of same molality prepared in different solvents will have the same freezing point depression.
Correct answer is option 'D'. Can you explain this answer?
Which one of the following statement is false? [AIEEE-2004]
a)
Raoult's law states that the vapour pressure of a component over a solution is proportional to its mole fraction
b)
The osmotic pressure (p) of a solution is given by the equation p = MRT where M is the molarity of the solution
c)
The correct order of osmotic pressure for 0.01 M aqueous solution of each compound is BaCl2 > KCl >CH3COOH > sucrose.
d)
Two sucrose solutions of same molality prepared in different solvents will have the same freezing point depression.
|
|
Anuj Unni answered |
The false statement is (c) The correct order of osmotic pressure for 0.01 M aqueous solution of each compound is BaCl2 < nacl="" />< cacl2.="" the="" correct="" order="" of="" osmotic="" pressure="" for="" 0.01="" m="" aqueous="" solution="" of="" each="" compound="" is="" nacl="" />< cacl2="" />< bacl2.="" />
The molal elevation constant is the elevation in boiling point of- a)1M solution
- b)1m solution
- c)1N solution
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
The molal elevation constant is the elevation in boiling point of
a)
1M solution
b)
1m solution
c)
1N solution
d)
None of the above
|
|
Swati Verma answered |
The correct answer is Option B.
Molal elevation constant is a characteristic constant for a given solvent. It is the elevation of boiling point produced when one mole of solute is dissolved in 1 kg of solvent. The proportionality constant, Kb, is called the molal boiling point elevation constant. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute.
Molal elevation constant is a characteristic constant for a given solvent. It is the elevation of boiling point produced when one mole of solute is dissolved in 1 kg of solvent. The proportionality constant, Kb, is called the molal boiling point elevation constant. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute.
Increasing the temperature of solution of a weak electrolyte will cause- a)No change in van’t Hoff factor
- b)Decrease in van’t Hoff factor
- c)Depends on nature of solute.
- d)Increase in van’t Hoff factor
Correct answer is option 'D'. Can you explain this answer?
Increasing the temperature of solution of a weak electrolyte will cause
a)
No change in van’t Hoff factor
b)
Decrease in van’t Hoff factor
c)
Depends on nature of solute.
d)
Increase in van’t Hoff factor
|
|
Kalyan Joshi answered |
There are a few possible outcomes when increasing the temperature of a solution of a weak electrolyte:
1) Increase in ionization: In some cases, increasing the temperature can cause more ions to dissociate from the weak electrolyte, leading to an increase in conductivity.
2) Decrease in ionization: On the other hand, increasing the temperature can also disrupt the equilibrium between the weak electrolyte and its ions, leading to a decrease in ionization and conductivity.
3) Change in equilibrium constant: The temperature can also affect the equilibrium constant of the weak electrolyte, which can in turn affect the degree of ionization and conductivity.
Overall, the effect of increasing the temperature on a solution of a weak electrolyte can vary depending on the specific properties of the electrolyte and the conditions of the experiment.
1) Increase in ionization: In some cases, increasing the temperature can cause more ions to dissociate from the weak electrolyte, leading to an increase in conductivity.
2) Decrease in ionization: On the other hand, increasing the temperature can also disrupt the equilibrium between the weak electrolyte and its ions, leading to a decrease in ionization and conductivity.
3) Change in equilibrium constant: The temperature can also affect the equilibrium constant of the weak electrolyte, which can in turn affect the degree of ionization and conductivity.
Overall, the effect of increasing the temperature on a solution of a weak electrolyte can vary depending on the specific properties of the electrolyte and the conditions of the experiment.
The exact mathematical expression of Raoult's law is- a)

- b)
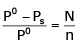
- c)

- d)
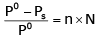
Correct answer is option 'C'. Can you explain this answer?
The exact mathematical expression of Raoult's law is
a)
b)
c)
d)

|
EduRev Support answered |
The exact mathematical expression of Raoult's law is 
Here, Po represents the vapour pressure of the pure solvent, P represents the vapour pressure of the solution, n represents the number of moles of solute and N represents the number of moles of the solvent.
Here, Po represents the vapour pressure of the pure solvent, P represents the vapour pressure of the solution, n represents the number of moles of solute and N represents the number of moles of the solvent.
In which unit, the concentration of solution remains independent of temperature- a)molarity
- b)normaility
- c)molality
- d)formality
Correct answer is option 'C'. Can you explain this answer?
In which unit, the concentration of solution remains independent of temperature
a)
molarity
b)
normaility
c)
molality
d)
formality

|
Ritika Kulkarni answered |
It is independent of volume hence independent of Temperature.
If the vapour pressure of pure solvent A is 17.5 mm and lowering of vapour pressure of solution formed by adding a non-volatile electrolyte is 0.0175 mm then what is the relative lowering of vapour pressure?- a)0.1
- b)1.01
- c)0.001
- d)0.01
Correct answer is option 'C'. Can you explain this answer?
If the vapour pressure of pure solvent A is 17.5 mm and lowering of vapour pressure of solution formed by adding a non-volatile electrolyte is 0.0175 mm then what is the relative lowering of vapour pressure?
a)
0.1
b)
1.01
c)
0.001
d)
0.01
|
|
Geetika Shah answered |
The correct answer is option C
Relative lowering of vapour pressure:
= lowering of vapour pressure of solution/ vapour pressure of pure solvent
=0.0175/17.5=0.001
Relative lowering of vapour pressure:
= lowering of vapour pressure of solution/ vapour pressure of pure solvent
=0.0175/17.5=0.001
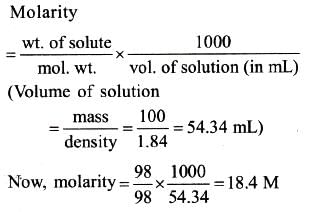
The density of a solution prepared by dissolving 120 g of urea (mol. mass = 60 u) in 1000g of water is 1.15 g/mL. The molarity of this solution is : [AIEEE-2012]- a)1.78 M
- b)1.02 M
- c) 2.05 M
- d)0.50 M
Correct answer is option 'C'. Can you explain this answer?
The density of a solution prepared by dissolving 120 g of urea (mol. mass = 60 u) in 1000g of water is 1.15 g/mL. The molarity of this solution is : [AIEEE-2012]
a)
1.78 M
b)
1.02 M
c)
2.05 M
d)
0.50 M
|
|
Sanaya Kumar answered |
Finding the Mass of Solution
The first step to solving this problem is to find the mass of the solution. This can be done by adding the mass of the solute (urea) to the mass of the solvent (water).
Mass of solution = Mass of solute + Mass of solvent
Mass of solution = 120 g + 1000 g
Mass of solution = 1120 g
Finding the Volume of Solution
The density of the solution is given as 1.15 g/mL. This means that for every 1 mL of solution, there is 1.15 g of solution. We can use this information to find the volume of the solution.
Density = Mass / Volume
1.15 g/mL = 1120 g / Volume
Volume = 1120 g / 1.15 g/mL
Volume = 973.91 mL
Converting to Liters
The unit of volume used in molarity is liters. Therefore, we need to convert the volume from milliliters to liters.
Volume = 973.91 mL x 1 L / 1000 mL
Volume = 0.97391 L
Finding the Number of Moles of Solute
The molarity of a solution is defined as the number of moles of solute per liter of solution. We have already found the volume of the solution, so we now need to find the number of moles of solute.
Number of moles of solute = Mass of solute / Molecular weight of solute
Number of moles of solute = 120 g / 60 g/mol
Number of moles of solute = 2 mol
Finding the Molarity
Now that we have the volume of the solution and the number of moles of solute, we can find the molarity of the solution.
Molarity = Number of moles of solute / Volume of solution
Molarity = 2 mol / 0.97391 L
Molarity = 2.05 M
Therefore, the molarity of the solution is 2.05 M.
The first step to solving this problem is to find the mass of the solution. This can be done by adding the mass of the solute (urea) to the mass of the solvent (water).
Mass of solution = Mass of solute + Mass of solvent
Mass of solution = 120 g + 1000 g
Mass of solution = 1120 g
Finding the Volume of Solution
The density of the solution is given as 1.15 g/mL. This means that for every 1 mL of solution, there is 1.15 g of solution. We can use this information to find the volume of the solution.
Density = Mass / Volume
1.15 g/mL = 1120 g / Volume
Volume = 1120 g / 1.15 g/mL
Volume = 973.91 mL
Converting to Liters
The unit of volume used in molarity is liters. Therefore, we need to convert the volume from milliliters to liters.
Volume = 973.91 mL x 1 L / 1000 mL
Volume = 0.97391 L
Finding the Number of Moles of Solute
The molarity of a solution is defined as the number of moles of solute per liter of solution. We have already found the volume of the solution, so we now need to find the number of moles of solute.
Number of moles of solute = Mass of solute / Molecular weight of solute
Number of moles of solute = 120 g / 60 g/mol
Number of moles of solute = 2 mol
Finding the Molarity
Now that we have the volume of the solution and the number of moles of solute, we can find the molarity of the solution.
Molarity = Number of moles of solute / Volume of solution
Molarity = 2 mol / 0.97391 L
Molarity = 2.05 M
Therefore, the molarity of the solution is 2.05 M.
The mass of sodium chloride in 2.5 M solution is- a)37.80 g
- b)146.25 g
- c)117 .00g
- d)58.50 g
Correct answer is option 'B'. Can you explain this answer?
The mass of sodium chloride in 2.5 M solution is
a)
37.80 g
b)
146.25 g
c)
117 .00g
d)
58.50 g
|
|
Rajeev Saxena answered |
For preparing one molar solution we are required to dissolve one mole of the NaCl ( 58.5g) , for 2.5 mol we require 2.5 * 58.5= 146.25 g
Which expression correctly represents Henry’s Law?- a)
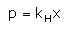
- b)
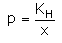
- c)
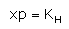
- d)
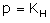
Correct answer is option 'A'. Can you explain this answer?
Which expression correctly represents Henry’s Law?
a)
b)
c)
d)
|
|
Arjun Singhania answered |
The Henry's law constant (KH) (also called the air–water partition coefficient) is the ratio of a compound's partial pressure in air to the concentration of the compound in water at a given temperature. Values for Henry's law constants are expressed in units of atmospheres for air to moles per cubic meter for water (atm-m3/mol) or in a dimensionless unit described as KH′ = KH/(RT) where KH′ is the dimensionless Henry's law constant, KH is the Henry's law constant (atm-m3/mol), R is the ideal gas constant (8.20575 x 10−5 atm-m3/mol-K) and T is the water temperature (K). As a rule of thumb, compounds with a Henry's law constant greater than 10−3 atm-m3/mol and a molecular weight less than 200 grams per mole are considered volatile.
In a solution if the solute is non- volatile, pA0 is the vapour pressure of pure solvent A and the mole fraction of A is xA , the vapour pressure of the solvent (pA) will be :- a)
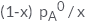
- b)
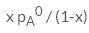
- c)
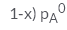
- d)
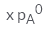
Correct answer is option 'D'. Can you explain this answer?
In a solution if the solute is non- volatile, pA0 is the vapour pressure of pure solvent A and the mole fraction of A is xA , the vapour pressure of the solvent (pA) will be :
a)
b)
c)
d)

|
Sushil Kumar answered |
The correct answer is option D
When a non - volatile solute is added into a volatile solvent to form an ideal solution. there will be a decrease in vapour pressure to that of solvent. According to roult's law the decrease in vapour is directly proportional to the mole fraction of solute in an ideal solution.
When a non - volatile solute is added into a volatile solvent to form an ideal solution. there will be a decrease in vapour pressure to that of solvent. According to roult's law the decrease in vapour is directly proportional to the mole fraction of solute in an ideal solution.
Can you explain the answer of this question below: Calculate the molality of 12.5% w/w sulphuric acid?
- A:
2.85m
- B:
3.15m
- C:
1.45m
- D:
2.50m
The answer is c.
Calculate the molality of 12.5% w/w sulphuric acid?
2.85m
3.15m
1.45m
2.50m
|
|
Arun Khanna answered |
12.5% w/w means 12.5 g in 100 g of solution.
Weight of solvent = 100 g – 12.5 g= 87.5 g. Number of moles of sulphuric acid = 12.5/ 98 =0.127 mol
So molality = 0.127 X 1000/87.5 = 1.45 m
Density of a 2.05 M solution of acetic acid in water is 1.02 g/mL. The molality of the solution is -[AIEEE 2006]- a)3.28 mol kg-1
- b) 2.28 mol kg-1
- c)0.44 mol kg-1
- d)1.14 mol kg-1
Correct answer is option 'B'. Can you explain this answer?
Density of a 2.05 M solution of acetic acid in water is 1.02 g/mL. The molality of the solution is -
[AIEEE 2006]
a)
3.28 mol kg-1
b)
2.28 mol kg-1
c)
0.44 mol kg-1
d)
1.14 mol kg-1
|
|
Hansa Sharma answered |
The correct answer is option B
weight of acetic acid =2.05×60=123g2.05×60=123g
Weight of solution =1000×1.02=1020g=1000×1.02=1020g
∴ Weight of water=(1020−123)=897g
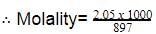
=2.285 mol kg-1
weight of acetic acid =2.05×60=123g2.05×60=123g
Weight of solution =1000×1.02=1020g=1000×1.02=1020g
∴ Weight of water=(1020−123)=897g
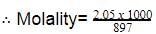
=2.285 mol kg-1
If α is the degree of dissociation of Na2SO4, the van’t Hoff factor ,i , used for calculating the molecular mass is- a)1 + α
- b)1 – 2α
- c)1 + 2α
- d)1 – α
Correct answer is option 'C'. Can you explain this answer?
If α is the degree of dissociation of Na2SO4, the van’t Hoff factor ,i , used for calculating the molecular mass is
a)
1 + α
b)
1 – 2α
c)
1 + 2α
d)
1 – α

|
Nilesh Saini answered |
Na2SO4 ↔ 2Na^+ + SO4^2–
1 0 0
1 – α 2α α
Vant Hoff factor (i) = (1 – α + 2α + α)/1
= 1 + 2α
The correct option is c.
Which of the following unit of concertration is independent of temperature?- a)Molarity
- b)Molality
- c)Mole fraction
- d)all
Correct answer is option 'B'. Can you explain this answer?
Which of the following unit of concertration is independent of temperature?
a)
Molarity
b)
Molality
c)
Mole fraction
d)
all

|
Kritika Singh answered |
Those concentration terms which involve volume are temperature dependent while those which not involve volume are temperature independent....
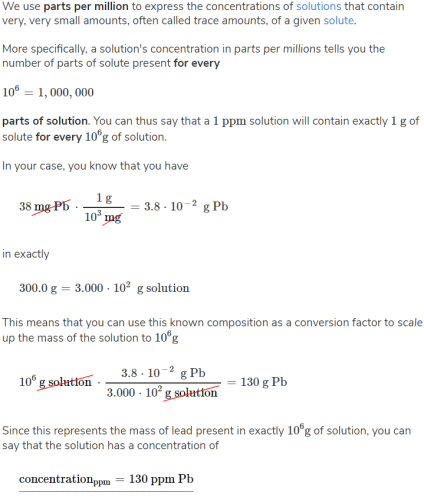
A sample of 300.0 g of drinking water is found to contain 38 mg Pb. What this concentration in parts per million?- a)3 x 102 ppm
- b)6.5 m
- c)130 ppm Pb
- d)21 ppm
Correct answer is option 'C'. Can you explain this answer?
A sample of 300.0 g of drinking water is found to contain 38 mg Pb. What this concentration in parts per million?
a)
3 x 102 ppm
b)
6.5 m
c)
130 ppm Pb
d)
21 ppm
|
|
Mira Sharma answered |
We use parts per million to express the concentrations of solutions that contain very, very small amounts, often called trace amounts, of a given solute.
More specifically, a solution's concentration in parts per millions tells you the number of parts of solute present for every
10^6=1,000,000
parts of solution. You can thus say that a 1 ppm
solution will contain exactly 1 g of solute for every 10^6g of solution.
In this case, you know that you have
38 mg Pb x (1 g/10^3 mg) = 3.8 x 10^-2 g Pb
in exactly
300.0 g = 3.000 x 10^2 g solution
This means that you can use this known composition as a conversion factor to scale up the mass of the solution to
10^6 g solution x (3.8 x 10^-2 g Pb/3.000 x 10^2 g solution)
= 130 g solution
Since this represents the mass of lead present in exactly 10^6 g of solution, you can say that the solution has a concentration of:
concentration (ppm) = 130 ppm Pb
Chapter doubts & questions for Solutions - Chapter-wise Tests for JEE Main & Advanced 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Solutions - Chapter-wise Tests for JEE Main & Advanced in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Chapter-wise Tests for JEE Main & Advanced
446 docs|930 tests
|
Related JEE Content
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup