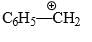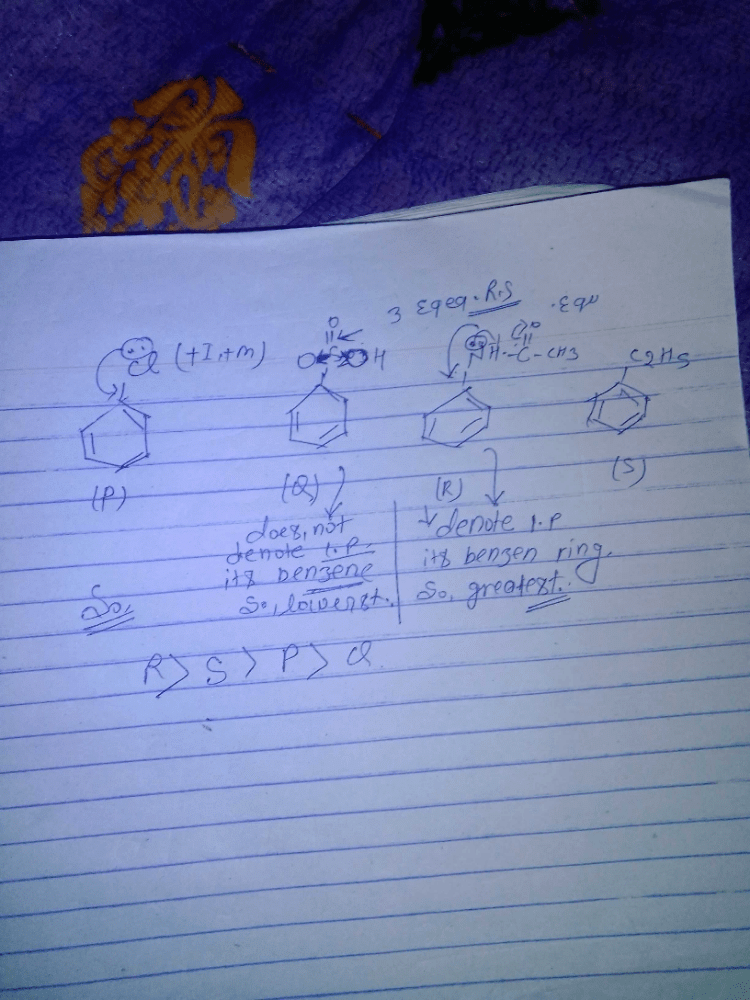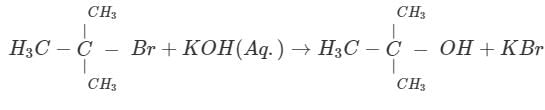All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Organic Reaction Mechanism for Chemistry Exam
For the reaction below if the concentration of KCN is increased four times, the rate of the reaction will be:
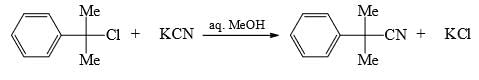
- a) Doubled
- b) Increased four times
- c)Unaffected
- d) Halved
Correct answer is option 'C'. Can you explain this answer?
For the reaction below if the concentration of KCN is increased four times, the rate of the reaction will be:


a)
Doubled
b)
Increased four times
c)
Unaffected
d)
Halved
|
|
Vikram Kapoor answered |
This is an SN1 reaction, so, rate of the reaction only depends on substrate, it doesn't depend on nucleophile. Hence change in concentration of the nucleophile won't affect the rate.
C is correct.
The major product formed in the following reaction is: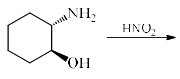
- a)
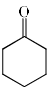
- b)
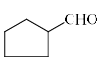
- c)
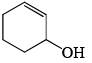
- d)
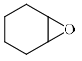
Correct answer is option 'B'. Can you explain this answer?
The major product formed in the following reaction is:
a)
b)
c)
d)
|
|
Chirag Verma answered |
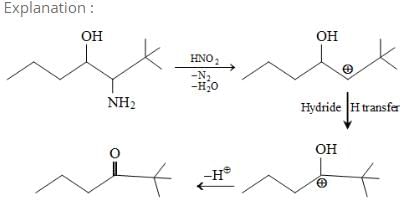
It is an example of semipinacol rearrangement. NH2 and OH group will remain in the diequatorial position in cyclohexane chair form. So after treatment with HNO2, NH2 becomes N2+. Then rearrangement takes place through C-C bond migration which is antiperiplanar to N2+. Thus ring contraction takes place and product will be B.
Consider the following carbocations is most stable:- a)C6H5CH2+
- b)C6H5CH2CH2+
- c)C6H5CH+CH3
- d)C6H5C+ (CH3)2
Correct answer is option 'D'. Can you explain this answer?
Consider the following carbocations is most stable:
a)
C6H5CH2+
b)
C6H5CH2CH2+
c)
C6H5CH+CH3
d)
C6H5C+ (CH3)2
|
|
Vedika Singh answered |
- The stability of C6H5CH2+ is due to resonance.
- The stability of C6H5CH2CH2+ is due to only inductive effect.
- The stability of C6H5CH+CH3 is due to resonance and +I effect of 1 methyl group.
- The stability of C6H5C+(CH3)2 is due to both resonance and +I effect of 2 methyl groups.
So, the order of stability of carbocation is d>c>a>b or b<a<c<d.
Solvolysis of the optically active compound X gives, mainly: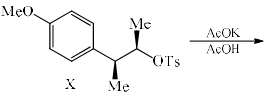
- a)
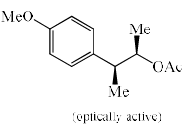
- b)
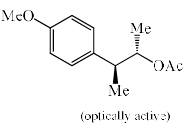
- c)
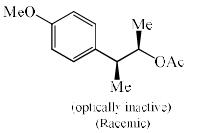
- d)
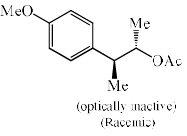
Correct answer is option 'C'. Can you explain this answer?
Solvolysis of the optically active compound X gives, mainly:
a)
b)
c)
d)

|
Asf Institute answered |
OTs is a good leaving group. So after departure of the leaving group (OTs-) a carbocation will form whose geometry is planer so there are two possibilities of attacking the nucleophile ( OAc- furnished by AcOK) :
1) above the plane.
2) down the plane.
So a racemic mixture will be formed which is optically inactive. If the nucleophile attacked the carbon centre from down the group then the configuration of the centre will either of the + or - then the racemic mixture was not obtained.
The correct order of reactivity of p-halonitrobenzens in the following reaction is 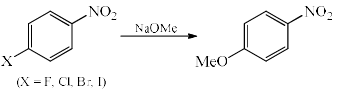
- a)p-Chloronitrobenzene > p-iodonitrobenezene > p-fluoronitrobenzene > p-bromonitrobenzene
- b)p-floronitrobenzene > p-chloronitrobenzene > p-bromonitrobenzene > p-iodonitrobenzene
- c)p-iodonitrobenzene > p-bromonitrobenzene > p-chloronitrobenzene > p-fluronitrobenzene
- d)p-bromonitrobenzene > p-fluronitrobenzene > p-iodonitrobenzene > p-chloronitrobenzene
Correct answer is option 'B'. Can you explain this answer?
The correct order of reactivity of p-halonitrobenzens in the following reaction is
a)
p-Chloronitrobenzene > p-iodonitrobenezene > p-fluoronitrobenzene > p-bromonitrobenzene
b)
p-floronitrobenzene > p-chloronitrobenzene > p-bromonitrobenzene > p-iodonitrobenzene
c)
p-iodonitrobenzene > p-bromonitrobenzene > p-chloronitrobenzene > p-fluronitrobenzene
d)
p-bromonitrobenzene > p-fluronitrobenzene > p-iodonitrobenzene > p-chloronitrobenzene
|
|
Dronacharya Institute answered |
- This is a classical example of aromatic nucleophilic substitution. Here the reaction proceeds through two steps, in the first step the nucleophile attacks and in the second step the leaving group detaches from the ring.
- The first step is slow, that is rate determining step because when the nucleophile attacks, the ring loses it's aromaticity and a negative charge arises which take part in resonance with NO2 group.
- So the departure of the leaving group is not the rate determining step (RDS) so it doesn't matter how the leaving group is, it depends on how much the leaving group stabilises the -ve charge. And it follows the order F > Cl > Br >I
Arrange stability of the given carbocations in decreasing order:

- a)I < II < III < IV
- b) IV < III < II < I
- c)IV < II < III < I
- d)II < IV < III < I
Correct answer is option 'C'. Can you explain this answer?
Arrange stability of the given carbocations in decreasing order:


a)
I < II < III < IV
b)
IV < III < II < I
c)
IV < II < III < I
d)
II < IV < III < I

|
Veda Institute answered |
Correct Answer :- c
Explanation : The carbocation here is stabilized when there is an electron-donating group present.
More the activating effect of the group, more stable is the cation. Out of given options, I is most stable due to ability of oxygen to activate the ring with its lone pair of electrons.
Out of III and I,III is more stable as the lone pair of nitrogen in II is less available due to Ac group. IV is least stable due to electron- withdrawing effect of Cl atom.
The correct answer is : IV < II < III < I
Reactive intermediate formed in the following reaction is: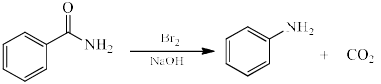
- a)Carbene
- b)Isocyanate
- c)Nitrene
- d)Carbanion
Correct answer is option 'B'. Can you explain this answer?
Reactive intermediate formed in the following reaction is:
a)
Carbene
b)
Isocyanate
c)
Nitrene
d)
Carbanion
|
|
Chirag Verma answered |
The reaction of bromine with sodium hydroxide forms sodium hypobromite in situ, which transforms the primary amide into an intermediate isocyanate. The formation of an intermediate nitrene is not possible because it also implies the formation of a hydroxamic acid as a byproduct, which has never been observed. The intermediate isocyanate is hydrolyzed to a primary amine, giving off carbon dioxide.
The major product P formed in the given: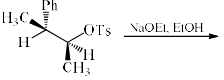
- a)

- b)
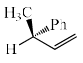
- c)
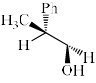
- d)
Correct answer is 'D'. Can you explain this answer?
The major product P formed in the given:
a)
b)
c)
d)
|
|
Nayak Satyaprakash Shyam answered |
In question cH3 is on cis position so D is correct there is no inversion of compound
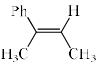
The major product formed in the following reaction is :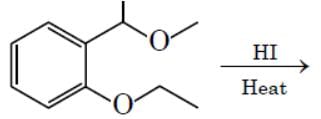
- a)
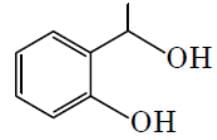
- b)
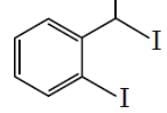
- c)
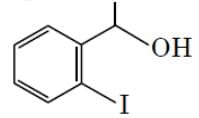
- d)
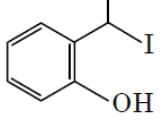
Correct answer is option 'D'. Can you explain this answer?
The major product formed in the following reaction is :

a)

b)

c)

d)


|
Edurev.iitjam answered |
Hydrolysis of ether. Lone pair of oxygen captures H atom from HI group that is present on the up gives rise to a stable carbocation(Resonance stabilized as well as well +i effect of 1 methyl group) and the reaction proceeds via SN1 pathway where I- acts as a nucleophile, but for other group, formed carbocation is highly unstable and ends up at alcohol.
An intermediate in racemization of (R)-3-phenyl-2-butanone is:
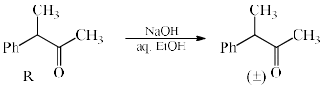
- a)
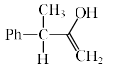
- b)
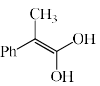
- c)
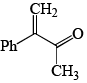
- d)
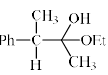
Correct answer is option 'B'. Can you explain this answer?
An intermediate in racemization of (R)-3-phenyl-2-butanone is:
a)
b)
c)
d)

|
Veda Institute answered |
Racemization by this mechanism occurs only at a carbon stereocenters with at least one a hydrogen. This process is usually an undesired side effect of acid impurities in a sample, because it is often, in medicine for example, important to have an enantiomerically pure form of a compound rather than a racemic mixture.
When enantiomerically pure (either R or S) 3-phenyl-2-butanone is dissolved in ethanol, no change occurs in the optical activity of the solution over time. If, however, a trace of acid (for example, HCl) is added, the optical activity of the solution begins to decrease and gradually drops to zero. When 3-phenyl-2-butanone is isolated from this solution, it is found to be a racemic mixture.
Identify correct C—O bond length order:
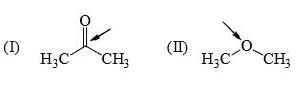
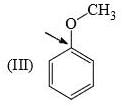
- a)I > II > III
- b)II > III > I
- c) I > III > II
- d) III > I > II
Correct answer is option 'B'. Can you explain this answer?
Identify correct C—O bond length order:
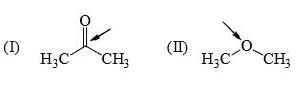

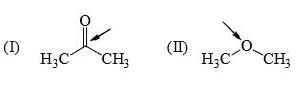

a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II
|
|
Vedika Singh answered |
I is the shortest because of double bond.
III is next because of resonance.
II is last as there is no kind of bond strengthening.
Hence, B is correct.
Identify correct acidic strength order in the following compounds:
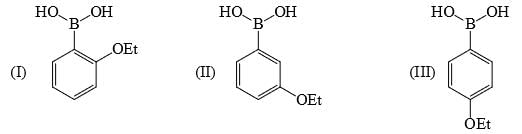
a)II > III > Ib) III > II > I c)I > II > IIId)III > I > IICorrect answer is option 'C'. Can you explain this answer?
b) III > II > I
c)I > II > III
d)III > I > II
Correct answer is option 'C'. Can you explain this answer?
|
|
Chirag Verma answered |
Correct Answer :- C
Explanation : The correct option is A. Since −OE+ which increases the acidity of the compound and is distance dependent. In I −OE+ group is nearer to the substituents. So, its more acidic than the other two. SO the correct order will be i>ii>iii.
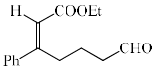
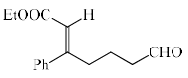
In the reaction, the major product X is: 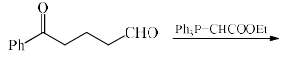
- a)
- b)
- c)
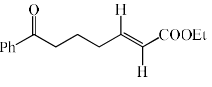
- d)
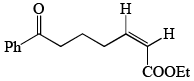
Correct answer is option 'C'. Can you explain this answer?
In the reaction, the major product X is: 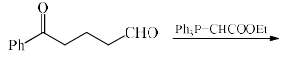
a)
b)
c)
d)
|
|
Pooja Choudhury answered |
Aldehyde is more reactive than ketone, so, E alkene is generated on aldehyde.
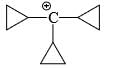
The relative stability of the following carbocations is:
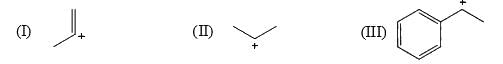
- a) I > II > III
- b) II > III > I
- c) I > III > II
- d)III > II > I
Correct answer is option 'D'. Can you explain this answer?
The relative stability of the following carbocations is:


a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > II > I

|
Veda Institute answered |
Correct Answer :- d
Explanation : The dispersal of the charge stabilizes the carbocation. More the number of alkyl groups, the greater the dispersal of positive charge and therefore, more the stability of carbocation,is an electron donating group, thus it will increases the stability of carbocation, hence the expected order is, (iii)>(ii)>(i).
The acidity for the following compounds increases in the order:CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH- a) I < II < III
- b) II < III < I
- c) III < II < I
- d) II < I < III
Correct answer is option 'C'. Can you explain this answer?
The acidity for the following compounds increases in the order:
CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH
a)
I < II < III
b)
II < III < I
c)
III < II < I
d)
II < I < III

|
Edurev.iitjam answered |
a. EWG ( e−− withdrawing groups) increases the acidic strength, whereas EDG ( e−− donating groups) decrease the acidic strength.
b. Nearer is the EWG to the source [(−COOH)group], stronger is the acid, i.e., α− substituted halo acid stronger than β−orγ− substituted halo acid.
Increasing order of acidic strength:
(III)<(II)<(I).
Increasing order of acidic strength:
(III)<(II)<(I).
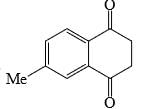
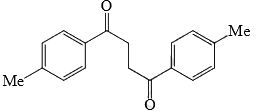
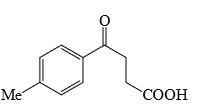
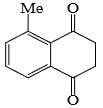
Identify that compounds that give Cannizaro reaction:
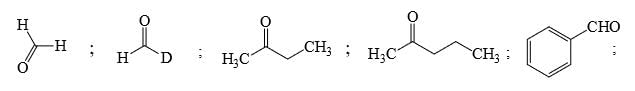
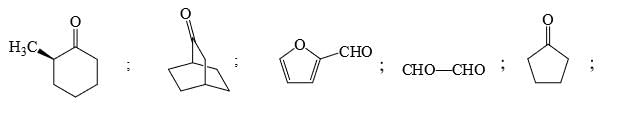
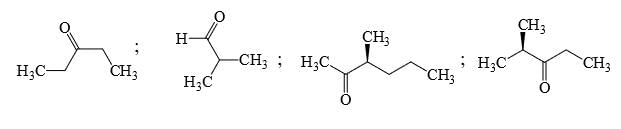
Correct answer is '6'. Can you explain this answer?
Identify that compounds that give Cannizaro reaction:





|
Adarsh Kumar Pathak answered |
Mere hisab se to 5 hi aaye ga
Which of the following statement(s) is/are true about the reaction given below:
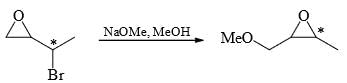 (1) it involves a carbocation intermediate
(1) it involves a carbocation intermediate
(2) rearrangement is due to SN1 reaction mechanism.
(3) it proceeds via a concerted SN2 pathway
(4) it involves neighbouring group participation.
- a)(1) only
- b)(3) and (4) only
- c)(2) and (4) only
- d)All of the above
Correct answer is option 'B'. Can you explain this answer?
Which of the following statement(s) is/are true about the reaction given below:
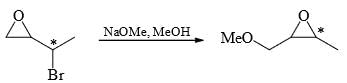
(1) it involves a carbocation intermediate
(2) rearrangement is due to SN1 reaction mechanism.
(3) it proceeds via a concerted SN2 pathway
(4) it involves neighbouring group participation.
(2) rearrangement is due to SN1 reaction mechanism.
(3) it proceeds via a concerted SN2 pathway
(4) it involves neighbouring group participation.
a)
(1) only
b)
(3) and (4) only
c)
(2) and (4) only
d)
All of the above

|
Veda Institute answered |
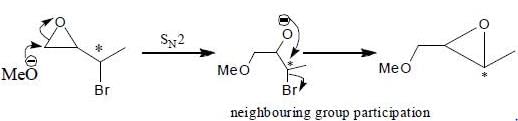 From above mechanism:
From above mechanism:If proceecs via a concerted SN2 pathway.
It involves neighbouring group participation.
The major product formed in the following reaction is: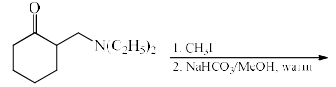
- a)
- b)
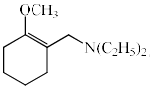
- c)
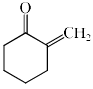
- d)
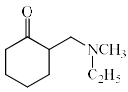
Correct answer is option 'C'. Can you explain this answer?
The major product formed in the following reaction is:
a)
b)
c)
d)
|
|
Pooja Choudhury answered |
In the first step of the reaction a quaternary ammonium salt is formed which is then eliminated to form the alkene. The alkene formation proceeds through the E1cB pathway where the acidic beta hydrogen is abstracted by the Base and the carbanion is stabilised by the positively charged leaving group i. e N+(C2H5)CH3 which is a powerful electron withdrawing group and the Hoffman product is predominant product.
The major product of the reaction
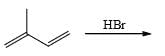
- a)
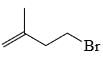
- b)
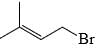
- c)
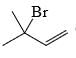
- d)
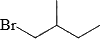
Correct answer is option 'C'. Can you explain this answer?
The major product of the reaction
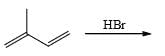
a)

b)

c)

d)

|
|
Pooja Choudhury answered |
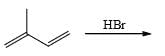

According to Markovnikov's rule, when an alkene undergoes hydrohalogenation, the proton is added to the carbon with the highest number of hydrogens, resulting in a stable carbocation intermediate before the nucleophile attack.
What is the nucleophilicity order for SN2 reaction:(I) C6H5S–
(II) C2H5O–
(III) NO3–
(IV) CN–
(V) I–- a)V > II > IV > I > III
- b)III > IV > V > II > I
- c)I > IV > V > II > III
- d)II > IV > V > III > I
Correct answer is option 'A'. Can you explain this answer?
What is the nucleophilicity order for SN2 reaction:
(I) C6H5S–
(II) C2H5O–
(III) NO3–
(IV) CN–
(V) I–
(II) C2H5O–
(III) NO3–
(IV) CN–
(V) I–
a)
V > II > IV > I > III
b)
III > IV > V > II > I
c)
I > IV > V > II > III
d)
II > IV > V > III > I
|
|
Pooja Choudhury answered |
PhS- is a better nucleophile in SN2 rxn than any other nucleophile given in the question.
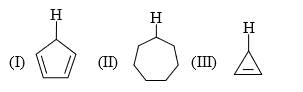
Increasing order of stability of following carbocations (give least stable first)?(I) Tropylium
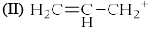
(III) (C6H5)2C+
(III) CH3+- a)III < I <II <IV
- b)IV < II <III <I
- c)I < III <II <IV
- d)IV < III <II <I
Correct answer is option 'B'. Can you explain this answer?
Increasing order of stability of following carbocations (give least stable first)?
(I) Tropylium
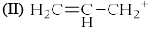
(III) (C6H5)2C+
(III) CH3+
(III) (C6H5)2C+
(III) CH3+
a)
III < I <II <IV
b)
IV < II <III <I
c)
I < III <II <IV
d)
IV < III <II <I

|
Pie Academy answered |
- Tropylium - aromatic with 6 pi-electrons (Most stable carbocation)
- Triphenylmethyl carbocation - Positive charge in resonance with two phenyl rings.
- Allylic carbonation - Positive charge delocalized over 3 carbons.
- Methyl carbonation - No stabilizing interactions.
Which is the most stable arenium carbocation:
- a)
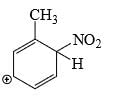
- b)
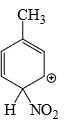
- c)
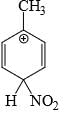
- d)
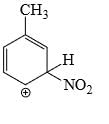
Correct answer is option 'C'. Can you explain this answer?
Which is the most stable arenium carbocation:
a)

b)

c)

d)


|
Beena Joshi answered |
No. of alpha hydrogens are more
For the reaction between alkyl halide and OH- increase in solvent polarity generally- a)Decreases the rate of SN1 reaction
- b)Increases the rate of SN1 reaction
- c)Increases the rate of SN2 reaction
- d)Does not alter the rate of SN1 and SN2 reaction
Correct answer is option 'B'. Can you explain this answer?
For the reaction between alkyl halide and OH- increase in solvent polarity generally
a)
Decreases the rate of SN1 reaction
b)
Increases the rate of SN1 reaction
c)
Increases the rate of SN2 reaction
d)
Does not alter the rate of SN1 and SN2 reaction
|
|
Pooja Choudhury answered |
A polar protic solvent favours SN1 mechanism because polar solvents has the below properties:
- It stabilizes the carbocation intermediate. Polar solvents like methanol have a permanent dipole which means that partial negative charge on the molecule will have dipole-dipole interactions with the carbocation, stabilizing it.
- It reduces the reactivity of the nucleophile. The polar solvent can interact electrostatically with the nucleophile. This reduces the reactivity of the nucleophile and enhances the SN1 reaction.
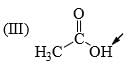
The relative acidity of the indicated H in each of the following is: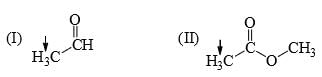
- a) I > II > III
- b)II > III > I
- c) I > III > II
- d) III > I > II
Correct answer is option 'D'. Can you explain this answer?
The relative acidity of the indicated H in each of the following is:
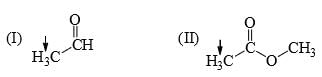

a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II
|
|
Subha Som answered |
In 3 the h is attached with acidic carboxyl gr
in 1 the -ve charge which generate after removing h will get stability by carbonyl gr of aldehyde
but in 2 the carbonyl gr is less available in ester gr
in 1 the -ve charge which generate after removing h will get stability by carbonyl gr of aldehyde
but in 2 the carbonyl gr is less available in ester gr
The relative nucleophilicity in polar, protic, solvents of the following is:
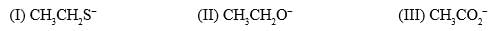
- a)I > II > III
- b)II > III > I
- c) I > III > II
- d) III > I > II
Correct answer is option 'A'. Can you explain this answer?
The relative nucleophilicity in polar, protic, solvents of the following is:
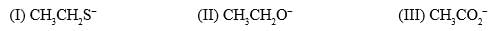
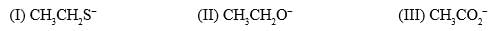
a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II

|
Veda Institute answered |
Correct Answer :- a
Explanation : In a polar protic solvent (CH3OH), nucleophilicity increases down a column of the periodic table. So S- is more nucleophilic than O-.
For two species with the same attacking atom, the more basic is the more nucleophilic, so CH3CH2O- is more nucleophilic than CH3CO2-
CO2- give identical str resonance hybrids more solvated, very less nucleophilic.
Which of the following carbonium ion is most stable?
- a)
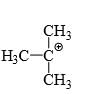
- b)
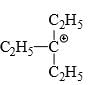
- c)
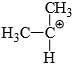
- d)
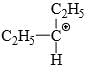
Correct answer is option 'A'. Can you explain this answer?
Which of the following carbonium ion is most stable?
a)

b)

c)

d)

|
|
Pooja Choudhury answered |
A is the correct option as 9- alpha Hydrogens are present. More alpha hydrogen, more hyperconjugation, more stability.
Cannizzaro reaction is not given by:- a)Formaldehyde
- b)Acetaldehyde
- c)Benzaldehyde
- d)Trimethylacetaldehyde
Correct answer is option 'B'. Can you explain this answer?
Cannizzaro reaction is not given by:
a)
Formaldehyde
b)
Acetaldehyde
c)
Benzaldehyde
d)
Trimethylacetaldehyde

|
Rohan Desai answered |
The Cannizzaro reaction is not given by Acetaldehyde. Aldehydes that contains alpha hydrogen atoms are not involved in Cannizzaro reaction and aldehyde like acetaldehyde contains alpha hydrogen in it. So, that's why it can't participate or involve in this reaction.
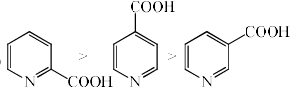
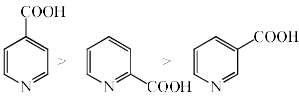
The rate of decarboxylation of isomeric carboxylic acids is:- a)
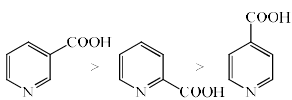
- b)
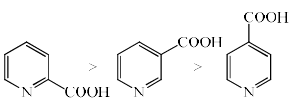
- c)
- d)
Correct answer is option 'C'. Can you explain this answer?
The rate of decarboxylation of isomeric carboxylic acids is:
a)
b)
c)
d)

|
Gaurav Singh answered |
Answer is wrong ....correct the ans...during the decarboxylation intermediate is carboanion...when acid reacts with NaOH+CaO decarboxylatuon takes place...now check the stability of carbaion...option is b which is coorrect
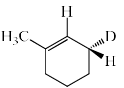
The major product formed in the following reaction is: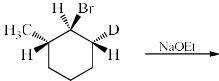
- a)
- b)
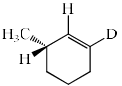
- c)
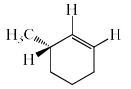
- d)
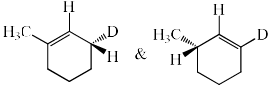
Correct answer is option 'C'. Can you explain this answer?
The major product formed in the following reaction is:
a)
b)
c)
d)
|
|
Dronacharya Institute answered |
NaOEt is a strong base and it will abstract the acidic proton from the alpha carbon. Dueterium is abstracted from alpha carbon because it is show more +I effect than hydrogen. After that then elimination will occur with bromide as leaving group.
Hence C
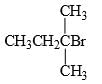
In this transformation,
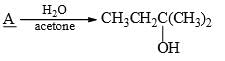 What is the best structure for A?
What is the best structure for A?
- a)
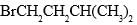
- b)
- c)
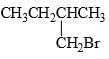
- d)

Correct answer is option 'B'. Can you explain this answer?
In this transformation,
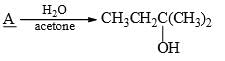
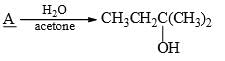
What is the best structure for A?
a)
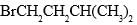
b)

c)
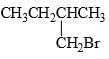
d)


|
Himalika Pantola answered |
B because the reaction reaction intermediate formed after removal of Br the more electronegative compound than carbon make it carboaction which is in case of B stabilized by hyperconjugation and +I effect of methyl group leading to stable product
Which of the following statements is correct:
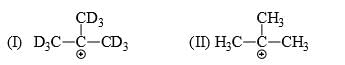
- a) I is more stable than II
- b)II is more stable than I
- c)Both are equally stable
- d) Stability criterion cannot be applied in this case
Correct answer is option 'B'. Can you explain this answer?
Which of the following statements is correct:
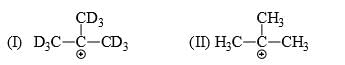
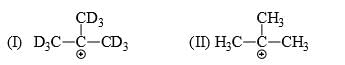
a)
I is more stable than II
b)
II is more stable than I
c)
Both are equally stable
d)
Stability criterion cannot be applied in this case

|
Adarsh Shukla answered |
Since C-D is a much stronger bond than C-H so there is more hyperconjugation in C-H bond and hence 2nd structure with 9 alpha hydrogen is more stable than 1st structure
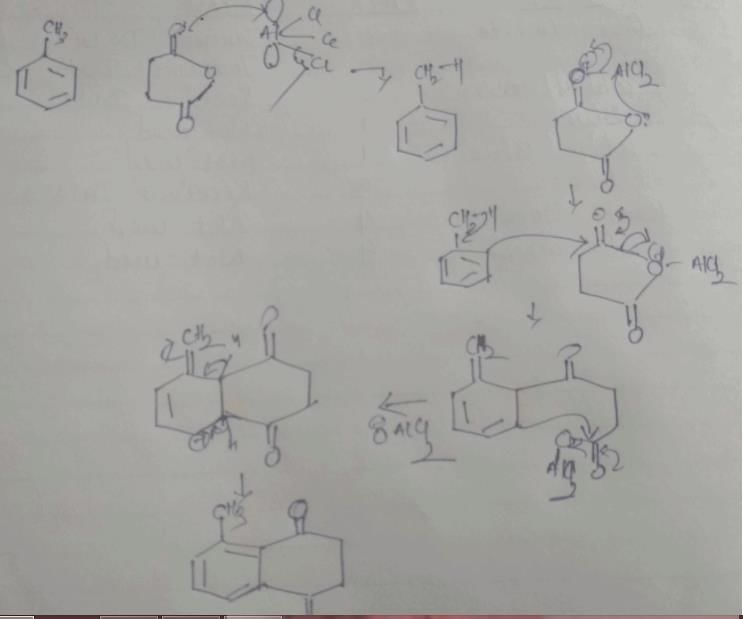
In the cyclisation reaction given below, the most probable product formed is:
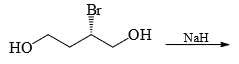
- a)
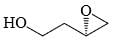
- b)
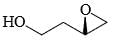
- c)
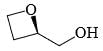
- d)
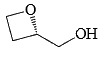
Correct answer is option 'C'. Can you explain this answer?
In the cyclisation reaction given below, the most probable product formed is:


a)

b)

c)

d)
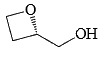

|
Asf Institute answered |
The given reaction involves cyclisation using sodium hydride (NaH) as a base. The mechanism can be understood as follows:
- Deprotonation by NaH: Sodium hydride (NaH) is a strong base and will deprotonate the hydroxyl (OH) group, forming an alkoxide ion (RO-).
- Nucleophilic Substitution: The alkoxide ion will act as a nucleophile and will attack the electrophilic carbon that is attached to the bromine (Br). This will lead to the displacement of the bromine atom and formation of a cyclic ether.
Analyzing the structure, the most probable cyclisation would be a 5-membered ring formation due to the stability and strain factors.
Let's consider the provided options:
- Option A: This forms a linear structure with no ring formation, which is not consistent with the given reagents and conditions.
- Option B: This forms a 3-membered ring, which is highly strained and less likely to form under these conditions.
- Option C: This forms a 4-membered ring where the oxygen is part of the ring and the hydroxyl group is on an adjacent carbon, which fits the expected cyclisation.
- Option D: This also forms a 4-membered ring but with the hydroxyl group on a different position than Option B, which is not consistent with the given starting material.
Based on the analysis, Option C is the most probable product formed in this cyclisation reaction.
The major product obtained treatment of compound X with H2SO4 at 80°C is: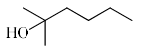
- a)

- b)
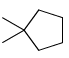
- c)
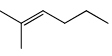
- d)
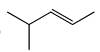
Correct answer is option 'C'. Can you explain this answer?
The major product obtained treatment of compound X with H2SO4 at 80°C is:
a)
b)
c)
d)
|
|
Rima Das answered |
This is an example of. dehydration of alkane.And there is a possibility of forming two products (a) and (c). In (c) the double bond is more substituted, so it is the major product.
A compound 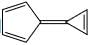 shows a large dipole moment. Which of the following resonance structures
shows a large dipole moment. Which of the following resonance structures
can be used to adequately explain this observation:
- a)I only
- b) III and IV
- c) II and III
- d) IV only
Correct answer is option 'B'. Can you explain this answer?
A compound  shows a large dipole moment. Which of the following resonance structures
shows a large dipole moment. Which of the following resonance structures
can be used to adequately explain this observation:
 shows a large dipole moment. Which of the following resonance structures
shows a large dipole moment. Which of the following resonance structurescan be used to adequately explain this observation:

a)
I only
b)
III and IV
c)
II and III
d)
IV only
|
|
Pooja Choudhury answered |
Both (IV) and (III) member ring are aromatic due to charge separation
in case of 5 member ring total 6 electron and 3 member ring 2 electron are present
according to 4n+2 rule they are aromatic
and all the carbon are sp2 hybridize
in case of 5 member ring total 6 electron and 3 member ring 2 electron are present
according to 4n+2 rule they are aromatic
and all the carbon are sp2 hybridize
Which of the following statements regarding the E2 mechanism is wrong?- a)Reactions by the E2 mechanism are always bimolecular.
- b)Reactions by the E2 mechanism are generally second order.
- c)Reactions by the E2 mechanism usually occur in one step.
- d)Reactions by the E2 mechanism usually occur in two steps.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements regarding the E2 mechanism is wrong?
a)
Reactions by the E2 mechanism are always bimolecular.
b)
Reactions by the E2 mechanism are generally second order.
c)
Reactions by the E2 mechanism usually occur in one step.
d)
Reactions by the E2 mechanism usually occur in two steps.
|
|
Pooja Choudhury answered |
E2 stands for bimolecular elimination. The reaction involves a one-step mechanism in which carbon-hydrogen and carbon-halogen bonds break to form a double bond (C=C Pi bond).
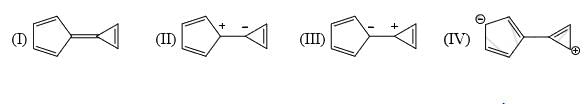
Reactivity of the following towards reaction with NaBH4 is:
- a) I > II > III
- b)II > III > I
- c) I > III > II
- d)III > I > II
Correct answer is option 'A'. Can you explain this answer?
Reactivity of the following towards reaction with NaBH4 is:

a)
I > II > III
b)
II > III > I
c)
I > III > II
d)
III > I > II
|
|
Subha Som answered |
As crowding increase the rate of reduction reduce
What will be the correct order of SN2/E2 ratio for the %yield of the product of the following halide?
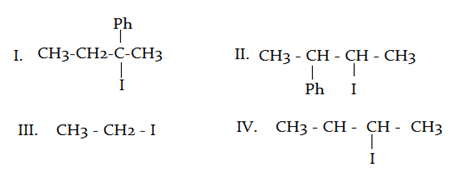
- a)III > IV > II > I
- b)III > II > IV > I
- c)I > III > IV > II
- d)II > I > III > IV
Correct answer is option 'A'. Can you explain this answer?
What will be the correct order of SN2/E2 ratio for the %yield of the product of the following halide?
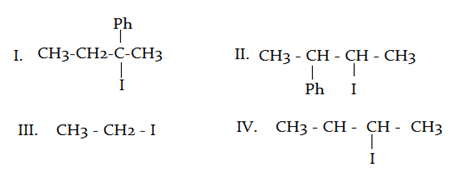
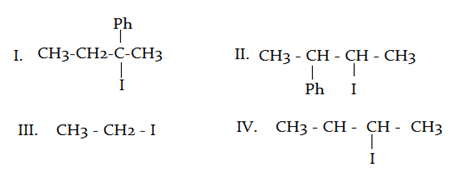
a)
III > IV > II > I
b)
III > II > IV > I
c)
I > III > IV > II
d)
II > I > III > IV

|
Pioneer Academy answered |
Least hindered halide give fastest SN2 reaction as the hindrance increases. As the hindrance increases, the occurrence of SN2 reaction decreases.
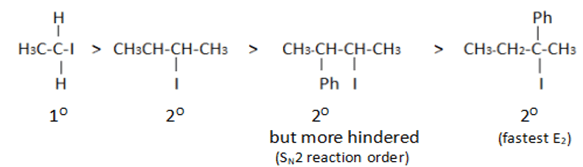
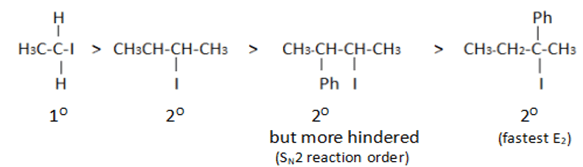
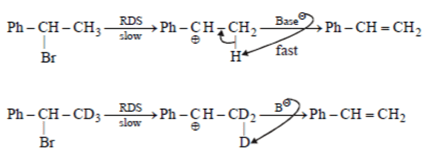
Assuming both the reactions as E1, where will the expected ratio between KH/KD lies between?
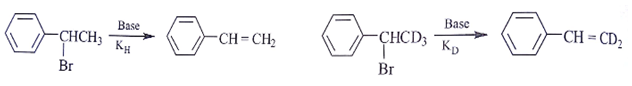
- a)nearly I
- b)nearly 3
- c)nearly 5
- d)anything in between 2 and 8
Correct answer is option 'A'. Can you explain this answer?
Assuming both the reactions as E1, where will the expected ratio between KH/KD lies between?


a)
nearly I
b)
nearly 3
c)
nearly 5
d)
anything in between 2 and 8

|
Asf Institute answered |
In E1 elimination RDS is the formation of carbocation from halide hence C – H and C – D are not the part of RDS there KH/KD is approx. 1.

Which of the following statement is correct regarding the following reaction?
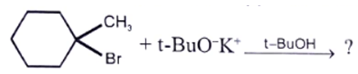
- a)Major product is endocyclic alkene formed according to Saytzeff
- b)Major product is exocyclic alkene formed according to Saytzeff
- c)Major product is exocyclic alkene formed according to Hoffmann
- d)Major product is endocyclic alkene formed according to Hoffmann
Correct answer is option 'C'. Can you explain this answer?
Which of the following statement is correct regarding the following reaction?
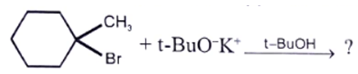
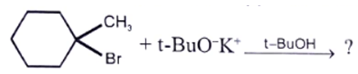
a)
Major product is endocyclic alkene formed according to Saytzeff
b)
Major product is exocyclic alkene formed according to Saytzeff
c)
Major product is exocyclic alkene formed according to Hoffmann
d)
Major product is endocyclic alkene formed according to Hoffmann

|
Pioneer Academy answered |
Exocyclic alkene are more stable than endo beacuse of but due to “steric hindrance” the less substituted alkene is formed.

Chapter doubts & questions for Organic Reaction Mechanism - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Organic Reaction Mechanism - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup