All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Organometallic Chemistry for Chemistry Exam
Among the following, the unstable carbonyl species is:- a)Mn(CO)5Cl
- b)[Mn(CO)5]–
- c)[Mn(CO)5]+
- d)Mn(CO)5
Correct answer is option 'D'. Can you explain this answer?
Among the following, the unstable carbonyl species is:
a)
Mn(CO)5Cl
b)
[Mn(CO)5]–
c)
[Mn(CO)5]+
d)
Mn(CO)5

|
Omkar Sahu answered |
C and d are unstable carbonyl because it is not follow 18e- rule.
The catalyst used for the oxidation of ethylene to acetaldehyde is:
- a)Ru(PPh3)3Cl
- b)Co2(CO)8 and H2
- c)TiCl4 and AlEt3
- d)PdCl2 and CuCl
Correct answer is option 'D'. Can you explain this answer?
The catalyst used for the oxidation of ethylene to acetaldehyde is:
a)
Ru(PPh3)3Cl
b)
Co2(CO)8 and H2
c)
TiCl4 and AlEt3
d)
PdCl2 and CuCl

|
Anirban Khanna answered |
The principle of the process currently in general use - the partial oxidation of ethylene to acetaldehyde - is based on the observation made by F. C. Phillips back in 1894 that platinum metal salts stoichiometrically oxidize ethylene selectively to acetaldehyde while themselves being reduced to the metal.
In the trans-PtCl2L(CO) complex, the CO stretching frequency for L = NH3, pyridine, NMe3 decreases in the order:- a)Pyridine > NH3 > NMe3
- b)NH3 > Pyridine > NMe3
- c)NMe3 > NH3 > Pyridine
- d)Pyridine > NMe3 > NH3
Correct answer is option 'A'. Can you explain this answer?
In the trans-PtCl2L(CO) complex, the CO stretching frequency for L = NH3, pyridine, NMe3 decreases in the order:
a)
Pyridine > NH3 > NMe3
b)
NH3 > Pyridine > NMe3
c)
NMe3 > NH3 > Pyridine
d)
Pyridine > NMe3 > NH3

|
Shreya Chauhan answered |
Explanation:
The CO stretching frequency in trans-PtCl2L(CO) complex is affected by the nature of the ligand L. The order of decreasing CO stretching frequency for different ligands is as follows:
a) Pyridine NH3 NMe3
b) NH3 Pyridine NMe3
c) NMe3 NH3 Pyridine
d) Pyridine NMe3 NH3
The reason for this order can be explained by the trans effect of the ligands on the CO molecule.
1. Pyridine has the lowest CO stretching frequency because it has the weakest trans effect on the CO molecule. This is because pyridine is a pi-donor ligand and does not have any lone pair of electrons to donate to the metal center. Therefore, it does not interact strongly with the CO molecule.
2. NH3 has a higher CO stretching frequency than pyridine because it has a stronger trans effect on the CO molecule. This is because NH3 is a stronger sigma-donor ligand and can donate its lone pair of electrons to the metal center. This interaction between the nitrogen lone pair and the metal center weakens the bond between the metal and the CO molecule, resulting in a higher CO stretching frequency.
3. NMe3 has the highest CO stretching frequency because it has the strongest trans effect on the CO molecule. This is because NMe3 is a stronger sigma-donor ligand than NH3 and can donate its lone pair of electrons more effectively to the metal center. This interaction weakens the bond between the metal and the CO molecule even further, resulting in a higher CO stretching frequency.
Therefore, the order of decreasing CO stretching frequency for different ligands in trans-PtCl2L(CO) complex is Pyridine < nh3="" />< nme3.="" />
The CO stretching frequency in trans-PtCl2L(CO) complex is affected by the nature of the ligand L. The order of decreasing CO stretching frequency for different ligands is as follows:
a) Pyridine NH3 NMe3
b) NH3 Pyridine NMe3
c) NMe3 NH3 Pyridine
d) Pyridine NMe3 NH3
The reason for this order can be explained by the trans effect of the ligands on the CO molecule.
1. Pyridine has the lowest CO stretching frequency because it has the weakest trans effect on the CO molecule. This is because pyridine is a pi-donor ligand and does not have any lone pair of electrons to donate to the metal center. Therefore, it does not interact strongly with the CO molecule.
2. NH3 has a higher CO stretching frequency than pyridine because it has a stronger trans effect on the CO molecule. This is because NH3 is a stronger sigma-donor ligand and can donate its lone pair of electrons to the metal center. This interaction between the nitrogen lone pair and the metal center weakens the bond between the metal and the CO molecule, resulting in a higher CO stretching frequency.
3. NMe3 has the highest CO stretching frequency because it has the strongest trans effect on the CO molecule. This is because NMe3 is a stronger sigma-donor ligand than NH3 and can donate its lone pair of electrons more effectively to the metal center. This interaction weakens the bond between the metal and the CO molecule even further, resulting in a higher CO stretching frequency.
Therefore, the order of decreasing CO stretching frequency for different ligands in trans-PtCl2L(CO) complex is Pyridine < nh3="" />< nme3.="" />
Which of the following will exhibit optical isomerism:- a)[Cr(en)(H2O)4]3+
- b)[Cr(en)3]3+
- c)trans-[Cr(en)Cl2(NH3)2]+
- d)[Cr(NH3)6]3+
Correct answer is option 'B'. Can you explain this answer?
Which of the following will exhibit optical isomerism:
a)
[Cr(en)(H2O)4]3+
b)
[Cr(en)3]3+
c)
trans-[Cr(en)Cl2(NH3)2]+
d)
[Cr(NH3)6]3+

|
Anisha Banerjee answered |
Explanation:
Optical isomerism is a form of stereoisomerism that arises when a molecule has a non-superimposable mirror image. Optical isomers are also known as enantiomers.
Enantiomers are defined as chiral molecules that are mirror images of each other and cannot be superimposed on each other. A chiral molecule has no plane of symmetry and is not superimposable on its mirror image. A molecule that is not chiral is known as an achiral molecule.
The formula for the complex ion is [Cr(en)3]3+. The complex ion contains three bidentate en ligands (ethylenediamine) coordinated to a central chromium(III) ion.
In [Cr(en)3]3+, the three en ligands are arranged around the central chromium ion in a trigonal prismatic geometry. Each en ligand forms two coordination bonds with the chromium ion: one through each of its nitrogen atoms.
The complex ion has optical isomerism because the three en ligands are arranged in a chiral manner around the central chromium ion. The complex ion has a non-superimposable mirror image, which is its enantiomer.
In contrast, the other complexes listed do not have optical isomerism because they have either a plane of symmetry or an internal mirror plane.
a) [Cr(en)(H2O)4]3 has a plane of symmetry due to the presence of the water ligands.
b) [Cr(en)3]3+ has optical isomerism as explained above.
c) trans-[Cr(en)Cl2(NH3)2] has an internal mirror plane due to the presence of the trans isomer.
d) [Cr(NH3)6]3+ has an octahedral geometry with no chiral centers and no plane of symmetry, but it has a center of inversion which makes it achiral.
Therefore, the correct answer is option B, [Cr(en)3]3+.
Optical isomerism is a form of stereoisomerism that arises when a molecule has a non-superimposable mirror image. Optical isomers are also known as enantiomers.
Enantiomers are defined as chiral molecules that are mirror images of each other and cannot be superimposed on each other. A chiral molecule has no plane of symmetry and is not superimposable on its mirror image. A molecule that is not chiral is known as an achiral molecule.
The formula for the complex ion is [Cr(en)3]3+. The complex ion contains three bidentate en ligands (ethylenediamine) coordinated to a central chromium(III) ion.
In [Cr(en)3]3+, the three en ligands are arranged around the central chromium ion in a trigonal prismatic geometry. Each en ligand forms two coordination bonds with the chromium ion: one through each of its nitrogen atoms.
The complex ion has optical isomerism because the three en ligands are arranged in a chiral manner around the central chromium ion. The complex ion has a non-superimposable mirror image, which is its enantiomer.
In contrast, the other complexes listed do not have optical isomerism because they have either a plane of symmetry or an internal mirror plane.
a) [Cr(en)(H2O)4]3 has a plane of symmetry due to the presence of the water ligands.
b) [Cr(en)3]3+ has optical isomerism as explained above.
c) trans-[Cr(en)Cl2(NH3)2] has an internal mirror plane due to the presence of the trans isomer.
d) [Cr(NH3)6]3+ has an octahedral geometry with no chiral centers and no plane of symmetry, but it has a center of inversion which makes it achiral.
Therefore, the correct answer is option B, [Cr(en)3]3+.
Among the following, the correct statement is:- a)CH is isolabal to Co(CO)3
- b)CH2 is isolabal to Ni(CO)2
- c)CH is isolabal to Fe(CO)4
- d)CH2 is isolabal to Mn(CO)4
Correct answer is option 'A'. Can you explain this answer?
Among the following, the correct statement is:
a)
CH is isolabal to Co(CO)3
b)
CH2 is isolabal to Ni(CO)2
c)
CH is isolabal to Fe(CO)4
d)
CH2 is isolabal to Mn(CO)4

|
Suman answered |
For this case we should count total valance electron and electron deficiency
TVE deficiency
a.
CH 4+1=5 8-5 =3
Co(CO)3 9+6=15 18- 15 =3
b.
CH2 4+2=6 8-6=2
Ni(CO)2 10+4=14 18-14=4
c.
CH 5 3
Fe(CO)4 8+8=16 18-16=2
d.
CH2 6 2
Mn(CO)4 7+8=15 18-15=3
so as deficiency of CH and Co(CO)3 is same so it is isolabal
TVE deficiency
a.
CH 4+1=5 8-5 =3
Co(CO)3 9+6=15 18- 15 =3
b.
CH2 4+2=6 8-6=2
Ni(CO)2 10+4=14 18-14=4
c.
CH 5 3
Fe(CO)4 8+8=16 18-16=2
d.
CH2 6 2
Mn(CO)4 7+8=15 18-15=3
so as deficiency of CH and Co(CO)3 is same so it is isolabal
In hydroformylation process, propene is converted into
- a)Butanol
- b)Butanal
- c)Propane
- d)Butanoic Acid
Correct answer is option 'B'. Can you explain this answer?
In hydroformylation process, propene is converted into
a)
Butanol
b)
Butanal
c)
Propane
d)
Butanoic Acid

|
Anirban Khanna answered |
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. It is important because aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents.
When propene reacts with CO and H2 then butanal is formed.
H2 + CO + CH3CH=CH2 → CH3CH2CH2CHO ("normal")
H2 + CO + CH3CH=CH2 → (CH3)2CHCHO ("iso")
Hence B is correct
In Monsanto acetic acid process shown below, the role of HI is:
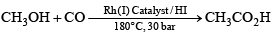
- a)To convert CH3OH to a stronger nucleophile CH3O–
- b)To convert CH3OH to CH3I
- c)To reduce a Rh(III) active species to a Rh(I) species in the catalytic cycle
- d)To reduce the Rh(I) catalyst to Rh(0) species
Correct answer is option 'B'. Can you explain this answer?
In Monsanto acetic acid process shown below, the role of HI is:
a)
To convert CH3OH to a stronger nucleophile CH3O–
b)
To convert CH3OH to CH3I
c)
To reduce a Rh(III) active species to a Rh(I) species in the catalytic cycle
d)
To reduce the Rh(I) catalyst to Rh(0) species
|
|
Vikram Kapoor answered |
Correct Answer :- B
Explanation : The Monsanto acetic acid process is the major commercial production method for acetic acid. Methanol, which can be generated from synthesis gas ("syn gas", a CO/H2 mixture), is reacted with carbon monoxide in the presence of a catalyst to afford acetic acid. In essence, the reaction can be thought of as the insertion of carbon monoxide into the C-O bond of methanol, i.e. the carbonylation of methanol.
The number of metal-metal bonds in [W2(OPh)6] is:- a)1
- b)2
- c)3
- d)4
Correct answer is option 'C'. Can you explain this answer?
The number of metal-metal bonds in [W2(OPh)6] is:
a)
1
b)
2
c)
3
d)
4

|
Anirban Khanna answered |
Total valency electron = 12 + 18 = 30 (A) [ (OPh) in bridging donate 3 electron]
B = (nx18–A) = 36–30 = 6
Metal-metal bond = B/2 = 6/2 = 3
The organometallic compound W(C5H5)2(CO)2 follows the 18-electron rule, The hapticities of the two cyclopentadienyl groups are:- a)5 and 5
- b)3 and 5
- c)3 and 3
- d)1 and 5
Correct answer is option 'B'. Can you explain this answer?
The organometallic compound W(C5H5)2(CO)2 follows the 18-electron rule, The hapticities of the two cyclopentadienyl groups are:
a)
5 and 5
b)
3 and 5
c)
3 and 3
d)
1 and 5
|
|
Vikram Kapoor answered |
W is 6 electron species while CO will give 2 electrons
So, 1W + 2(CO) = 10 electrons.
Remaining 8 electrons will be coming from 2Cp.
Hapticity means no of electrons donated by the ligand.
Here 5 + 3 = 8. So one Cp = 5
Hapticity One Cp = 3
Hapticity Total 1 W + 2 (CO) + 1 ( Cp-5) + 1 (Cp-3)
= 6+4+5+3
= 18
Therefore, correct answer is B.
If the bond length of CO bond in carbon monoxide is 1.128 Å. Then what is the value of CO bond length in Fe(CO)5:- a)1.15 Å
- b)1.128 Å
- c)1.72 Å
- d)1.118 Å
Correct answer is option 'A'. Can you explain this answer?
If the bond length of CO bond in carbon monoxide is 1.128 Å. Then what is the value of CO bond length in Fe(CO)5:
a)
1.15 Å
b)
1.128 Å
c)
1.72 Å
d)
1.118 Å
|
|
Nabanita Nayak answered |
Both a and c mat be correct. bond length must be greater than 1.128A
due to CO attached with metal. whatever it's terminal or bridged it's bond order decreases than free CO hence bond length increases.
due to CO attached with metal. whatever it's terminal or bridged it's bond order decreases than free CO hence bond length increases.
Zintl Ion 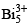 is cluster of:
is cluster of:- a)Closo
- b)Nido
- c)Arachno
- d)Hypo
Correct answer is option 'A'. Can you explain this answer?
Zintl Ion 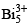 is cluster of:
is cluster of:
a)
Closo
b)
Nido
c)
Arachno
d)
Hypo

|
Yaseen Khan answered |
Valency of Bi × n - charge= 5×5-3=22 =4n+2 is called closo , here n=5 . When 4n+4 is called nido and 4n+6 is called arachno.
What kind of isomerism is exhibited by octahedral [Co(NH3)4Br2]Cl:- a)Geometrical and ionization
- b)Geometrical and optical
- c)Optical and ionization
- d)Geometrical only
Correct answer is option 'A'. Can you explain this answer?
What kind of isomerism is exhibited by octahedral [Co(NH3)4Br2]Cl:
a)
Geometrical and ionization
b)
Geometrical and optical
c)
Optical and ionization
d)
Geometrical only

|
Swara Reddy answered |
Geometrical and ionization isomerism is exhibited by octahedral [Co(NH3)4Br2]Cl.
Explanation:
Isomerism is the phenomenon in which two or more compounds have the same molecular formula but different arrangements of atoms. In coordination compounds, there are three types of isomerism:
1. Geometrical Isomerism: It arises due to the different possible arrangements of ligands around a central metal ion.
2. Optical Isomerism: It arises due to the presence of chiral ligands in a coordination compound.
3. Ionization Isomerism: It arises due to the different possible ways of distributing ligands between the cation and anion present in a coordination compound.
In the given complex, [Co(NH3)4Br2]Cl, there are two possible isomers:
1. Geometrical isomerism: The two bromine atoms can either be adjacent to each other in a cis-arrangement or opposite to each other in a trans-arrangement.
2. Ionization isomerism: The chloride ion can either be present in the cationic part or the anionic part of the complex.
Since both isomers are present in the given complex, it exhibits both geometrical and ionization isomerism.
Therefore, the correct option is 'A' - Geometrical and ionization.
Explanation:
Isomerism is the phenomenon in which two or more compounds have the same molecular formula but different arrangements of atoms. In coordination compounds, there are three types of isomerism:
1. Geometrical Isomerism: It arises due to the different possible arrangements of ligands around a central metal ion.
2. Optical Isomerism: It arises due to the presence of chiral ligands in a coordination compound.
3. Ionization Isomerism: It arises due to the different possible ways of distributing ligands between the cation and anion present in a coordination compound.
In the given complex, [Co(NH3)4Br2]Cl, there are two possible isomers:
1. Geometrical isomerism: The two bromine atoms can either be adjacent to each other in a cis-arrangement or opposite to each other in a trans-arrangement.
2. Ionization isomerism: The chloride ion can either be present in the cationic part or the anionic part of the complex.
Since both isomers are present in the given complex, it exhibits both geometrical and ionization isomerism.
Therefore, the correct option is 'A' - Geometrical and ionization.
The number of IR bands will be obtain in M(CO)5- a) 1
- b) 2
- c) 3
- d) 5
Correct answer is option 'B'. Can you explain this answer?
The number of IR bands will be obtain in M(CO)5
a)
1b)
2c)
3 d)
5

|
Shivani Thakur answered |
Since carbonyl is π acceptor ligand so it accepts electron density from metal ion as well as as a ligand it donates electron density.At a when one carbonyl ligand donates electron density to metal,at the same time another carbonyl accept that electron density from metal.so stretching frequency of donating carbonyl decreases while the stretching frequency of accepting carbonyl increases.thus when carbonyl acts as donar,and when carbonyl acts as acceptor has two different frequencies.
The ionization isomer of [Cr(H2O)4Cl(NO2)]Cl is:- a)[Cr(H2O)4(O2N)Cl2
- b)[Cr(H2O)4Cl2](NO2)
- c)[Cr(H2O)4Cl(ONO)Cl
- d)[Cr(H2O)4Cl2(NO2)]H2O
Correct answer is option 'B'. Can you explain this answer?
The ionization isomer of [Cr(H2O)4Cl(NO2)]Cl is:
a)
[Cr(H2O)4(O2N)Cl2
b)
[Cr(H2O)4Cl2](NO2)
c)
[Cr(H2O)4Cl(ONO)Cl
d)
[Cr(H2O)4Cl2(NO2)]H2O

|
Sagarika Patel answered |
Ionisation isomers: where the isomers can be thought of as occurring because of the formation of different ions in solution. one isomer [PtBr(NH3)3]NO2 -> NO2- anions in solution
another isomer [Pt(NO2)(NH3)3]Br -> Br- anions in solution
Therefore the ansewr of the question is
[Cr(H2O)4Cl2](NO2)
Wikinson’s catalyst:- a)Is coordinatively saturated
- b)Does not obey the 18-electron rule
- c)Is used for oxidation of alcohols
- d)Is an Ir complex used in preparation of important pharmaceutical products
Correct answer is option 'B'. Can you explain this answer?
Wikinson’s catalyst:
a)
Is coordinatively saturated
b)
Does not obey the 18-electron rule
c)
Is used for oxidation of alcohols
d)
Is an Ir complex used in preparation of important pharmaceutical products

|
Baishali Bajaj answered |
Wilkinson's catalyst, is the common name for chloridotris(triphenylphosphane)rhodium(I), a coordination complex of rhodium with the formula RhCl(PPh3)3 (Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel Laureate, Sir Geoffrey Wilkinson, who first popularized its use.
Which one of the following specie will be most easily reduced:- a)V(CO)6
- b)Cr(CO)6
- c)Fe(CO)5
- d)Ni(CO)4
Correct answer is option 'A'. Can you explain this answer?
Which one of the following specie will be most easily reduced:
a)
V(CO)6
b)
Cr(CO)6
c)
Fe(CO)5
d)
Ni(CO)4

|
Sahana Roy answered |
Reduction Potential of Metal Carbonyl Complexes
Metal carbonyl complexes are compounds that contain a metal atom bound to one or more carbon monoxide (CO) ligands. The metal-carbon bond in these complexes is polar, with carbon being the electron donor and the metal being the electron acceptor. This polar bond makes metal carbonyl complexes susceptible to reduction, with the metal ion being reduced to a lower oxidation state.
Factors Affecting the Reduction Potential of Metal Carbonyl Complexes
The ease of reduction of metal carbonyl complexes depends on several factors, including:
1. The oxidation state of the metal ion: Generally, the lower the oxidation state of the metal ion, the easier it is to reduce the metal carbonyl complex.
2. The identity of the metal ion: Some metals are more easily reduced than others. For example, Group 5 and 6 metals such as vanadium and chromium are more difficult to reduce than Group 7 and 8 metals such as manganese, iron, nickel, and cobalt.
3. The nature of the ligands: The strength of the metal-carbon bond in a metal carbonyl complex depends on the nature of the ligands. Stronger ligands such as phosphines and amines tend to stabilize the metal ion in a higher oxidation state and make it more difficult to reduce the metal carbonyl complex.
4. The steric hindrance around the metal ion: The presence of bulky ligands around the metal ion can hinder the approach of reducing agents and make it more difficult to reduce the metal carbonyl complex.
Answer
Out of the given options, V(CO)6 is the most easily reduced metal carbonyl complex. This is because:
1. The oxidation state of the vanadium ion in V(CO)6 is +2, which is the lowest oxidation state among the given options.
2. Vanadium is a Group 5 metal, which is more difficult to reduce than Group 7 and 8 metals. However, the low oxidation state of the vanadium ion in V(CO)6 makes it easier to reduce compared to other Group 5 and 6 metal carbonyl complexes.
3. The CO ligands in V(CO)6 are weaker than other ligands such as phosphines and amines, which makes the metal-carbon bond easier to break and the complex easier to reduce.
4. V(CO)6 has a more open structure compared to other metal carbonyl complexes, which reduces steric hindrance and makes it easier for reducing agents to approach the vanadium ion.
Therefore, V(CO)6 is the most easily reduced metal carbonyl complex among the given options.
Metal carbonyl complexes are compounds that contain a metal atom bound to one or more carbon monoxide (CO) ligands. The metal-carbon bond in these complexes is polar, with carbon being the electron donor and the metal being the electron acceptor. This polar bond makes metal carbonyl complexes susceptible to reduction, with the metal ion being reduced to a lower oxidation state.
Factors Affecting the Reduction Potential of Metal Carbonyl Complexes
The ease of reduction of metal carbonyl complexes depends on several factors, including:
1. The oxidation state of the metal ion: Generally, the lower the oxidation state of the metal ion, the easier it is to reduce the metal carbonyl complex.
2. The identity of the metal ion: Some metals are more easily reduced than others. For example, Group 5 and 6 metals such as vanadium and chromium are more difficult to reduce than Group 7 and 8 metals such as manganese, iron, nickel, and cobalt.
3. The nature of the ligands: The strength of the metal-carbon bond in a metal carbonyl complex depends on the nature of the ligands. Stronger ligands such as phosphines and amines tend to stabilize the metal ion in a higher oxidation state and make it more difficult to reduce the metal carbonyl complex.
4. The steric hindrance around the metal ion: The presence of bulky ligands around the metal ion can hinder the approach of reducing agents and make it more difficult to reduce the metal carbonyl complex.
Answer
Out of the given options, V(CO)6 is the most easily reduced metal carbonyl complex. This is because:
1. The oxidation state of the vanadium ion in V(CO)6 is +2, which is the lowest oxidation state among the given options.
2. Vanadium is a Group 5 metal, which is more difficult to reduce than Group 7 and 8 metals. However, the low oxidation state of the vanadium ion in V(CO)6 makes it easier to reduce compared to other Group 5 and 6 metal carbonyl complexes.
3. The CO ligands in V(CO)6 are weaker than other ligands such as phosphines and amines, which makes the metal-carbon bond easier to break and the complex easier to reduce.
4. V(CO)6 has a more open structure compared to other metal carbonyl complexes, which reduces steric hindrance and makes it easier for reducing agents to approach the vanadium ion.
Therefore, V(CO)6 is the most easily reduced metal carbonyl complex among the given options.
Amongst the following, the metal that does not form homoleptic polynuclear metal carbonyl is:- a)Mn
- b)Fe
- c)Cr
- d)Co
Correct answer is option 'C'. Can you explain this answer?
Amongst the following, the metal that does not form homoleptic polynuclear metal carbonyl is:
a)
Mn
b)
Fe
c)
Cr
d)
Co

|
Baishali Bajaj answered |
These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl (Ni(CO)4), but more commonly metal carbonyls are heteroleptic and contain a mixture of ligands. Mononuclear metal carbonyls contain only one metal atom as the central atom.
The order of CO bond strengths in the following metal hexacarbonyls is likely to be:- a)
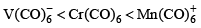
- b)
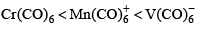
- c)
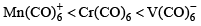
- d)
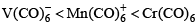
Correct answer is option 'A'. Can you explain this answer?
The order of CO bond strengths in the following metal hexacarbonyls is likely to be:
a)
b)
c)
d)
|
|
Pooja Choudhury answered |
- In the isoelectronic series of metal carbonyl, the CO bond strength is expected to increase in the order [V(CO)-6]< [Cr(CO)6]< [Mn(CO)+6]
- The oxidation state of the central metal atom increases in the order V(−) < Cr(0) < Mn(+) .
- As the oxidation state of the central metal atom increases, less and less electron density is donated to the carbonyl ligand.
- This increases the CO bond strength as the added electron density occupies the anti-bonding orbital.
The existence of two different coloured complexes with the composition of [Co(NH3)4Cl2]+ is due to:- a)Ionization isomerism
- b)Linkage isomerism
- c)Geometrical isomerism
- d)Coordination isomerism
Correct answer is option 'C'. Can you explain this answer?
The existence of two different coloured complexes with the composition of [Co(NH3)4Cl2]+ is due to:
a)
Ionization isomerism
b)
Linkage isomerism
c)
Geometrical isomerism
d)
Coordination isomerism

|
Akshat Saini answered |
Complexes of [MA4B2] type exhibit geometrical isomerism.
The complex [Co(NH3)4Cl2]+ is a [MA4B2] type complex and thus, fulfils the conditions that are necessary to exhibit geometrical isomerism. Hence, it has two geometrical isomers.
The infra–red starching frequency vCO of P-S follows the order: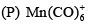

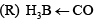
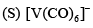
- a)P > R > S > Q
- b)S > P > R > Q
- c)Q > S > P > R
- d)R > Q > P > S
Correct answer is option 'D'. Can you explain this answer?
The infra–red starching frequency vCO of P-S follows the order:
a)
P > R > S > Q
b)
S > P > R > Q
c)
Q > S > P > R
d)
R > Q > P > S

|
Suman answered |
If we look at the M.O of CO we find HOMO is the non bonding orbital which has some antibonding character also so when CO adduct with BH3 it acts only as sigma bond donner .For this purpose CO give electron from non bonding orbital which has some antibonding character also so bond strangth increases for BH3 adduct ..and adduct with Mn(+) and V(-) it works as sigma donner aswell as pi accepter and accept pi bond in antibonding orbital . so as pi boding strangth increases bond strangth of CO bond decrease .. as V(-) give highest back bonding so CO bond strangth in this case is lowest ..so follow D order
Solid Co2(CO)8 shows infrared CO stretching bands at 1857, 1886, 2001, 2031, 2044, 2059, 2071 and 2112 cm–1. When Co2(CO)8 is dissolved in hexane, the carbonyl bands at 1857 and 1886 cm–1 disappear. These changes in the infrared spectrum in hexane are due to:- a)Loss of terminal CO
- b)Structural change of Co2(CO)8 involving conversion of terminal CO to bridging CO
- c)Dissociation of Co2(CO)8 to Co(CO)4
- d)Structural changes of Co2(CO)8 involving conversion of bridging CO to terminal CO
Correct answer is option 'D'. Can you explain this answer?
Solid Co2(CO)8 shows infrared CO stretching bands at 1857, 1886, 2001, 2031, 2044, 2059, 2071 and 2112 cm–1. When Co2(CO)8 is dissolved in hexane, the carbonyl bands at 1857 and 1886 cm–1 disappear. These changes in the infrared spectrum in hexane are due to:
a)
Loss of terminal CO
b)
Structural change of Co2(CO)8 involving conversion of terminal CO to bridging CO
c)
Dissociation of Co2(CO)8 to Co(CO)4
d)
Structural changes of Co2(CO)8 involving conversion of bridging CO to terminal CO

|
Anisha Pillai answered |
Explanation:
When Co2(CO)8 is dissolved in hexane, the carbonyl bands at 1857 and 1886 cm1 disappear. This suggests that there is a structural change in Co2(CO)8 in hexane solution.
The possible reasons for this change are:
a) Loss of terminal CO: If terminal CO is lost, there should be a decrease in the number of CO stretching bands. However, in this case, the number of CO stretching bands remains the same.
b) Structural change of Co2(CO)8 involving conversion of terminal CO to bridging CO: If terminal CO is converted to bridging CO, there should be a decrease in the number of terminal CO stretching bands and an increase in the number of bridging CO stretching bands. However, in this case, the number of CO stretching bands remains the same.
c) Dissociation of Co2(CO)8 to Co(CO)4: If Co2(CO)8 dissociates to Co(CO)4, there should be a decrease in the number of CO stretching bands. However, in this case, the number of CO stretching bands remains the same.
d) Structural changes of Co2(CO)8 involving conversion of bridging CO to terminal CO: If bridging CO is converted to terminal CO, there should be an increase in the number of terminal CO stretching bands and a decrease in the number of bridging CO stretching bands. This is consistent with the observation that the carbonyl bands at 1857 and 1886 cm1 disappear in hexane, which suggests that some of the bridging CO groups have been converted to terminal CO groups.
Therefore, the correct answer is option 'D', which suggests that there is a structural change in Co2(CO)8 involving the conversion of bridging CO to terminal CO.
When Co2(CO)8 is dissolved in hexane, the carbonyl bands at 1857 and 1886 cm1 disappear. This suggests that there is a structural change in Co2(CO)8 in hexane solution.
The possible reasons for this change are:
a) Loss of terminal CO: If terminal CO is lost, there should be a decrease in the number of CO stretching bands. However, in this case, the number of CO stretching bands remains the same.
b) Structural change of Co2(CO)8 involving conversion of terminal CO to bridging CO: If terminal CO is converted to bridging CO, there should be a decrease in the number of terminal CO stretching bands and an increase in the number of bridging CO stretching bands. However, in this case, the number of CO stretching bands remains the same.
c) Dissociation of Co2(CO)8 to Co(CO)4: If Co2(CO)8 dissociates to Co(CO)4, there should be a decrease in the number of CO stretching bands. However, in this case, the number of CO stretching bands remains the same.
d) Structural changes of Co2(CO)8 involving conversion of bridging CO to terminal CO: If bridging CO is converted to terminal CO, there should be an increase in the number of terminal CO stretching bands and a decrease in the number of bridging CO stretching bands. This is consistent with the observation that the carbonyl bands at 1857 and 1886 cm1 disappear in hexane, which suggests that some of the bridging CO groups have been converted to terminal CO groups.
Therefore, the correct answer is option 'D', which suggests that there is a structural change in Co2(CO)8 involving the conversion of bridging CO to terminal CO.
Complexes of general formula, fac–[Mo(CO)3(Phosphine)3] have the C—O stretching frequency bands as given below,
Phosphines: PF3 (P), PCl3 (Q), P(Cl)Ph2 (R), PMe3(S)
ΝCO, cm–1 : 2090 (I); 2040 (II); 1977 (III); 1945 (IV)- a)(P –I), (Q–II), (R–III), (S–IV)
- b)(P –II), (Q–I), (R–IV), (S–III)
- c)(P –IV), (Q–III), (R–II), (S–I)
- d)(P –III), (Q–IV), (R–I), (S–II)
Correct answer is option 'A'. Can you explain this answer?
Complexes of general formula, fac–[Mo(CO)3(Phosphine)3] have the C—O stretching frequency bands as given below,
Phosphines: PF3 (P), PCl3 (Q), P(Cl)Ph2 (R), PMe3(S)
ΝCO, cm–1 : 2090 (I); 2040 (II); 1977 (III); 1945 (IV)
Phosphines: PF3 (P), PCl3 (Q), P(Cl)Ph2 (R), PMe3(S)
ΝCO, cm–1 : 2090 (I); 2040 (II); 1977 (III); 1945 (IV)
a)
(P –I), (Q–II), (R–III), (S–IV)
b)
(P –II), (Q–I), (R–IV), (S–III)
c)
(P –IV), (Q–III), (R–II), (S–I)
d)
(P –III), (Q–IV), (R–I), (S–II)

|
Rutuja Choudhary answered |
CO stretching frequency bands of complexes
• The complexes have general formula fac[Mo(CO)3(Phosphine)3]
• The CO stretching frequency bands are given below for different phosphines:
- PF3 (P) - 2090 (I)
- PCl3 (Q) - 2040 (II)
- P(Cl)Ph2 (R) - 1977 (III)
- PMe3(S) - 1945 (IV)
• Three different phosphines are coordinated with Mo(CO)3 in each complex
• The fac notation indicates that three phosphine ligands are arranged around the central metal atom in a face-capping fashion.
Answer explanation
• The correct answer is option 'A': (P I), (QII), (RIII), (SIV)
• This means that the CO stretching frequency band for complex with PF3 is I (2090 cm-1), for complex with PCl3 is II (2040 cm-1), for complex with P(Cl)Ph2 is III (1977 cm-1), and for complex with PMe3 is IV (1945 cm-1).
• The answer is obtained by comparing the CO stretching frequency bands for each phosphine with the given values and matching them with the options.
• The CO stretching frequency bands are affected by the nature of the ligands and the coordination environment around the metal atom.
• In general, the CO stretching frequency decreases with an increase in electron density around the metal atom.
• The phosphines have different electron-donating abilities due to the presence of different groups such as F, Cl, Ph, and Me.
• The order of electron-donating ability is PMe3 > PF3 > P(Cl)Ph2 > PCl3.
• This order is reflected in the CO stretching frequency bands, where the complex with the most electron-donating phosphine (PMe3) has the lowest frequency band (IV) and the complex with the least electron-donating phosphine (PCl3) has the highest frequency band (II).
• The complexes have general formula fac[Mo(CO)3(Phosphine)3]
• The CO stretching frequency bands are given below for different phosphines:
- PF3 (P) - 2090 (I)
- PCl3 (Q) - 2040 (II)
- P(Cl)Ph2 (R) - 1977 (III)
- PMe3(S) - 1945 (IV)
• Three different phosphines are coordinated with Mo(CO)3 in each complex
• The fac notation indicates that three phosphine ligands are arranged around the central metal atom in a face-capping fashion.
Answer explanation
• The correct answer is option 'A': (P I), (QII), (RIII), (SIV)
• This means that the CO stretching frequency band for complex with PF3 is I (2090 cm-1), for complex with PCl3 is II (2040 cm-1), for complex with P(Cl)Ph2 is III (1977 cm-1), and for complex with PMe3 is IV (1945 cm-1).
• The answer is obtained by comparing the CO stretching frequency bands for each phosphine with the given values and matching them with the options.
• The CO stretching frequency bands are affected by the nature of the ligands and the coordination environment around the metal atom.
• In general, the CO stretching frequency decreases with an increase in electron density around the metal atom.
• The phosphines have different electron-donating abilities due to the presence of different groups such as F, Cl, Ph, and Me.
• The order of electron-donating ability is PMe3 > PF3 > P(Cl)Ph2 > PCl3.
• This order is reflected in the CO stretching frequency bands, where the complex with the most electron-donating phosphine (PMe3) has the lowest frequency band (IV) and the complex with the least electron-donating phosphine (PCl3) has the highest frequency band (II).
The rate of alkene coordination to [PtCl4]2- is highest for- a)norbornene
- b)ethylene
- c)cyclohexene
- d)1-butene
Correct answer is option 'A'. Can you explain this answer?
The rate of alkene coordination to [PtCl4]2- is highest for
a)
norbornene
b)
ethylene
c)
cyclohexene
d)
1-butene

|
Asf Institute answered |
The reactivity order of alkene follows: Norbornene > cyclohexane > 1-butene > ethylene.
Norbornene compound is more reactive due to more substituted than Cyclohexane. We know that More substituted alkene is highly reactive compared to less substituted alkyl. That is why cyclohexane is less reactive compared to norbornene.
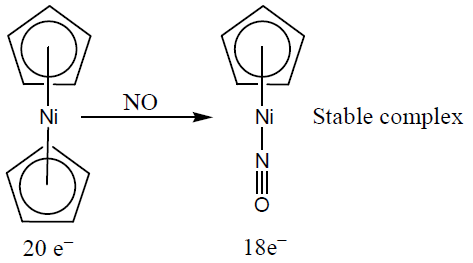
Nitrosyl ligand binds to d-metal atoms in linear and bent fashion and behaves, respectively, as- a)NO+ and NO
- b)NO+ and NO–
- c)NO– and NO
- d)NO– and NO+
Correct answer is option 'B'. Can you explain this answer?
Nitrosyl ligand binds to d-metal atoms in linear and bent fashion and behaves, respectively, as
a)
NO+ and NO
b)
NO+ and NO–
c)
NO– and NO
d)
NO– and NO+

|
Madhavan Iyer answered |
Nitrosyl Ligand Binding to d-Metal Atoms
Introduction:
Nitrosyl ligand is a chemical species consisting of one nitrogen atom and one oxygen atom, bonded together with a triple bond. This ligand can bind to transition metals in both linear and bent fashion. The behavior of nitrosyl ligand depends on the nature of the metal atom to which it is bound.
Linear Binding:
When the nitrosyl ligand binds to a transition metal atom in a linear fashion, it is referred to as nitric oxide or NO. In this case, the nitrogen and oxygen atoms are in a straight line. The nitrosyl ligand acts as a one-electron donor and forms a sigma bond with the metal atom. This type of bonding is often observed in complexes of d-block metals with low oxidation states.
Bent Binding:
When the nitrosyl ligand binds to a transition metal atom in a bent fashion, it is referred to as nitrosyl ion or NO-. In this case, the nitrogen and oxygen atoms are not in a straight line, but form an angle. The nitrosyl ligand acts as a two-electron donor and forms a pi bond with the metal atom. This type of bonding is often observed in complexes of d-block metals with high oxidation states.
Conclusion:
In summary, the nitrosyl ligand can bind to transition metal atoms in both linear and bent fashion, depending on the nature of the metal atom. When it binds in a linear fashion, it is referred to as nitric oxide or NO, and when it binds in a bent fashion, it is referred to as nitrosyl ion or NO-.
Introduction:
Nitrosyl ligand is a chemical species consisting of one nitrogen atom and one oxygen atom, bonded together with a triple bond. This ligand can bind to transition metals in both linear and bent fashion. The behavior of nitrosyl ligand depends on the nature of the metal atom to which it is bound.
Linear Binding:
When the nitrosyl ligand binds to a transition metal atom in a linear fashion, it is referred to as nitric oxide or NO. In this case, the nitrogen and oxygen atoms are in a straight line. The nitrosyl ligand acts as a one-electron donor and forms a sigma bond with the metal atom. This type of bonding is often observed in complexes of d-block metals with low oxidation states.
Bent Binding:
When the nitrosyl ligand binds to a transition metal atom in a bent fashion, it is referred to as nitrosyl ion or NO-. In this case, the nitrogen and oxygen atoms are not in a straight line, but form an angle. The nitrosyl ligand acts as a two-electron donor and forms a pi bond with the metal atom. This type of bonding is often observed in complexes of d-block metals with high oxidation states.
Conclusion:
In summary, the nitrosyl ligand can bind to transition metal atoms in both linear and bent fashion, depending on the nature of the metal atom. When it binds in a linear fashion, it is referred to as nitric oxide or NO, and when it binds in a bent fashion, it is referred to as nitrosyl ion or NO-.
The catalyst used in the conversion of ethylene to acetaldehyde using Wacker process:- a)HCo(CO)4
- b)[PdCl4]2–
- c)V2O5
- d)TiCl4 in the presence of Al(C2H5)3
Correct answer is option 'B'. Can you explain this answer?
The catalyst used in the conversion of ethylene to acetaldehyde using Wacker process:
a)
HCo(CO)4
b)
[PdCl4]2–
c)
V2O5
d)
TiCl4 in the presence of Al(C2H5)3

|
Preethi Joshi answered |
Wacker process is a chemical reaction used to convert ethylene to acetaldehyde. The catalyst used in this reaction is [PdCl4]2.
Explanation:
[PdCl4]2 is a palladium-based catalyst that is widely used in organic chemistry reactions. In the Wacker process, it acts as an oxidizing agent that helps in the conversion of ethylene to acetaldehyde.
The reaction takes place in the presence of water and oxygen. Ethylene and the catalyst are added to the reaction vessel, and the reaction is carried out at high pressure and temperature. The catalyst helps in the formation of a palladium-ethylene complex, which undergoes oxidative addition with water to form a palladium-hydroxyethyl complex. This complex then undergoes a series of steps to form acetaldehyde.
The use of [PdCl4]2 as a catalyst in the Wacker process has several advantages. It is a highly efficient catalyst that can be easily separated from the reaction mixture. It is also a relatively cheap catalyst, making the process cost-effective.
In conclusion, [PdCl4]2 is the catalyst used in the conversion of ethylene to acetaldehyde using the Wacker process. It acts as an oxidizing agent and helps in the formation of a palladium-ethylene complex, which undergoes a series of steps to form acetaldehyde.
Explanation:
[PdCl4]2 is a palladium-based catalyst that is widely used in organic chemistry reactions. In the Wacker process, it acts as an oxidizing agent that helps in the conversion of ethylene to acetaldehyde.
The reaction takes place in the presence of water and oxygen. Ethylene and the catalyst are added to the reaction vessel, and the reaction is carried out at high pressure and temperature. The catalyst helps in the formation of a palladium-ethylene complex, which undergoes oxidative addition with water to form a palladium-hydroxyethyl complex. This complex then undergoes a series of steps to form acetaldehyde.
The use of [PdCl4]2 as a catalyst in the Wacker process has several advantages. It is a highly efficient catalyst that can be easily separated from the reaction mixture. It is also a relatively cheap catalyst, making the process cost-effective.
In conclusion, [PdCl4]2 is the catalyst used in the conversion of ethylene to acetaldehyde using the Wacker process. It acts as an oxidizing agent and helps in the formation of a palladium-ethylene complex, which undergoes a series of steps to form acetaldehyde.
The cluster having arachno type structure is:- a)[Os5(CO)16]
- b)[Os3(CO)12]
- c)[Ir4(CO)12]
- d)[Ph6(CO)16]
Correct answer is option 'B'. Can you explain this answer?
The cluster having arachno type structure is:
a)
[Os5(CO)16]
b)
[Os3(CO)12]
c)
[Ir4(CO)12]
d)
[Ph6(CO)16]

|
Athul Menon answered |
Arachno type structure refers to a type of cluster geometry where the metal atoms are arranged in a three-dimensional shape resembling a spider or spider web. The correct answer is option B, [Os3(CO)12], which has an arachno type structure.
Explanation:
1. Cluster Compounds:
Cluster compounds are molecules consisting of metal atoms or ions, typically in a small cluster of two to several atoms, surrounded by other atoms or ligands.
2. Arachno Type Structure:
Arachno type structure is a type of cluster geometry where the metal atoms are arranged in a three-dimensional shape resembling a spider or spider web. This structure is characterized by the presence of three types of vertices, which are called arachno, meta and ortho vertices.
3. Option A [Os5(CO)16]:
This cluster compound contains five osmium atoms and sixteen carbon monoxide ligands. It has a trigonal bipyramidal structure, not arachno type.
4. Option B [Os3(CO)12]:
This cluster compound contains three osmium atoms and twelve carbon monoxide ligands. It has an arachno type structure, which is characterized by the presence of three types of vertices: arachno, meta, and ortho vertices.
5. Option C [Ir4(CO)12]:
This cluster compound contains four iridium atoms and twelve carbon monoxide ligands. It has a tetrahedral structure, not arachno type.
6. Option D [Ph6(CO)16]:
This cluster compound contains six phenyl groups and sixteen carbon monoxide ligands. It has a cuboctahedral structure, not arachno type.
Conclusion:
The correct answer is option B, [Os3(CO)12], which has an arachno type structure.
Explanation:
1. Cluster Compounds:
Cluster compounds are molecules consisting of metal atoms or ions, typically in a small cluster of two to several atoms, surrounded by other atoms or ligands.
2. Arachno Type Structure:
Arachno type structure is a type of cluster geometry where the metal atoms are arranged in a three-dimensional shape resembling a spider or spider web. This structure is characterized by the presence of three types of vertices, which are called arachno, meta and ortho vertices.
3. Option A [Os5(CO)16]:
This cluster compound contains five osmium atoms and sixteen carbon monoxide ligands. It has a trigonal bipyramidal structure, not arachno type.
4. Option B [Os3(CO)12]:
This cluster compound contains three osmium atoms and twelve carbon monoxide ligands. It has an arachno type structure, which is characterized by the presence of three types of vertices: arachno, meta, and ortho vertices.
5. Option C [Ir4(CO)12]:
This cluster compound contains four iridium atoms and twelve carbon monoxide ligands. It has a tetrahedral structure, not arachno type.
6. Option D [Ph6(CO)16]:
This cluster compound contains six phenyl groups and sixteen carbon monoxide ligands. It has a cuboctahedral structure, not arachno type.
Conclusion:
The correct answer is option B, [Os3(CO)12], which has an arachno type structure.
Active catalytic species for hydroformylation is:- a)RuCl2(PPh3)3
- b)HCo(CO)3
- c)RuCl(PPh3)3
- d)K2PtCl6
Correct answer is option 'B'. Can you explain this answer?
Active catalytic species for hydroformylation is:
a)
RuCl2(PPh3)3
b)
HCo(CO)3
c)
RuCl(PPh3)3
d)
K2PtCl6

|
Rashi Choudhury answered |
Active Catalytic Species for Hydroformylation
Hydroformylation is a process in which alkenes react with carbon monoxide and hydrogen to produce aldehydes. The reaction is catalyzed by transition metal complexes, which form active catalytic species.
Active Catalytic Species
The active catalytic species for hydroformylation is HCo(CO)3, which is a cobalt carbonyl complex. Cobalt carbonyl complexes are widely used as catalysts for hydroformylation reactions because they are highly efficient and selective.
Mechanism of Hydroformylation
The mechanism of hydroformylation involves the following steps:
1. Formation of the active catalytic species: The cobalt carbonyl complex is activated by reacting with hydrogen gas to form HCo(CO)3H.
2. Insertion of the alkene: The alkene inserts into the cobalt-hydrogen bond to form a cobalt-alkyl complex.
3. Coordination of the carbon monoxide: Carbon monoxide coordinates to the cobalt-alkyl complex to form a cobalt-alkyl-carbonyl complex.
4. Migration of the alkyl group: The alkyl group migrates from the cobalt to the carbon monoxide to form an acyl complex.
5. Elimination of the aldehyde: The acyl complex eliminates the aldehyde product, regenerating the cobalt carbonyl complex.
Conclusion
In conclusion, the active catalytic species for hydroformylation is HCo(CO)3, which is a cobalt carbonyl complex. The mechanism of hydroformylation involves the formation of the active catalytic species, insertion of the alkene, coordination of the carbon monoxide, migration of the alkyl group, and elimination of the aldehyde product.
Hydroformylation is a process in which alkenes react with carbon monoxide and hydrogen to produce aldehydes. The reaction is catalyzed by transition metal complexes, which form active catalytic species.
Active Catalytic Species
The active catalytic species for hydroformylation is HCo(CO)3, which is a cobalt carbonyl complex. Cobalt carbonyl complexes are widely used as catalysts for hydroformylation reactions because they are highly efficient and selective.
Mechanism of Hydroformylation
The mechanism of hydroformylation involves the following steps:
1. Formation of the active catalytic species: The cobalt carbonyl complex is activated by reacting with hydrogen gas to form HCo(CO)3H.
2. Insertion of the alkene: The alkene inserts into the cobalt-hydrogen bond to form a cobalt-alkyl complex.
3. Coordination of the carbon monoxide: Carbon monoxide coordinates to the cobalt-alkyl complex to form a cobalt-alkyl-carbonyl complex.
4. Migration of the alkyl group: The alkyl group migrates from the cobalt to the carbon monoxide to form an acyl complex.
5. Elimination of the aldehyde: The acyl complex eliminates the aldehyde product, regenerating the cobalt carbonyl complex.
Conclusion
In conclusion, the active catalytic species for hydroformylation is HCo(CO)3, which is a cobalt carbonyl complex. The mechanism of hydroformylation involves the formation of the active catalytic species, insertion of the alkene, coordination of the carbon monoxide, migration of the alkyl group, and elimination of the aldehyde product.
Which of the following in not suitable as catalyst for hydroformylation:- a)HCo(CO)4
- b)HCo(CO)4PBu3
- c)HRh(CO)(PPh3)3
- d)H2Rh(PPh3)2Cl
Correct answer is option 'D'. Can you explain this answer?
Which of the following in not suitable as catalyst for hydroformylation:
a)
HCo(CO)4
b)
HCo(CO)4PBu3
c)
HRh(CO)(PPh3)3
d)
H2Rh(PPh3)2Cl

|
Sagarika Yadav answered |
Answer:
Not suitable catalyst for hydroformylation is H2Rh(PPh3)2Cl.
Explanation:
Hydroformylation is a process that involves the addition of a mixture of carbon monoxide and hydrogen to an unsaturated organic compound in the presence of a catalyst to form an aldehyde. The catalyst used for this process is usually a metal complex.
The following are suitable catalysts for hydroformylation:
a) HCo(CO)4: This is a cobalt complex that is widely used as a catalyst for hydroformylation. It is a stable solid that can be easily handled and stored.
b) HCo(CO)4PBu3: This is a modified cobalt complex that contains a bulky phosphine ligand. The bulky ligand helps to modify the steric properties of the catalyst, improving its selectivity and activity.
c) HRh(CO)(PPh3)3: This is a rhodium complex that is also widely used as a catalyst for hydroformylation. It is a highly active catalyst that can be easily prepared and used.
d) H2Rh(PPh3)2Cl: This is a rhodium complex that is not suitable as a catalyst for hydroformylation. The chloride ligand is a weak coordinating ligand that can easily dissociate from the metal center, leading to deactivation of the catalyst. Additionally, the presence of the chloride ligand can lead to unwanted side reactions and reduced selectivity.
In summary, H2Rh(PPh3)2Cl is not a suitable catalyst for hydroformylation due to its weak ligand and potential for unwanted side reactions.
Not suitable catalyst for hydroformylation is H2Rh(PPh3)2Cl.
Explanation:
Hydroformylation is a process that involves the addition of a mixture of carbon monoxide and hydrogen to an unsaturated organic compound in the presence of a catalyst to form an aldehyde. The catalyst used for this process is usually a metal complex.
The following are suitable catalysts for hydroformylation:
a) HCo(CO)4: This is a cobalt complex that is widely used as a catalyst for hydroformylation. It is a stable solid that can be easily handled and stored.
b) HCo(CO)4PBu3: This is a modified cobalt complex that contains a bulky phosphine ligand. The bulky ligand helps to modify the steric properties of the catalyst, improving its selectivity and activity.
c) HRh(CO)(PPh3)3: This is a rhodium complex that is also widely used as a catalyst for hydroformylation. It is a highly active catalyst that can be easily prepared and used.
d) H2Rh(PPh3)2Cl: This is a rhodium complex that is not suitable as a catalyst for hydroformylation. The chloride ligand is a weak coordinating ligand that can easily dissociate from the metal center, leading to deactivation of the catalyst. Additionally, the presence of the chloride ligand can lead to unwanted side reactions and reduced selectivity.
In summary, H2Rh(PPh3)2Cl is not a suitable catalyst for hydroformylation due to its weak ligand and potential for unwanted side reactions.
Among the following which option is correct about the thermal stability of given compounds:(P) HMn(CO)5
(Q) HTe(CO)5
(R) HRe(CO)5- a)P > Q > R
- b)R > Q > P
- c)Q < P < R
- d)Q < R < P
Correct answer is option 'B'. Can you explain this answer?
Among the following which option is correct about the thermal stability of given compounds:
(P) HMn(CO)5
(Q) HTe(CO)5
(R) HRe(CO)5
(Q) HTe(CO)5
(R) HRe(CO)5
a)
P > Q > R
b)
R > Q > P
c)
Q < P < R
d)
Q < R < P

|
Jyoti Sharma answered |
Because coordinated compound become more stable on going down group, as cfse gets increase
In the complex, [Ni2(η5-Cp)2(CO)2] the IR stretching frequency appears at 1857 cm-1 (strong) and 1897 cm-1 (weak). The valence electron count and the nature of the M—CO bond respectively are:- a)16 e-, bridging
- b)17 e-, bridging
- c)18 e-, terminal
- d)18 e-, bridging
Correct answer is option 'C'. Can you explain this answer?
In the complex, [Ni2(η5-Cp)2(CO)2] the IR stretching frequency appears at 1857 cm-1 (strong) and 1897 cm-1 (weak). The valence electron count and the nature of the M—CO bond respectively are:
a)
16 e-, bridging
b)
17 e-, bridging
c)
18 e-, terminal
d)
18 e-, bridging

|
Prerna Sheoran answered |
Stretching frequency of terminal CO varies from 2143 to 1850 cm^-1 and the valence electron count is 2×10+5×2+2×2+2=36. means 18 electrons per metal.....so...oprionC is correct
Metals used in automobile catalytic converters are:- a)Pt and Pd
- b)Pt and Rh
- c)Pd and Rh
- d)All of the above.
Correct answer is option 'D'. Can you explain this answer?
Metals used in automobile catalytic converters are:
a)
Pt and Pd
b)
Pt and Rh
c)
Pd and Rh
d)
All of the above.
|
|
Sandeep Sahoo answered |
In order to properly filter hydrocarbons and reduce your car's emissions, catalytic converters use precious metals such as platinum, palladium and rhodium.
How many M — M bonds are present in [Cp Mo(CO3)]2?- a)1
- b)2
- c)3
- d)Zero
Correct answer is option 'A'. Can you explain this answer?
How many M — M bonds are present in [Cp Mo(CO3)]2?
a)
1
b)
2
c)
3
d)
Zero
|
|
Pooja Choudhury answered |
Cyclopentadienylmolybdenum tricarbonyl dimer is the chemical compound with the formula Cp2Mo2(CO)6, where Cp is C5H5.
An intermediate formed during the hydroformylation of olefins using Co2(CO)8 as catalyst is:- a)HCo(CO)8
- b)H4Co(CO)3
- c)H2Co(CO)4
- d)HCo(CO)4
Correct answer is option 'D'. Can you explain this answer?
An intermediate formed during the hydroformylation of olefins using Co2(CO)8 as catalyst is:
a)
HCo(CO)8
b)
H4Co(CO)3
c)
H2Co(CO)4
d)
HCo(CO)4

|
Anisha Banerjee answered |
Hydroformylation of Olefins and Co2(CO)8 as Catalyst
Hydroformylation is an industrial process that involves the reaction of an olefin with carbon monoxide and hydrogen to form an aldehyde. The reaction is catalyzed by transition metal complexes, with Co2(CO)8 being one of the most commonly used catalysts. During the reaction, an intermediate is formed which is HCo(CO)4.
Explanation
The hydroformylation reaction involves the insertion of a carbonyl group into the olefin, which is initiated by the Co2(CO)8 catalyst. The first step in the reaction is the formation of a cobalt carbonyl complex, which is followed by the insertion of the olefin into the Co-C bond. This results in the formation of the HCo(CO)4 intermediate.
The HCo(CO)4 intermediate is highly reactive and can undergo further reactions, such as the migration of the carbonyl group to form a new Co-C bond. This leads to the formation of higher-order cobalt carbonyl complexes, which can then react with hydrogen and carbon monoxide to form the aldehyde product.
Conclusion
In summary, the intermediate formed during the hydroformylation of olefins using Co2(CO)8 as a catalyst is HCo(CO)4. This intermediate is crucial for the reaction to proceed, as it undergoes further reactions to form higher-order cobalt carbonyl complexes, which are responsible for the formation of the aldehyde product.
Hydroformylation is an industrial process that involves the reaction of an olefin with carbon monoxide and hydrogen to form an aldehyde. The reaction is catalyzed by transition metal complexes, with Co2(CO)8 being one of the most commonly used catalysts. During the reaction, an intermediate is formed which is HCo(CO)4.
Explanation
The hydroformylation reaction involves the insertion of a carbonyl group into the olefin, which is initiated by the Co2(CO)8 catalyst. The first step in the reaction is the formation of a cobalt carbonyl complex, which is followed by the insertion of the olefin into the Co-C bond. This results in the formation of the HCo(CO)4 intermediate.
The HCo(CO)4 intermediate is highly reactive and can undergo further reactions, such as the migration of the carbonyl group to form a new Co-C bond. This leads to the formation of higher-order cobalt carbonyl complexes, which can then react with hydrogen and carbon monoxide to form the aldehyde product.
Conclusion
In summary, the intermediate formed during the hydroformylation of olefins using Co2(CO)8 as a catalyst is HCo(CO)4. This intermediate is crucial for the reaction to proceed, as it undergoes further reactions to form higher-order cobalt carbonyl complexes, which are responsible for the formation of the aldehyde product.
The correct order of νNO (cm–1) in the following compounds is:- a)NO+ > NO > [NiCp(NO)] > [Cr(Cp)2(NO)4]
- b)[Cr(Cp)2(NO)4] > [NiCp(NO)] > NO+ > NO
- c)NO+ > [Cr(Cp)2(NO)4] > NO > [NiCp(NO)]
- d)[NiCp(NO)] > NO > [Cr(Cp)2(NO)4] > NO+
Correct answer is option 'A'. Can you explain this answer?
The correct order of νNO (cm–1) in the following compounds is:
a)
NO+ > NO > [NiCp(NO)] > [Cr(Cp)2(NO)4]
b)
[Cr(Cp)2(NO)4] > [NiCp(NO)] > NO+ > NO
c)
NO+ > [Cr(Cp)2(NO)4] > NO > [NiCp(NO)]
d)
[NiCp(NO)] > NO > [Cr(Cp)2(NO)4] > NO+

|
Niharika Choudhary answered |
The correct order of NO (cm-1) in the given compounds is option 'A' which is NO, NO, [NiCp(NO)], [Cr(Cp)2(NO)4].
Explanation:
To determine the order of NO (cm-1) in the given compounds, we need to consider the stretching frequency of the NO group in each of the compounds. The stretching frequency of NO group is dependent on the electronic environment surrounding the NO group.
In general, the stretching frequency of NO group decreases in the order:
NO+ > NO > NO-
This is due to the fact that the NO group is electron-withdrawing and the presence of electron-withdrawing groups lowers the stretching frequency of NO.
Now, let's analyze each of the given compounds and determine the order of NO (cm-1):
a) NO, NO, [NiCp(NO)], [Cr(Cp)2(NO)4]
- The first two compounds are simple diatomic molecules of NO, so they have the highest stretching frequency of NO.
- [NiCp(NO)] is a complex containing NO group coordinated to nickel. The presence of nickel as a transition metal shifts the stretching frequency of NO to a lower frequency.
- [Cr(Cp)2(NO)4] is also a complex containing NO group coordinated to chromium. The presence of chromium as a transition metal also shifts the stretching frequency of NO to a lower frequency.
Therefore, the correct order of NO (cm-1) in this compound is NO, NO, [NiCp(NO)], [Cr(Cp)2(NO)4].
b) [Cr(Cp)2(NO)4], [NiCp(NO)], NO, NO
- [Cr(Cp)2(NO)4] and [NiCp(NO)] are the same as in option 'A' and have the same order.
- NO is a simple diatomic molecule and has the highest stretching frequency of NO.
Therefore, the correct order of NO (cm-1) in this compound is [Cr(Cp)2(NO)4], [NiCp(NO)], NO, NO.
c) NO, [Cr(Cp)2(NO)4], NO, [NiCp(NO)]
- The first and third compounds are simple diatomic molecules of NO, so they have the highest stretching frequency of NO.
- [Cr(Cp)2(NO)4] is a complex containing NO group coordinated to chromium. The presence of chromium as a transition metal shifts the stretching frequency of NO to a lower frequency.
- [NiCp(NO)] is a complex containing NO group coordinated to nickel. However, since it is the last compound in the list, we cannot compare its stretching frequency with the others.
Therefore, the correct order of NO (cm-1) in this compound is NO, [Cr(Cp)2(NO)4], NO, [NiCp(NO)].
d) [NiCp(NO)], NO, [Cr(Cp)2(NO)4], NO
- The first and fourth compounds are simple diatomic molecules of NO, so they have the highest stretching frequency of NO.
- [NiCp(NO)] is a complex containing NO group coordinated to nickel. The presence of nickel as a transition metal shifts the stretching frequency of NO to a lower frequency.
- [Cr(Cp)2(NO)4] is a complex containing NO
Explanation:
To determine the order of NO (cm-1) in the given compounds, we need to consider the stretching frequency of the NO group in each of the compounds. The stretching frequency of NO group is dependent on the electronic environment surrounding the NO group.
In general, the stretching frequency of NO group decreases in the order:
NO+ > NO > NO-
This is due to the fact that the NO group is electron-withdrawing and the presence of electron-withdrawing groups lowers the stretching frequency of NO.
Now, let's analyze each of the given compounds and determine the order of NO (cm-1):
a) NO, NO, [NiCp(NO)], [Cr(Cp)2(NO)4]
- The first two compounds are simple diatomic molecules of NO, so they have the highest stretching frequency of NO.
- [NiCp(NO)] is a complex containing NO group coordinated to nickel. The presence of nickel as a transition metal shifts the stretching frequency of NO to a lower frequency.
- [Cr(Cp)2(NO)4] is also a complex containing NO group coordinated to chromium. The presence of chromium as a transition metal also shifts the stretching frequency of NO to a lower frequency.
Therefore, the correct order of NO (cm-1) in this compound is NO, NO, [NiCp(NO)], [Cr(Cp)2(NO)4].
b) [Cr(Cp)2(NO)4], [NiCp(NO)], NO, NO
- [Cr(Cp)2(NO)4] and [NiCp(NO)] are the same as in option 'A' and have the same order.
- NO is a simple diatomic molecule and has the highest stretching frequency of NO.
Therefore, the correct order of NO (cm-1) in this compound is [Cr(Cp)2(NO)4], [NiCp(NO)], NO, NO.
c) NO, [Cr(Cp)2(NO)4], NO, [NiCp(NO)]
- The first and third compounds are simple diatomic molecules of NO, so they have the highest stretching frequency of NO.
- [Cr(Cp)2(NO)4] is a complex containing NO group coordinated to chromium. The presence of chromium as a transition metal shifts the stretching frequency of NO to a lower frequency.
- [NiCp(NO)] is a complex containing NO group coordinated to nickel. However, since it is the last compound in the list, we cannot compare its stretching frequency with the others.
Therefore, the correct order of NO (cm-1) in this compound is NO, [Cr(Cp)2(NO)4], NO, [NiCp(NO)].
d) [NiCp(NO)], NO, [Cr(Cp)2(NO)4], NO
- The first and fourth compounds are simple diatomic molecules of NO, so they have the highest stretching frequency of NO.
- [NiCp(NO)] is a complex containing NO group coordinated to nickel. The presence of nickel as a transition metal shifts the stretching frequency of NO to a lower frequency.
- [Cr(Cp)2(NO)4] is a complex containing NO
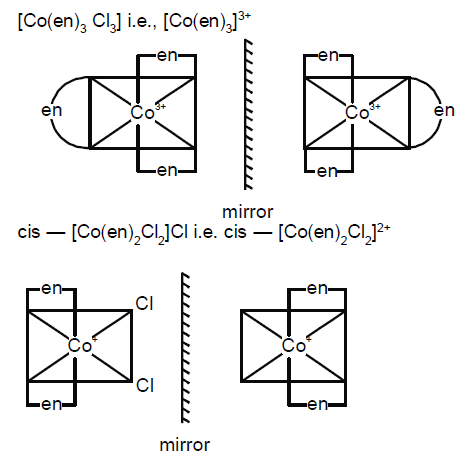
Which one of the following has an optical isomer:- a)[Co(H2O)4(en)]3+
- b)[CoCl(NH3)5]Cl2
- c)[Zn(en)(NH3)2]2+
- d)[Co(en)3]3+
Correct answer is option 'D'. Can you explain this answer?
Which one of the following has an optical isomer:
a)
[Co(H2O)4(en)]3+
b)
[CoCl(NH3)5]Cl2
c)
[Zn(en)(NH3)2]2+
d)
[Co(en)3]3+

|
Ishaan Sengupta answered |
Optical Isomerism in Coordination Compounds
In coordination compounds, optical isomerism arises due to the presence of a chiral center in the coordination sphere. A chiral center is a carbon atom that is attached to four different groups. In coordination compounds, the chiral center can be a metal ion, a ligand, or a combination of both.
Answer and Explanation:
The correct answer is option 'D' [Co(en)3]3.
- In this complex, there are three bidentate ethylenediamine (en) ligands attached to the central cobalt ion.
- Each ethylenediamine ligand has two nitrogen atoms that can act as donor atoms to form coordination bonds with the cobalt ion.
- Since the two nitrogen atoms in the ethylenediamine ligand are different, the complex has a chiral center.
- The cobalt ion is surrounded by three chiral ligands, which gives rise to optical isomerism.
- The complex exists in two enantiomeric forms that are non-superimposable mirror images of each other.
- These two enantiomers rotate plane-polarized light in opposite directions and are therefore optically active.
Hence, the correct option is D.
In coordination compounds, optical isomerism arises due to the presence of a chiral center in the coordination sphere. A chiral center is a carbon atom that is attached to four different groups. In coordination compounds, the chiral center can be a metal ion, a ligand, or a combination of both.
Answer and Explanation:
The correct answer is option 'D' [Co(en)3]3.
- In this complex, there are three bidentate ethylenediamine (en) ligands attached to the central cobalt ion.
- Each ethylenediamine ligand has two nitrogen atoms that can act as donor atoms to form coordination bonds with the cobalt ion.
- Since the two nitrogen atoms in the ethylenediamine ligand are different, the complex has a chiral center.
- The cobalt ion is surrounded by three chiral ligands, which gives rise to optical isomerism.
- The complex exists in two enantiomeric forms that are non-superimposable mirror images of each other.
- These two enantiomers rotate plane-polarized light in opposite directions and are therefore optically active.
Hence, the correct option is D.
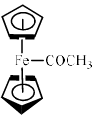
The reaction of acetyl chloride and AlCl3 with ferrocene gives:- a)
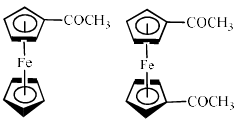
- b)
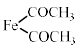
- c)
- d)FeCl3 and Al(COCl3)3
Correct answer is option 'A'. Can you explain this answer?
The reaction of acetyl chloride and AlCl3 with ferrocene gives:
a)
b)
c)
d)
FeCl3 and Al(COCl3)3

|
Kalpana Pandey answered |
Because in option (a) there are 18 electrons and therefore it is most stable ..
Regarding the catalytic cycle of hydrogenation of alkanes involving RhCl(PPh3)3 as t he catalyst, the correct statement is:- a)Only 18-electron Rh complex is involved
- b)14-and 16-and 18-electron Rh complexes are involved
- c)14-and 16-electron Rh complexes are involved
- d)16-and 18-electron Rh complexes are involved
Correct answer is option 'D'. Can you explain this answer?
Regarding the catalytic cycle of hydrogenation of alkanes involving RhCl(PPh3)3 as t he catalyst, the correct statement is:
a)
Only 18-electron Rh complex is involved
b)
14-and 16-and 18-electron Rh complexes are involved
c)
14-and 16-electron Rh complexes are involved
d)
16-and 18-electron Rh complexes are involved

|
Yashvi Roy answered |
Catalytic cycle of hydrogenation of alkanes involving RhCl(PPh3)3 as the catalyst:
Formation of 16-electron Rh intermediate:
- RhCl(PPh3)3 acts as a source of Rh(I) ion that reacts with H2 to form Rh(H)Cl(PPh3)3.
- The Rh(H)Cl(PPh3)3 intermediate reacts with alkane to form Rh(alkane)(Cl)(PPh3)2, which is a 16-electron Rh intermediate.
Formation of 18-electron Rh intermediate:
- The 16-electron Rh intermediate then reacts with H2 to form a dihydride intermediate, Rh(H)2(alkane)(Cl)(PPh3)2.
- The dihydride intermediate then reacts with another H2 to form the 18-electron Rh intermediate, Rh(alkane)(H)2(Cl)(PPh3)2.
Product formation:
- The 18-electron Rh intermediate then reacts with another molecule of alkane to release the product, along with the regeneration of the 16-electron Rh intermediate.
- The 16-electron Rh intermediate can then undergo another cycle of hydrogenation with H2 to form the dihydride intermediate and then the 18-electron intermediate, leading to the formation of more product.
Therefore, in the catalytic cycle of hydrogenation of alkanes involving RhCl(PPh3)3 as the catalyst, both 16-electron and 18-electron Rh complexes are involved. Hence, option 'D' is the correct answer.
Formation of 16-electron Rh intermediate:
- RhCl(PPh3)3 acts as a source of Rh(I) ion that reacts with H2 to form Rh(H)Cl(PPh3)3.
- The Rh(H)Cl(PPh3)3 intermediate reacts with alkane to form Rh(alkane)(Cl)(PPh3)2, which is a 16-electron Rh intermediate.
Formation of 18-electron Rh intermediate:
- The 16-electron Rh intermediate then reacts with H2 to form a dihydride intermediate, Rh(H)2(alkane)(Cl)(PPh3)2.
- The dihydride intermediate then reacts with another H2 to form the 18-electron Rh intermediate, Rh(alkane)(H)2(Cl)(PPh3)2.
Product formation:
- The 18-electron Rh intermediate then reacts with another molecule of alkane to release the product, along with the regeneration of the 16-electron Rh intermediate.
- The 16-electron Rh intermediate can then undergo another cycle of hydrogenation with H2 to form the dihydride intermediate and then the 18-electron intermediate, leading to the formation of more product.
Therefore, in the catalytic cycle of hydrogenation of alkanes involving RhCl(PPh3)3 as the catalyst, both 16-electron and 18-electron Rh complexes are involved. Hence, option 'D' is the correct answer.
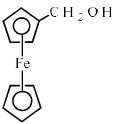
Ferrocene undergoes Vilsmeir reaction to yield:- a)
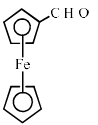
- b)
- c)
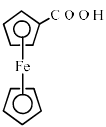
- d)
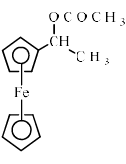
Correct answer is option 'A'. Can you explain this answer?
Ferrocene undergoes Vilsmeir reaction to yield:
a)
b)
c)
d)

|
Pie Academy answered |
Ferrocene reacts through the Vilsmeier reaction to produce a range of products. This reaction is typically used to introduce formyl groups into aromatic compounds. In the case of ferrocene, the following points highlight the process:
- The Vilsmeier reaction involves the use of phosphorus oxychloride and dimethylformamide (DMF).
- During the reaction, the ferrocene undergoes electrophilic substitution.
- This leads to the formation of a substituted product, which can be further analysed.
- The reaction conditions can influence the position and number of substitutions on the ferrocene ring.
Through this process, ferrocene can yield various substituted derivatives, expanding its potential applications in organic synthesis and materials science.
Which second row transition metal is present in the following compound: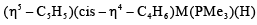
- a)Zr
- b)Nb
- c)Mo
- d)R4
Correct answer is option 'C'. Can you explain this answer?
Which second row transition metal is present in the following compound:
a)
Zr
b)
Nb
c)
Mo
d)
R4

|
Edurev.iitjam answered |
The given complex is written as:
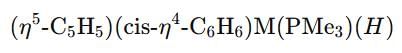
This indicates a metal complex with cyclopentadienyl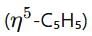 and cyclohexadienyl
and cyclohexadienyl 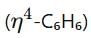 ligands, along with phosphine (PMe3) and hydride (H) groups. The central metal is likely a second-row transition metal from the periodic table, and the coordination environment suggests it is from group 6.
ligands, along with phosphine (PMe3) and hydride (H) groups. The central metal is likely a second-row transition metal from the periodic table, and the coordination environment suggests it is from group 6.
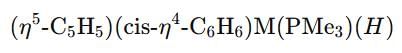
This indicates a metal complex with cyclopentadienyl
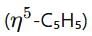 and cyclohexadienyl
and cyclohexadienyl 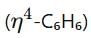 ligands, along with phosphine (PMe3) and hydride (H) groups. The central metal is likely a second-row transition metal from the periodic table, and the coordination environment suggests it is from group 6.
ligands, along with phosphine (PMe3) and hydride (H) groups. The central metal is likely a second-row transition metal from the periodic table, and the coordination environment suggests it is from group 6.- Zr (Zirconium) and Nb (Niobium) are in the 4th and 5th rows, respectively.
- Mo (Molybdenum) is a second-row transition metal in group 6, which fits the given compound, and it is known to form complexes with the described ligands.
- R₄ is not a valid metal, so it is not an option.
Thus, the correct second-row transition metal in the complex is Mo (Molybdenum), making Option C the correct answer.
The homogeneous catalyst that is used in the hydroformylation or hydrocarbonylation is based on:- a)Co
- b)Cr
- c)Ti
- d)V
Correct answer is option 'A'. Can you explain this answer?
The homogeneous catalyst that is used in the hydroformylation or hydrocarbonylation is based on:
a)
Co
b)
Cr
c)
Ti
d)
V

|
Pranavi Mishra answered |
Homogeneous Catalyst for Hydroformylation
Hydroformylation, also known as oxo synthesis, is a chemical reaction that involves the addition of a formyl group (-CHO) and a hydrogen atom to an unsaturated compound. The reaction is catalyzed by a homogeneous catalyst, which is a soluble compound that is present in the same phase as the reactants. The most commonly used homogeneous catalyst for hydroformylation is based on cobalt.
Cobalt-Based Homogeneous Catalyst
The cobalt-based homogeneous catalyst is typically a complex of cobalt with a phosphine ligand, such as triphenylphosphine (PPh3) or tris(2-diphenylphosphinoethyl)amine (PNP). The cobalt-phosphine complex is soluble in the reaction medium, which is typically a mixture of carbon monoxide (CO), hydrogen (H2), and an unsaturated compound, such as an alkene or an alkyne.
Mechanism of Hydroformylation
The mechanism of hydroformylation involves the activation of the CO and H2 molecules by the cobalt-phosphine complex, followed by the insertion of the unsaturated compound into the cobalt-carbon bond. The resulting intermediate undergoes a series of migratory insertions and eliminations to yield the final product, which is a mixture of aldehydes with different chain lengths.
Advantages of Cobalt-Based Homogeneous Catalyst
The cobalt-based homogeneous catalyst has several advantages over other catalysts, such as rhodium or iridium. These include:
- Low cost: Cobalt is abundant and relatively cheap compared to other metals.
- High activity: The cobalt-phosphine complex is highly active and selective for hydroformylation.
- Wide substrate scope: The cobalt-based catalyst can be used for a wide range of unsaturated compounds, including linear and branched alkenes and alkynes.
- Mild reaction conditions: Hydroformylation can be carried out at moderate temperatures and pressures, which reduces the energy and cost of the process.
In conclusion, the cobalt-based homogeneous catalyst is the most commonly used catalyst for hydroformylation. It offers several advantages over other catalysts, including low cost, high activity, wide substrate scope, and mild reaction conditions.
Hydroformylation, also known as oxo synthesis, is a chemical reaction that involves the addition of a formyl group (-CHO) and a hydrogen atom to an unsaturated compound. The reaction is catalyzed by a homogeneous catalyst, which is a soluble compound that is present in the same phase as the reactants. The most commonly used homogeneous catalyst for hydroformylation is based on cobalt.
Cobalt-Based Homogeneous Catalyst
The cobalt-based homogeneous catalyst is typically a complex of cobalt with a phosphine ligand, such as triphenylphosphine (PPh3) or tris(2-diphenylphosphinoethyl)amine (PNP). The cobalt-phosphine complex is soluble in the reaction medium, which is typically a mixture of carbon monoxide (CO), hydrogen (H2), and an unsaturated compound, such as an alkene or an alkyne.
Mechanism of Hydroformylation
The mechanism of hydroformylation involves the activation of the CO and H2 molecules by the cobalt-phosphine complex, followed by the insertion of the unsaturated compound into the cobalt-carbon bond. The resulting intermediate undergoes a series of migratory insertions and eliminations to yield the final product, which is a mixture of aldehydes with different chain lengths.
Advantages of Cobalt-Based Homogeneous Catalyst
The cobalt-based homogeneous catalyst has several advantages over other catalysts, such as rhodium or iridium. These include:
- Low cost: Cobalt is abundant and relatively cheap compared to other metals.
- High activity: The cobalt-phosphine complex is highly active and selective for hydroformylation.
- Wide substrate scope: The cobalt-based catalyst can be used for a wide range of unsaturated compounds, including linear and branched alkenes and alkynes.
- Mild reaction conditions: Hydroformylation can be carried out at moderate temperatures and pressures, which reduces the energy and cost of the process.
In conclusion, the cobalt-based homogeneous catalyst is the most commonly used catalyst for hydroformylation. It offers several advantages over other catalysts, including low cost, high activity, wide substrate scope, and mild reaction conditions.
Which of the following is correct about the basicity:(P) [Mn(CO)5]–
(Q) [Tc(CO)5]–
(R) [Re(CO)5]–- a)P > Q > R
- b)R > Q > P
- c)Q < P < R
- d)Q < R < P
Correct answer is option 'B'. Can you explain this answer?
Which of the following is correct about the basicity:
(P) [Mn(CO)5]–
(Q) [Tc(CO)5]–
(R) [Re(CO)5]–
(Q) [Tc(CO)5]–
(R) [Re(CO)5]–
a)
P > Q > R
b)
R > Q > P
c)
Q < P < R
d)
Q < R < P

|
Niti Mukherjee answered |
Basicity of [Mn(CO)5], [Tc(CO)5] and [Re(CO)5]
Basicity refers to the ability of a molecule or ion to accept a proton or donate a pair of electrons. It is determined by the presence of lone pairs of electrons on the central atom of a molecule or ion.
[P] [Mn(CO)5]
- Mn is the central atom in [Mn(CO)5].
- It has 7 valence electrons and no lone pair of electrons.
- Therefore, it cannot donate a pair of electrons or accept a proton.
- Hence, it is not basic.
[Q] [Tc(CO)5]
- Tc is the central atom in [Tc(CO)5].
- It has 7 valence electrons and no lone pair of electrons.
- Therefore, it cannot donate a pair of electrons or accept a proton.
- Hence, it is not basic.
[R] [Re(CO)5]
- Re is the central atom in [Re(CO)5].
- It has 7 valence electrons and one lone pair of electrons.
- Therefore, it can donate the lone pair of electrons to accept a proton.
- Hence, it is basic.
Therefore, the correct order of basicity is R > Q > P, which is given in option B.
Basicity refers to the ability of a molecule or ion to accept a proton or donate a pair of electrons. It is determined by the presence of lone pairs of electrons on the central atom of a molecule or ion.
[P] [Mn(CO)5]
- Mn is the central atom in [Mn(CO)5].
- It has 7 valence electrons and no lone pair of electrons.
- Therefore, it cannot donate a pair of electrons or accept a proton.
- Hence, it is not basic.
[Q] [Tc(CO)5]
- Tc is the central atom in [Tc(CO)5].
- It has 7 valence electrons and no lone pair of electrons.
- Therefore, it cannot donate a pair of electrons or accept a proton.
- Hence, it is not basic.
[R] [Re(CO)5]
- Re is the central atom in [Re(CO)5].
- It has 7 valence electrons and one lone pair of electrons.
- Therefore, it can donate the lone pair of electrons to accept a proton.
- Hence, it is basic.
Therefore, the correct order of basicity is R > Q > P, which is given in option B.
Chapter doubts & questions for Organometallic Chemistry - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Organometallic Chemistry - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup