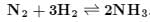All Exams >
Chemistry >
Physical Chemistry >
All Questions
All questions of Chemical Equilibrium for Chemistry Exam
Can you explain the answer of this question below:Under the equilibrium condition for the reaction, 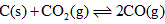 the total pressure is 12 atm. The value of Kp is:
the total pressure is 12 atm. The value of Kp is:
- A:
16
- B:
0.5
- C:
2
- D:
32
The answer is a.
Under the equilibrium condition for the reaction, the total pressure is 12 atm. The value of Kp is:
16
0.5
2
32
|
|
Aditya Deshmukh answered |
If 50% of CO2 reacts: C(s) + CO2(g) --> 2CO(g) P(total) = 12 atm If half the CO2 reacted then CO2 is x/2 and CO is x, and Pt = 12. Then x/2 + x = 12 1.5x = 12 x = 8 P(CO2) = 4 atm P(CO) = 8 atm The initial pressure of CO2 is 8 atm. When half of it reacts then 4 atm is left and the pressure of CO is twice as great, which is 8 atm. Kp = P(CO)^2 / P(CO2) Kp = 8^2 / 4 Kp = 16
Among the following, the equilibrium which is not affected by an increase in pressure is:- a)2SO3 (g) ⇔ 2SO2 (g) + O2 (g)
- b)H2 (g) + I2 (s) ⇔ 2HI (g)
- c)C(s) + H2O (g) ⇔ CO (g) + H2 (g)
- d)3Fe(s) + 4H2O (g) ⇔ Fe3O4 (s) + 4H2 (g)
Correct answer is option 'D'. Can you explain this answer?
Among the following, the equilibrium which is not affected by an increase in pressure is:
a)
2SO3 (g) ⇔ 2SO2 (g) + O2 (g)
b)
H2 (g) + I2 (s) ⇔ 2HI (g)
c)
C(s) + H2O (g) ⇔ CO (g) + H2 (g)
d)
3Fe(s) + 4H2O (g) ⇔ Fe3O4 (s) + 4H2 (g)
|
|
Vedika Singh answered |
As several moles of gaseous species are equal on both sides. Hence, for option (D), equilibrium is not affected by an increase in pressure.
Ag+(aq)+NH3(aq) 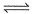 [Ag(NH3)(aq)]+ ; K, = 3.5x 10-3
[Ag(NH3)(aq)]+ ; K, = 3.5x 10-3
[Ag(NH3)]+ (aq)+NH3(aq) 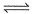 [Ag(NH3)2]2+(aq); K2 = 1.7x 10-3
[Ag(NH3)2]2+(aq); K2 = 1.7x 10-3
Formation constant of [Ag(NH3)2]+(aq) is- a)2.06
- b)5.2 x 10-3
- c)5.95 x 10-6
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Ag+(aq)+NH3(aq) 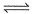 [Ag(NH3)(aq)]+ ; K, = 3.5x 10-3
[Ag(NH3)(aq)]+ ; K, = 3.5x 10-3
[Ag(NH3)]+ (aq)+NH3(aq)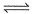 [Ag(NH3)2]2+(aq); K2 = 1.7x 10-3
[Ag(NH3)2]2+(aq); K2 = 1.7x 10-3
Formation constant of [Ag(NH3)2]+(aq) is
[Ag(NH3)]+ (aq)+NH3(aq)
Formation constant of [Ag(NH3)2]+(aq) is
a)
2.06
b)
5.2 x 10-3
c)
5.95 x 10-6
d)
None of these
|
|
Krishna Iyer answered |
The correct answer is Option C.
To get the formation constant add both reactions-
So the resultant K = K1 × K2
= 3.5×1.7×10−3
= 5.95×10−6
To get the formation constant add both reactions-
So the resultant K = K1 × K2
= 3.5×1.7×10−3
= 5.95×10−6
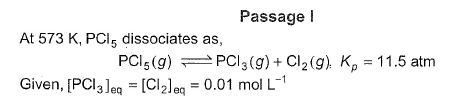 Q. What is [PCI5] eq?
Q. What is [PCI5] eq?- a)4.09 x 10-4 mol L-1
- b)2.444 x 103 mol L-1
- c)4.09 x 10-6 mol L-1
- d)2.444 x 105 mol L-1
Correct answer is option 'A'. Can you explain this answer?
Q. What is [PCI5] eq?
a)
4.09 x 10-4 mol L-1
b)
2.444 x 103 mol L-1
c)
4.09 x 10-6 mol L-1
d)
2.444 x 105 mol L-1
|
|
Riya Banerjee answered |
The correct answer is Option A.
PPCl3 = [PCl3]RT
PCl2 = [Cl2]RT
PPCl3 = [PCl3]RT
PCl2 = [Cl2]RT

Passage IIThe complex ion of Fe2+ with the chelating agent dipyridyl (abbreviated dipy) has been studied kinetically in both the forward and backward directions. For the complexion reaction,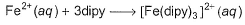
the rate of the formation of the complex at 298 K is given by rate = (1. 45 x 1013 L3mol -3s-1) [Fe 2+] [dipy]3 and for the reverse reaction , the rate of disapperance of the complex is
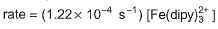 Q. What is equilibrium constant for the equilibrium ?
Q. What is equilibrium constant for the equilibrium ? 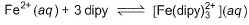
- a)8.4138x 10-18
- b)2.3770x 1017
- c)1.1885x 1017
- d)5.9426x 106
Correct answer is option 'C'. Can you explain this answer?
Passage II
The complex ion of Fe2+ with the chelating agent dipyridyl (abbreviated dipy) has been studied kinetically in both the forward and backward directions. For the complexion reaction,
the rate of the formation of the complex at 298 K is given by rate = (1. 45 x 1013 L3mol -3s-1) [Fe 2+] [dipy]3 and for the reverse reaction , the rate of disapperance of the complex is
Q. What is equilibrium constant for the equilibrium ?
a)
8.4138x 10-18
b)
2.3770x 1017
c)
1.1885x 1017
d)
5.9426x 106
|
|
Suresh Iyer answered |
The correct answer is Option C.
Fe2+ + 3 dipy -----> Fe(dipy)32+
Rf = Kf [Fe2+] [dipy]3
Rb = Kb [Fe(dipy)32+]
Keq = Kb [Fe(dipy)32+] / Kf [Fe2+] [dipy]3
Rf = Rb
Keq = Kf / Kb
= 1. 45 x 1013 / 1.24 x 10-4
= 1.885 x 1017
Fe2+ + 3 dipy -----> Fe(dipy)32+
Rf = Kf [Fe2+] [dipy]3
Rb = Kb [Fe(dipy)32+]
Keq = Kb [Fe(dipy)32+] / Kf [Fe2+] [dipy]3
Rf = Rb
Keq = Kf / Kb
= 1. 45 x 1013 / 1.24 x 10-4
= 1.885 x 1017
When NaNO3 is heated in a closed vessel, oxygen is... more liberated and NaNO2 is left behind. At equilibrium.a)Addition of NaNO2 favours reverse reaction.b)Addition of NaNO3 favours forward reaction.c)Increasing temperature favours forward reaction.d)Increasing pressure favours reverse reaction.Correct answer is option 'C,D'. Can you explain this answer?

|
Mrinalini Sen answered |
Since NaNo3 and NaNo2 are in solid state change in concentration doesn't affect the equilibrium.
For the reaction H2(g)+I2(g)⇌2HI (g) at 721 K
value of equilibrium constant is 50, when molar concentration of both hydrogen and iodine is 0.5 M at equilibrium value of KpKp under the same conditions will be- a)0.05
- b)50
- c)0.1
- d)25 RT
Correct answer is option 'B'. Can you explain this answer?
For the reaction H2(g)+I2(g)⇌2HI (g) at 721 K
value of equilibrium constant is 50, when molar concentration of both hydrogen and iodine is 0.5 M at equilibrium value of KpKp under the same conditions will be
value of equilibrium constant is 50, when molar concentration of both hydrogen and iodine is 0.5 M at equilibrium value of KpKp under the same conditions will be
a)
0.05
b)
50
c)
0.1
d)
25 RT
|
|
Vikram Kapoor answered |
The equilibrium reaction is H2(g)+I2(g)⇔2HI(g).
The relationship between Kp and Kc is Kp=Kc(RT)Δn.
For the equilibrium reaction, Δn=2−(1+1)=0.
Hence, Kp=Kc=50.
The relationship between Kp and Kc is Kp=Kc(RT)Δn.
For the equilibrium reaction, Δn=2−(1+1)=0.
Hence, Kp=Kc=50.
Consider the following gaseous equilibria given below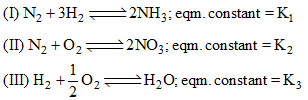 The equilibrium constant for the reaction,
The equilibrium constant for the reaction, 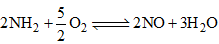 in terms of K1, K2 and K3 will be
in terms of K1, K2 and K3 will be- a)K1K2K3
- b)
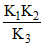
- c)
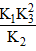
- d)
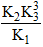
Correct answer is option 'D'. Can you explain this answer?
Consider the following gaseous equilibria given below
The equilibrium constant for the reaction, 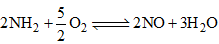 in terms of K1, K2 and K3 will be
in terms of K1, K2 and K3 will be
a)
K1K2K3
b)
c)
d)
|
|
Vikram Kapoor answered |
Correct Answer :- d
Explanation : K1 = [NH3]2/[N2] [H2]3
K2 = [NO]2/[N2] [O2]3
K3 = [H2O]/[O2]1/2 [H2]
For the reaction :
K = {[NO]2 [H2O]3}/{[NH3]2 [O2]3/2}
= (K2 K33)/K1
pH of a saturated solution of Ba(OH)2 is 12. The value of solubility product (Ksp) of Ba(OH)2 is- a)4.0 × 10–6
- b)5.0 × 10–6
- c)3.3 × 10–7
- d)5.0 × 10–7
Correct answer is option 'D'. Can you explain this answer?
pH of a saturated solution of Ba(OH)2 is 12. The value of solubility product (Ksp) of Ba(OH)2 is
a)
4.0 × 10–6
b)
5.0 × 10–6
c)
3.3 × 10–7
d)
5.0 × 10–7

|
Sagarika Patel answered |
pH + pOH = 14
pOH = 14 -12 =2 (given pH =12)
pOH = -log[OH-]
[OH-] = 10-pOH= 10-2....1
Ba(OH)2---> Ba+2+ 2OH-
At equilibrium:Ba+2= x andOH-= 2x
since 2x =10-2as eq. 1
therefore x = 10-2/2 = 0.5 * 10-2
ksp= [Ba+2]*[OH-]2= [0.5* 10-2][10-2]2= 0.5 *10-6=5 *10-7
Does Le-Chatelier’s principle predict a change of equilibrium concentrations for the following reaction if the gas mixture is compressed: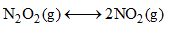
- a)Yes, backward reaction is favoured
- b)Yes, forward reaction is favoured
- c)No change in equilibrium composition is predicted
- d)No information is obtained about concentration changes
Correct answer is option 'A'. Can you explain this answer?
Does Le-Chatelier’s principle predict a change of equilibrium concentrations for the following reaction if the gas mixture is compressed:
a)
Yes, backward reaction is favoured
b)
Yes, forward reaction is favoured
c)
No change in equilibrium composition is predicted
d)
No information is obtained about concentration changes

|
Asf Institute answered |
N2O4(g)⇌2NO2(g)
One mole of N2O4 gives 2 moles of NO2 gas. The pressure of the gas increases. Upon compressing the gaseous mixture, there is a change in an equilibrium concentration & backward reaction is favoured. This is according to Le Chatlier's Principle
For the following gaseous phase equilibrium,
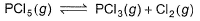
Kp is found to be equal to Kx (Kx is equilibrium constant when concentration are taken in terms of mole fraction. This is attained when pressure is - a)1 atm
- b)0.5 atm
- c)2 atm
- d)4 atm
Correct answer is option 'A'. Can you explain this answer?
For the following gaseous phase equilibrium,
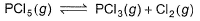
Kp is found to be equal to Kx (Kx is equilibrium constant when concentration are taken in terms of mole fraction. This is attained when pressure is
Kp is found to be equal to Kx (Kx is equilibrium constant when concentration are taken in terms of mole fraction. This is attained when pressure is
a)
1 atm
b)
0.5 atm
c)
2 atm
d)
4 atm
|
|
Preeti Iyer answered |
The correct answer is Option A.
Kp = Equilibrium constant in terms of partial pressure
Kc = Equilibrium constant in terms of concentration
Kx = Equilibrium constant in terms of mole fraction
Kp = KcRTΔn ---(1)
Kp = K * (Pt)Δn ---(2)
a) 1 atm
Given PCl5 (g) ---> PCl3 (g) + Cl2 (g)
Δn = 2 – 1
Given Kp = Kx
From (2)
Kp = Kx when PT = 1
Kp = Equilibrium constant in terms of partial pressure
Kc = Equilibrium constant in terms of concentration
Kx = Equilibrium constant in terms of mole fraction
Kp = KcRTΔn ---(1)
Kp = K * (Pt)Δn ---(2)
a) 1 atm
Given PCl5 (g) ---> PCl3 (g) + Cl2 (g)
Δn = 2 – 1
Given Kp = Kx
From (2)
Kp = Kx when PT = 1
9.2 grams of N2O4(g) is taken in a closed on litre vessel and heated till the following equilibrium is reached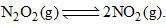 At equilibrium, 50% N2O4(g) is dissociated. What is the equilibrium constant (in mol litre–1) (molecular weight of N2O4 = 92)
At equilibrium, 50% N2O4(g) is dissociated. What is the equilibrium constant (in mol litre–1) (molecular weight of N2O4 = 92)- a)0.1
- b)0.4
- c)0.2
- d)2
Correct answer is option 'C'. Can you explain this answer?
9.2 grams of N2O4(g) is taken in a closed on litre vessel and heated till the following equilibrium is reached
At equilibrium, 50% N2O4(g) is dissociated. What is the equilibrium constant (in mol litre–1) (molecular weight of N2O4 = 92)
a)
0.1
b)
0.4
c)
0.2
d)
2

|
Nepal Dey answered |
For the reaction: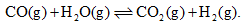 At a given temperature, the equilibrium amount of CO2(g) can be increased by.
At a given temperature, the equilibrium amount of CO2(g) can be increased by.- a)Adding a suitable catalyst
- b)Adding an inert gas
- c)Decreasing the volume of the container
- d)Increasing the amount of CO(g)
Correct answer is option 'D'. Can you explain this answer?
For the reaction:
At a given temperature, the equilibrium amount of CO2(g) can be increased by.
a)
Adding a suitable catalyst
b)
Adding an inert gas
c)
Decreasing the volume of the container
d)
Increasing the amount of CO(g)

|
Bhawesh answered |
According to the Le chatlier principle if a constraint (such as a change in pressure, temperature, or concentration of a reactant) is applied to a system in equilibrium, the equilibrium will shift so as to tend to counteract the effect of the constraint. Here if we increase the concentration of CO more CO2 will be formed.
Direction (Q. Nos. 21) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.The progress of the reaction 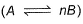 with time t is shown below.
with time t is shown below.
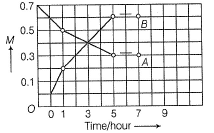
Match the parameters in Column l with their respective values in Column II.

Codes
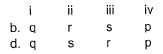
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 21) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
The progress of the reaction 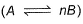 with time t is shown below.
with time t is shown below.

Match the parameters in Column l with their respective values in Column II.

Codes
Match the parameters in Column l with their respective values in Column II.
Codes

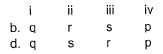
a)
a
b)
b
c)
c
d)
d
|
|
Rajesh Gupta answered |
The correct answer is Option A.
Loss in concentration of A in I hour = 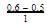 = 0.1
= 0.1
Gain in concentration of B in I hour =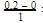 0.2
0.2
(i) ∵0.1 mole of A changes to 0.2 mole of B in a given time and thus, n=2
(ii) Equilibrium constant,
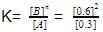 = 1.2mollitre−1
= 1.2mollitre−1
(iii) Initial rate of conversion of A = changes in conc. of A during I hour =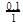
= 0.1 mol litre−1hour−1
(iv) ∵ Equilibrium is attained after 5 hr, where [B]=0.6 and [A]=0.3
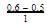 = 0.1
= 0.1Gain in concentration of B in I hour =
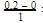 0.2
0.2(i) ∵0.1 mole of A changes to 0.2 mole of B in a given time and thus, n=2
(ii) Equilibrium constant,
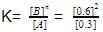 = 1.2mollitre−1
= 1.2mollitre−1(iii) Initial rate of conversion of A = changes in conc. of A during I hour =
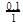
= 0.1 mol litre−1hour−1
(iv) ∵ Equilibrium is attained after 5 hr, where [B]=0.6 and [A]=0.3
For the chemical reaction,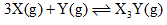 The amount of X3 Y at equilibrium is affected by
The amount of X3 Y at equilibrium is affected by- a)Temperature and pressure
- b)Temperature only
- c)Pressure only
- d)Temperature, pressure and catalyst
Correct answer is option 'A'. Can you explain this answer?
For the chemical reaction,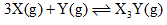
The amount of X3 Y at equilibrium is affected by
a)
Temperature and pressure
b)
Temperature only
c)
Pressure only
d)
Temperature, pressure and catalyst

|
Srishti Kulkarni answered |
There are 3 main factors that affect the equilibrium of a reaction:
- Concentration of reactants or products
- Temperature
- Pressure (Affects only gaseous reactions)
Select the correct statement (s) about the following equilibrium.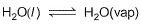
- a)At constant vapour pressure, number of molecules leaving the liquid equals the number returning to liquid from vapour
- b)This does not represent the static equilibrium
- c)This represents dynamic equilibrium and the rate of the forward and backward reactions become equal
- d)This represents dynamic as well as static equilibrium
Correct answer is option 'A,B,C'. Can you explain this answer?
Select the correct statement (s) about the following equilibrium.
a)
At constant vapour pressure, number of molecules leaving the liquid equals the number returning to liquid from vapour
b)
This does not represent the static equilibrium
c)
This represents dynamic equilibrium and the rate of the forward and backward reactions become equal
d)
This represents dynamic as well as static equilibrium
|
|
Lavanya Menon answered |
The correct answers are Options A, B and C.
During constant vapor pressure the no of molecules of H2O leaving the surface = the no of H2O molecules coming to the surface from the atmosphere. This process is dynamic as there is continuous movement of molecules.
During constant vapor pressure the no of molecules of H2O leaving the surface = the no of H2O molecules coming to the surface from the atmosphere. This process is dynamic as there is continuous movement of molecules.
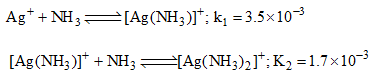 Then the formation constant of [Ag(NH3)2]+ is:
Then the formation constant of [Ag(NH3)2]+ is:- a)6.08 × 10–6
- b)6.08 × 106
- c)6.08 × 10–9
- d)None of these.
Correct answer is option 'D'. Can you explain this answer?
Then the formation constant of [Ag(NH3)2]+ is:
a)
6.08 × 10–6
b)
6.08 × 106
c)
6.08 × 10–9
d)
None of these.

|
Shoaib Ali answered |
If u add those equation than k1 and k2 goes in multiply, so u get 5.95 × 10^-6
At constant temperature, the equilibrium (Kp) for ... morethe decomposition reaction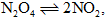 is expressed by
is expressed by  where, p = pressure,X = extent of decomposition. Which one of the following statement is true:a)Kp Increases with increase of pb)KpIncreases with increase of xc)KpIncreases with decrease of xd)KpRemains constant with change in p and x
where, p = pressure,X = extent of decomposition. Which one of the following statement is true:a)Kp Increases with increase of pb)KpIncreases with increase of xc)KpIncreases with decrease of xd)KpRemains constant with change in p and x
Correct answer is option 'D'. Can you explain this answer?

|
Aryan Choudhary answered |
Kp is a characteristic constant for a given reaction and changes only with temperature.
When a reversible reaction 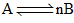 is followed with time with initial concentration of A being 0.6 mol. L–1, the following graph is obtained:
is followed with time with initial concentration of A being 0.6 mol. L–1, the following graph is obtained: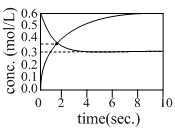 The equilibrium constant K, for the above reaction is:
The equilibrium constant K, for the above reaction is:- a)2 mol L–1
- b)1.2 mol L–1
- c)0.5 mol L–1
- d)0.4 mol L–1
Correct answer is option 'B'. Can you explain this answer?
When a reversible reaction 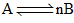 is followed with time with initial concentration of A being 0.6 mol. L–1, the following graph is obtained:
is followed with time with initial concentration of A being 0.6 mol. L–1, the following graph is obtained:
The equilibrium constant K, for the above reaction is:
a)
2 mol L–1
b)
1.2 mol L–1
c)
0.5 mol L–1
d)
0.4 mol L–1

|
Jyoti Sharma answered |
Equilibrium arrives at 0.3 mol/l and take stoichiometry of b as 2
For the complete oxidation of 100 g of Cyclohexanol to cyclohexanon, the quantity of CrO3 required is (assuming 100% chemical yield) [Atomic wt. of Cr = 52) - a)50 grams
- b)67 grams
- c)133 grams
- d)100 grams.
Correct answer is option 'D'. Can you explain this answer?
For the complete oxidation of 100 g of Cyclohexanol to cyclohexanon, the quantity of CrO3 required is (assuming 100% chemical yield) [Atomic wt. of Cr = 52)
a)
50 grams
b)
67 grams
c)
133 grams
d)
100 grams.
|
|
Vedika Singh answered |
As we know that, Cr = 52,
O3 = 16*3 = 48
CrO3 = 52 = 48
= 100 gm
O3 = 16*3 = 48
CrO3 = 52 = 48
= 100 gm
For the following equilibrium, N2O4 (g) 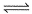 2NO2(g)Kp = KC. This is attained when
2NO2(g)Kp = KC. This is attained when- a)T = 1.0K
- b)T = 12.18 K
- c)T = 27.3 K
- d)T = 273 K
Correct answer is option 'B'. Can you explain this answer?
For the following equilibrium, N2O4 (g) 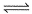 2NO2(g)
2NO2(g)
Kp = KC. This is attained when
a)
T = 1.0K
b)
T = 12.18 K
c)
T = 27.3 K
d)
T = 273 K
|
|
Riya Banerjee answered |
The correct answer is option B
KP=KC(RT)Δn
Δn=1
KP=KC(RT)
RT=1
T=1/R=1/0.0821=12.18
KP=KC(RT)Δn
Δn=1
KP=KC(RT)
RT=1
T=1/R=1/0.0821=12.18
The concentration of the oxides of nitrogen are monitored in air-pollution reports. At 25°C, the equilibrium constant for the reaction,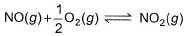
is 1.3 x 106 and that for 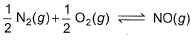
is 6.5 x 10-16 (when each species is expressed in terms of partial pressure).
For the reaction,
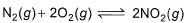
equilibrium constant is- a)
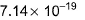
- b)
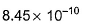
- c)
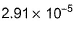
- d)
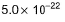
Correct answer is option 'A'. Can you explain this answer?
The concentration of the oxides of nitrogen are monitored in air-pollution reports. At 25°C, the equilibrium constant for the reaction,
is 1.3 x 106 and that for
is 6.5 x 10-16 (when each species is expressed in terms of partial pressure).
For the reaction,
equilibrium constant is
a)
b)
c)
d)
|
|
Raghav Bansal answered |
Given equations are
NO (g) + ½ O2 (g) ⇌ NO2 (g) ----------(i) k1 = 1.3×106
And ½ N2 (g) + ½ O2 (g) ⇌ NO(g) ----------(ii) k2 = 6.5×10-16
To get the reaction, N2(g) + 2O2(g) ⇌ 2NO2(g) ----------(iii) k3
We multiply eqn (i) and eqn (ii) by 2 and adding both reaction, we get eqn (iii)
k3 = k12×k22
= (1.3×106)2×(6.5×10-16)2
= 1.69×1012×42.25×10-32
= 7.14×10-19
NO (g) + ½ O2 (g) ⇌ NO2 (g) ----------(i) k1 = 1.3×106
And ½ N2 (g) + ½ O2 (g) ⇌ NO(g) ----------(ii) k2 = 6.5×10-16
To get the reaction, N2(g) + 2O2(g) ⇌ 2NO2(g) ----------(iii) k3
We multiply eqn (i) and eqn (ii) by 2 and adding both reaction, we get eqn (iii)
k3 = k12×k22
= (1.3×106)2×(6.5×10-16)2
= 1.69×1012×42.25×10-32
= 7.14×10-19
Direction (Q. Nos. 1-12) This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.Conversion factor for converting partial pressures (in Kp) to active masses (in Kc) is- a)nRT
- b)1/RT
- c)(RT)2
- d)1/RT2
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 1-12) This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.Conversion factor for converting partial pressures (in Kp) to active masses (in Kc) is
a)
nRT
b)
1/RT
c)
(RT)2
d)
1/RT2
|
|
Hansa Sharma answered |
Kp = kc(RT)∆ng
So to convert kp to kc we have, kc = kp(1/RT)∆ng.
So, the converting factor is 1/RT
So to convert kp to kc we have, kc = kp(1/RT)∆ng.
So, the converting factor is 1/RT
For the reaction, 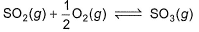
if Kp = Kc (RT)X, when the symbols have usual meaning, the value of x is (assuming ideality)[jee Main 2014]- a)-1
- b)-1/2
- c)+1/2
- d)+1
Correct answer is option 'B'. Can you explain this answer?
For the reaction, 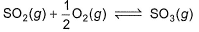
if Kp = Kc (RT)X, when the symbols have usual meaning, the value of x is (assuming ideality)
if Kp = Kc (RT)X, when the symbols have usual meaning, the value of x is (assuming ideality)
[jee Main 2014]
a)
-1
b)
-1/2
c)
+1/2
d)
+1
|
|
Gaurav Kumar answered |
The correct answer is Option B.
SO2(g) + 1/2O2(g) ⇌ SO3(g)
KP = KC(RT)Δn
SO2(g) + 1/2O2(g) ⇌ SO3(g)
KP = KC(RT)Δn
Δn= no. of gaseous moles of product minus no. of gaseous moles of reactant
Δn = 1−1−1/2
∴Δn = −1/2
Δn = 1−1−1/2
∴Δn = −1/2
For the reaction in equilibrium, A 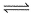 B
B
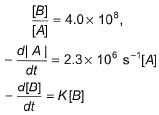
Thus, K is- a)
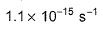
- b)
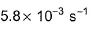
- c)
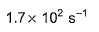
- d)
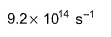
Correct answer is option 'B'. Can you explain this answer?
For the reaction in equilibrium, A  B
B
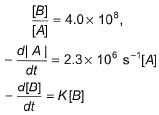
Thus, K is
Thus, K is
a)
b)
c)
d)

|
Sushil Kumar answered |
From the reaction
-d[A]/dt = d[B] /dt
⇒2.3 × 106 [A] = k [B]
as given in question [B] /[A] = 4 × 108
so [A] / [B] = 1/ 4 ×108
⇒ 2.3 × 106 . [A] /[B] = k
⇒ 2.3 × 106 / 4 × 108 = k
Or k = 5.8 × 10-3 /sec¹
-d[A]/dt = d[B] /dt
⇒2.3 × 106 [A] = k [B]
as given in question [B] /[A] = 4 × 108
so [A] / [B] = 1/ 4 ×108
⇒ 2.3 × 106 . [A] /[B] = k
⇒ 2.3 × 106 / 4 × 108 = k
Or k = 5.8 × 10-3 /sec¹
Pure ammonia is placed in a vessel at a temperature where its dissociation constant (α) is appreciable. At equilibrium,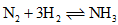
- a)Kp does not change significantly with pressure.
- b)α Does not change with pressure.
- c)Concentration of NH3 does not change with pressure.
- d)Concentration of hydrogen is less than that of nitrogen.
Correct answer is option 'A'. Can you explain this answer?
Pure ammonia is placed in a vessel at a temperature where its dissociation constant (α) is appreciable. At equilibrium,
a)
Kp does not change significantly with pressure.
b)
α Does not change with pressure.
c)
Concentration of NH3 does not change with pressure.
d)
Concentration of hydrogen is less than that of nitrogen.

|
Dheeraj Verma answered |
KP only affected by change in temperature
An example of a reversible reaction is:- a)Pb(NO3)2 (aq) + 2NaI (aq) = PbI2(s) + 2NaNO3(aq)
- b)AgNO3(aq) + HCl(aq) = AgCl(s) + HNO3(aq)
- c)2Na(s) + 2H2O(l) = 2NaOH(aq) + H2(g)
- d)KNO3(aq) + NaCl(aq) = KCl(aq) + NaNO3(aq)
Correct answer is option 'D'. Can you explain this answer?
An example of a reversible reaction is:
a)
Pb(NO3)2 (aq) + 2NaI (aq) = PbI2(s) + 2NaNO3(aq)
b)
AgNO3(aq) + HCl(aq) = AgCl(s) + HNO3(aq)
c)
2Na(s) + 2H2O(l) = 2NaOH(aq) + H2(g)
d)
KNO3(aq) + NaCl(aq) = KCl(aq) + NaNO3(aq)
|
|
Avinash Mehta answered |
Weak acids and bases may undergo reversible reactions. For example, carbonic acid and water react this way:
H2CO3 (l) + H2O(l) ⇌ HCO−3 (aq) + H3O+(aq)
Another example of a reversible reaction is:
N2O4 ⇆ 2 NO2
Two chemical reactions occur simultaneously:
N2O4 → 2 NO2
2 NO2 → N2O4
Reversible reactions do not necessarily occur at the same rate in both directions, but they do lead to an equilibrium condition. If dynamic equilibrium occurs, the product of one reaction is forming at the same rate as it is used up for the reverse reaction. Equilibrium constants are calculated or provided to help determine how much reactant and product is formed.
The equilibrium of a reversible reaction depends on the initial concentrations of the reactants and products and the equilibrium constant, K.
A 2 L vessel containing 2g of H2 gas at 27°C is connected to a 2L vessel containing 176 g of CO2 gas at 27°C. Assuming ideal behaviour of H2 and CO2, the partial pressure of H2 at equilibrium is………bar.
Correct answer is '6.25'. Can you explain this answer?
A 2 L vessel containing 2g of H2 gas at 27°C is connected to a 2L vessel containing 176 g of CO2 gas at 27°C. Assuming ideal behaviour of H2 and CO2, the partial pressure of H2 at equilibrium is………bar.

|
Raghav Rane answered |
H2 + CO2 - mixture of non-reacting gases
2g H2 = 1 mol
176g CO2 = 4 mol
Total no. of moles = 5
Total volume = 2+2 = 4L
Temperature = 27+273 = 300K
Total pressure, P=nRT/V=(5*0.083*300/4)= 31.125 bar
According to Dalton's law, p1 = P*(x1)
Mole fraction of H2=1/(1+4)=1/5
Therefore, p(H2)= 31.125*0.2 = 6.225 bar
2g H2 = 1 mol
176g CO2 = 4 mol
Total no. of moles = 5
Total volume = 2+2 = 4L
Temperature = 27+273 = 300K
Total pressure, P=nRT/V=(5*0.083*300/4)= 31.125 bar
According to Dalton's law, p1 = P*(x1)
Mole fraction of H2=1/(1+4)=1/5
Therefore, p(H2)= 31.125*0.2 = 6.225 bar
For the following equation, 2HBr(g) ⇌ H2(g) + Br2(g); are both KP and KC are equal?- a)yes
- b)cannot say
- c)no
- d)depends on the temperature
Correct answer is option 'A'. Can you explain this answer?
For the following equation, 2HBr(g) ⇌ H2(g) + Br2(g); are both KP and KC are equal?
a)
yes
b)
cannot say
c)
no
d)
depends on the temperature
|
|
Vivek Khatri answered |
We have here KC = [H2][Br2]/[HBr]2; KP = [pH2][pBr2]/[pHBr]2, where pH2 = [H2]RT, pBr2 = [Br2]RT and [pHBr] = [HBr]RT. So in this case as Δng = 0, where Δng = moles of products – moles of reactants which are in gaseous state only, both KP and KC are equal.
The pH of a solution of hydrochloric acid is 4. The molarity of the solution is:- a) 4.0
- b)0.4
- c)0.0001
- d)0.04
Correct answer is option 'C'. Can you explain this answer?
The pH of a solution of hydrochloric acid is 4. The molarity of the solution is:
a)
4.0
b)
0.4
c)
0.0001
d)
0.04

|
Aryan Choudhary answered |
Explanation:
pH and Molarity Relationship:
- The pH of a solution is a measure of its acidity or alkalinity. It is defined as the negative logarithm of the hydrogen ion concentration in moles per liter.
- The formula to calculate pH is: pH = -log[H+].
- A pH of 4 indicates a hydrogen ion concentration of 10^-4 moles per liter.
Calculating Molarity:
- The molarity (M) of a solution is defined as the number of moles of solute per liter of solution.
- Since the hydrogen ion concentration is 10^-4 moles per liter in this case, the molarity of the hydrochloric acid solution is also 10^-4 M.
- Therefore, the correct answer is option (c) 0.0001.
Summary:
- The pH of a solution of hydrochloric acid is 4, which corresponds to a hydrogen ion concentration of 10^-4 moles per liter.
- The molarity of the solution is equal to the hydrogen ion concentration, so the molarity is 0.0001 M.
pH and Molarity Relationship:
- The pH of a solution is a measure of its acidity or alkalinity. It is defined as the negative logarithm of the hydrogen ion concentration in moles per liter.
- The formula to calculate pH is: pH = -log[H+].
- A pH of 4 indicates a hydrogen ion concentration of 10^-4 moles per liter.
Calculating Molarity:
- The molarity (M) of a solution is defined as the number of moles of solute per liter of solution.
- Since the hydrogen ion concentration is 10^-4 moles per liter in this case, the molarity of the hydrochloric acid solution is also 10^-4 M.
- Therefore, the correct answer is option (c) 0.0001.
Summary:
- The pH of a solution of hydrochloric acid is 4, which corresponds to a hydrogen ion concentration of 10^-4 moles per liter.
- The molarity of the solution is equal to the hydrogen ion concentration, so the molarity is 0.0001 M.
When two reactants, A and B are mixed to give products, C and D, the reaction quotient, (Q) at the initial stages of the reaction:- a)Is zero
- b)Decreases with time
- c)Is independent of time
- d)Increases with time
Correct answer is option 'D'. Can you explain this answer?
When two reactants, A and B are mixed to give products, C and D, the reaction quotient, (Q) at the initial stages of the reaction:
a)
Is zero
b)
Decreases with time
c)
Is independent of time
d)
Increases with time

|
Juhi Sen answered |
The reaction quotient (Q) and its relation to reactants and products
The reaction quotient (Q) is a measure of the relative concentrations of reactants and products in a chemical reaction at any given point in time. It is calculated in the same way as the equilibrium constant (K), but it is determined using the concentrations of reactants and products at any moment during the reaction, rather than at equilibrium.
Initial stages of the reaction
In the initial stages of a reaction, before equilibrium is reached, the concentrations of reactants are typically higher compared to the concentrations of products. This is because the reaction has just started, and the products have not had enough time to form in significant amounts. As a result, the value of Q in the initial stages of the reaction is generally smaller than the equilibrium constant, K.
Understanding the options
a) Is zero: This option is incorrect because it implies that no products are formed at the initial stages of the reaction, which is not true. Some products will be present, although in smaller amounts compared to the reactants.
b) Decreases with time: This option is also incorrect because the value of Q does not necessarily decrease with time. It depends on the specific reaction and the rate at which reactants are converted to products.
c) Is independent of time: This option is incorrect because Q changes with time as the reaction progresses. Initially, Q will be smaller than K, but as the reaction proceeds, Q will approach the value of K.
d) Increases with time: This option is the correct answer. As the reaction progresses, reactants are consumed, and products are formed. This leads to an increase in the concentrations of products and a decrease in the concentrations of reactants. Consequently, the value of Q increases with time and approaches the value of K as equilibrium is approached.
Conclusion
In summary, the reaction quotient (Q) at the initial stages of a reaction is smaller than the equilibrium constant (K). As the reaction progresses, Q increases with time and approaches the value of K. The correct answer to the given question is option 'D'.
The reaction quotient (Q) is a measure of the relative concentrations of reactants and products in a chemical reaction at any given point in time. It is calculated in the same way as the equilibrium constant (K), but it is determined using the concentrations of reactants and products at any moment during the reaction, rather than at equilibrium.
Initial stages of the reaction
In the initial stages of a reaction, before equilibrium is reached, the concentrations of reactants are typically higher compared to the concentrations of products. This is because the reaction has just started, and the products have not had enough time to form in significant amounts. As a result, the value of Q in the initial stages of the reaction is generally smaller than the equilibrium constant, K.
Understanding the options
a) Is zero: This option is incorrect because it implies that no products are formed at the initial stages of the reaction, which is not true. Some products will be present, although in smaller amounts compared to the reactants.
b) Decreases with time: This option is also incorrect because the value of Q does not necessarily decrease with time. It depends on the specific reaction and the rate at which reactants are converted to products.
c) Is independent of time: This option is incorrect because Q changes with time as the reaction progresses. Initially, Q will be smaller than K, but as the reaction proceeds, Q will approach the value of K.
d) Increases with time: This option is the correct answer. As the reaction progresses, reactants are consumed, and products are formed. This leads to an increase in the concentrations of products and a decrease in the concentrations of reactants. Consequently, the value of Q increases with time and approaches the value of K as equilibrium is approached.
Conclusion
In summary, the reaction quotient (Q) at the initial stages of a reaction is smaller than the equilibrium constant (K). As the reaction progresses, Q increases with time and approaches the value of K. The correct answer to the given question is option 'D'.
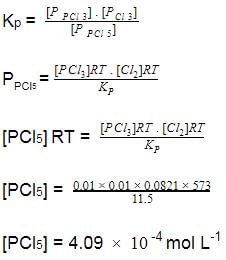
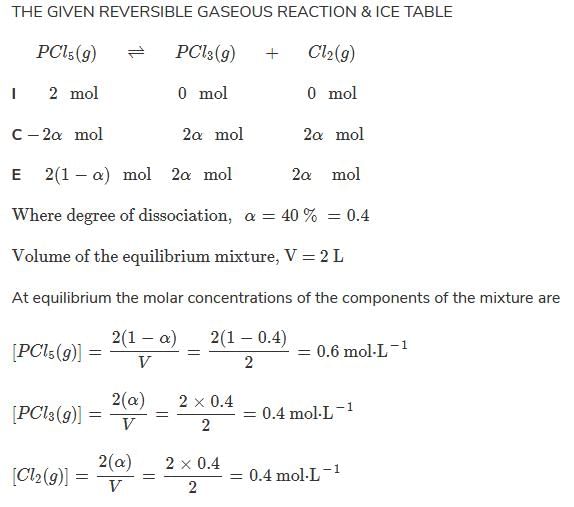
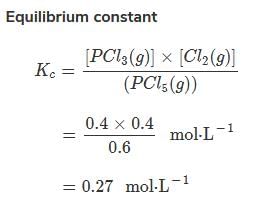
Direction (Q. Nos. 15-18) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)Passage IAt 573 K, PCI5 dissociates as,
PCI5 (g) ⇔ PCI3(g)+ Cl2 (g), Kp= 11.5 atm
Given, [PCI3]eq, = [Cl2]eq = 0.01 mol L-1
Q.
Kc of this equilibrium is- a)2.444 x 10-3
- b)4.09
- c)4.09 x 102
- d)0.244 mol L-1
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 15-18) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
Passage I
At 573 K, PCI5 dissociates as,
PCI5 (g) ⇔ PCI3(g)+ Cl2 (g), Kp= 11.5 atm
Given, [PCI3]eq, = [Cl2]eq = 0.01 mol L-1
PCI5 (g) ⇔ PCI3(g)+ Cl2 (g), Kp= 11.5 atm
Given, [PCI3]eq, = [Cl2]eq = 0.01 mol L-1
Q.
Kc of this equilibrium is
Kc of this equilibrium is
a)
2.444 x 10-3
b)
4.09
c)
4.09 x 102
d)
0.244 mol L-1

|
Maheshwar Malik answered |
Method to Solve :
If the equilibrium reaction 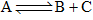 is heated, it is observed that the concentration of A increases. Then,
is heated, it is observed that the concentration of A increases. Then,- a)A must be a gas
- b)Either B or C must be solid
- c)The reaction is exothermic
- d)The reaction is endothermic
Correct answer is option 'C'. Can you explain this answer?
If the equilibrium reaction 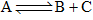 is heated, it is observed that the concentration of A increases. Then,
is heated, it is observed that the concentration of A increases. Then,
a)
A must be a gas
b)
Either B or C must be solid
c)
The reaction is exothermic
d)
The reaction is endothermic
|
|
Ramandeep Singh answered |
Basically when temperature increases,the reactant A utilizes the heat and decomposes into B and C...leading to endothermic reaction( in which heat is utilized)..
However in above case it is given that when temperature increases,concentration of A increase,it means that A is not utilizing the heat to proceed a forward reaction .....
so reaction comes to be exothermic....so correct option is C.
However in above case it is given that when temperature increases,concentration of A increase,it means that A is not utilizing the heat to proceed a forward reaction .....
so reaction comes to be exothermic....so correct option is C.
What is Kp for the equation,
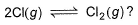
When the system contains equal number of Cl (g)atom and Cl2(g) molecules at 1 bar and 300 K?
Correct answer is '2'. Can you explain this answer?
What is Kp for the equation,
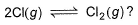
When the system contains equal number of Cl (g)atom and Cl2(g) molecules at 1 bar and 300 K?
When the system contains equal number of Cl (g)atom and Cl2(g) molecules at 1 bar and 300 K?
|
|
Lavanya Menon answered |
The correct answer is 2
2Cl(g) ⇌ Cl2(g)
Initially take moles of Cl on right hand side to be 1 then
2Cl Cl2
1 -x
=1 - x.x
kP will be equal to Kc because no. of moles are equal so
1 - x = x
2x = 1
X = ½
Kp = x/1 - x. 1 - x
= ½ ÷ (½ * ½ )
=2
2Cl(g) ⇌ Cl2(g)
Initially take moles of Cl on right hand side to be 1 then
2Cl Cl2
1 -x
=1 - x.x
kP will be equal to Kc because no. of moles are equal so
1 - x = x
2x = 1
X = ½
Kp = x/1 - x. 1 - x
= ½ ÷ (½ * ½ )
=2
Br2(l) ⇌ Br2(g) is in ________- a)homogeneous equilibrium
- b)not in both Homogeneous and heterogeneous equilibrium
- c)cannot say
- d)may or may not be in Homogeneous equilibrium
Correct answer is option 'B'. Can you explain this answer?
Br2(l) ⇌ Br2(g) is in ________
a)
homogeneous equilibrium
b)
not in both Homogeneous and heterogeneous equilibrium
c)
cannot say
d)
may or may not be in Homogeneous equilibrium

|
Vandana Gupta answered |
Explanation:
Homogeneous and Heterogeneous Equilibrium:
- In a chemical reaction, equilibrium is a state in which the forward and reverse reactions occur at equal rates.
- A homogeneous equilibrium refers to a reaction in which all the reactants and products are in the same phase (either all gases, all liquids, or all solids).
- A heterogeneous equilibrium refers to a reaction in which the reactants and products are in different phases (such as a gas and a solid, or a liquid and a solid).
The given reaction:
- The given reaction is Br2(l) ⇌ Br2(g).
- Here, the reactant (Br2) is a liquid and the product (Br2) is a gas.
- Since the reactant and product are in different phases (liquid and gas), the reaction is in a heterogeneous equilibrium.
Answer:
- The correct answer is option 'B' - the reaction is not in both homogeneous and heterogeneous equilibrium.
- As explained above, the reaction is in a heterogeneous equilibrium due to the different phases of the reactant and product.
- It is important to note that a reaction can only be in either a homogeneous or a heterogeneous equilibrium, not both at the same time.
Summary:
- The given reaction Br2(l) ⇌ Br2(g) is in a heterogeneous equilibrium because the reactant and product are in different phases. Therefore, the correct answer is option 'B'.
Homogeneous and Heterogeneous Equilibrium:
- In a chemical reaction, equilibrium is a state in which the forward and reverse reactions occur at equal rates.
- A homogeneous equilibrium refers to a reaction in which all the reactants and products are in the same phase (either all gases, all liquids, or all solids).
- A heterogeneous equilibrium refers to a reaction in which the reactants and products are in different phases (such as a gas and a solid, or a liquid and a solid).
The given reaction:
- The given reaction is Br2(l) ⇌ Br2(g).
- Here, the reactant (Br2) is a liquid and the product (Br2) is a gas.
- Since the reactant and product are in different phases (liquid and gas), the reaction is in a heterogeneous equilibrium.
Answer:
- The correct answer is option 'B' - the reaction is not in both homogeneous and heterogeneous equilibrium.
- As explained above, the reaction is in a heterogeneous equilibrium due to the different phases of the reactant and product.
- It is important to note that a reaction can only be in either a homogeneous or a heterogeneous equilibrium, not both at the same time.
Summary:
- The given reaction Br2(l) ⇌ Br2(g) is in a heterogeneous equilibrium because the reactant and product are in different phases. Therefore, the correct answer is option 'B'.
Given that for the equilibrium constants of two reactions,
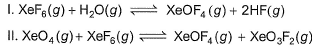
areK1 and K2. Equilibrium constant k3 of the following reaction in terms of k1, and K2.
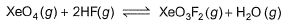
- a)K1 K2
- b)K1 / K2
- c)K2 / K1
- d)
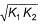
Correct answer is option 'C'. Can you explain this answer?
Given that for the equilibrium constants of two reactions,
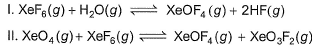
areK1 and K2. Equilibrium constant k3 of the following reaction in terms of k1, and K2.
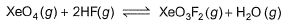
areK1 and K2. Equilibrium constant k3 of the following reaction in terms of k1, and K2.
a)
K1 K2
b)
K1 / K2
c)
K2 / K1
d)
|
|
Lavanya Menon answered |
The correct answer is Option C
XeF6(g) + H2O(g) ⇌ XeOF4(g)+2HF(g)
XeF6(g) + H2O(g) ⇌ XeOF4(g)+2HF(g)
K1 = ([XeOF4][HF]2 ) / ( [XeF6][H2O] ) ...(i)
XeO4(g) + XeF6(g) ⇌ XeOF4(g) + XeO3F2(g)
K2 = ([XeOF4][XeO3F2] ) / ( [XeO4][XeF6] ) ...(ii)
For the reaction,
XeO4(g) + 2HF(g) ⇌ XeO3F2(g) + H2O(g)
K=[XeO3F2][H2O]) / ([XeO4][HF]2 ) ...(iii)
∴ From Eqs. (i), (ii) and (iii)
K= K2 / K1
The units of KP and KC are equal.- a)true
- b)flase
Correct answer is option 'B'. Can you explain this answer?
The units of KP and KC are equal.
a)
true
b)
flase

|
Sahana Roy answered |
Explanation:
Units of KP and KC:
The equilibrium constant, KP, is used to express the equilibrium constant in terms of partial pressures of the reactants and products. The units of KP are typically expressed in atmospheres (atm) or pascals (Pa).
On the other hand, the equilibrium constant, KC, is used to express the equilibrium constant in terms of the molar concentrations of the reactants and products. The units of KC are typically expressed in moles per liter (mol/L) or molar (M).
Why the statement is false:
The statement "The units of KP and KC are equal" is false. KP and KC have different units because they are based on different properties - partial pressures and molar concentrations.
Example:
Let's consider the reaction:
A(g) + B(g) ⇌ C(g)
The equilibrium constant for this reaction can be expressed as KP or KC.
If we express the equilibrium constant as KP, it would be:
KP = (PC / P A * P B)
where PC, PA, and PB are the partial pressures of C, A, and B, respectively.
If we express the equilibrium constant as KC, it would be:
KC = (C / [A] * [B])
where C, [A], and [B] are the molar concentrations of C, A, and B, respectively.
Conclusion:
In conclusion, KP and KC have different units because they are based on different properties - partial pressures and molar concentrations. Therefore, the statement "The units of KP and KC are equal" is false.
Units of KP and KC:
The equilibrium constant, KP, is used to express the equilibrium constant in terms of partial pressures of the reactants and products. The units of KP are typically expressed in atmospheres (atm) or pascals (Pa).
On the other hand, the equilibrium constant, KC, is used to express the equilibrium constant in terms of the molar concentrations of the reactants and products. The units of KC are typically expressed in moles per liter (mol/L) or molar (M).
Why the statement is false:
The statement "The units of KP and KC are equal" is false. KP and KC have different units because they are based on different properties - partial pressures and molar concentrations.
Example:
Let's consider the reaction:
A(g) + B(g) ⇌ C(g)
The equilibrium constant for this reaction can be expressed as KP or KC.
If we express the equilibrium constant as KP, it would be:
KP = (PC / P A * P B)
where PC, PA, and PB are the partial pressures of C, A, and B, respectively.
If we express the equilibrium constant as KC, it would be:
KC = (C / [A] * [B])
where C, [A], and [B] are the molar concentrations of C, A, and B, respectively.
Conclusion:
In conclusion, KP and KC have different units because they are based on different properties - partial pressures and molar concentrations. Therefore, the statement "The units of KP and KC are equal" is false.
CO2(g) + C(s) ⇌ 2CO(g) is an example of _____________- a)homogeneous equilibrium
- b)heterogeneous equilibrium
- c)neither homogeneous nor heterogeneous
- d)both homogeneous and heterogeneous
Correct answer is option 'B'. Can you explain this answer?
CO2(g) + C(s) ⇌ 2CO(g) is an example of _____________
a)
homogeneous equilibrium
b)
heterogeneous equilibrium
c)
neither homogeneous nor heterogeneous
d)
both homogeneous and heterogeneous
|
|
Vivek Khatri answered |
In heterogeneous equilibrium, the reactants and products are present in two or more physical States or phases. Here carbon dioxide is present in the gaseous state while carbon is present in the solid state, so it is an example of heterogeneous equilibrium.
If KC of a reaction N2(g) + O2(g) ⇌ 2NO(g) is 2 x 10-3, then what is the KP?- a)4 x 10-3
- b)1 x 10-3
- c)3 x 10-3
- d)2 x 10-3
Correct answer is option 'D'. Can you explain this answer?
If KC of a reaction N2(g) + O2(g) ⇌ 2NO(g) is 2 x 10-3, then what is the KP?
a)
4 x 10-3
b)
1 x 10-3
c)
3 x 10-3
d)
2 x 10-3

|
Saanvi Roy answered |
To determine the value of KP, the equation relating KP and KC needs to be used. The equation is:
KP = KC(RT)^(Δn)
Where:
KP is the equilibrium constant in terms of partial pressures
KC is the equilibrium constant in terms of concentrations
R is the ideal gas constant
T is the temperature in Kelvin
Δn is the difference in the number of moles of gas between the products and reactants
In this reaction, the balanced equation is:
N2(g) + O2(g) ⇌ 2NO(g)
The reaction involves 2 moles of gas on the left side (N2 and O2) and 2 moles of gas on the right side (2NO). Therefore, Δn = 2 - 2 = 0.
Given that KC = 2 x 10^-3, we can use the equation to solve for KP:
KP = KC(RT)^Δn
Since Δn = 0, the equation simplifies to:
KP = KC
Substituting the given value of KC, we get:
KP = 2 x 10^-3
Therefore, the correct answer is option 'D', which is 2 x 10^-3.
KP = KC(RT)^(Δn)
Where:
KP is the equilibrium constant in terms of partial pressures
KC is the equilibrium constant in terms of concentrations
R is the ideal gas constant
T is the temperature in Kelvin
Δn is the difference in the number of moles of gas between the products and reactants
In this reaction, the balanced equation is:
N2(g) + O2(g) ⇌ 2NO(g)
The reaction involves 2 moles of gas on the left side (N2 and O2) and 2 moles of gas on the right side (2NO). Therefore, Δn = 2 - 2 = 0.
Given that KC = 2 x 10^-3, we can use the equation to solve for KP:
KP = KC(RT)^Δn
Since Δn = 0, the equation simplifies to:
KP = KC
Substituting the given value of KC, we get:
KP = 2 x 10^-3
Therefore, the correct answer is option 'D', which is 2 x 10^-3.
Consider the following equilibrium in a closed container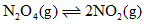 At a fixed temperature, the volume of the reaction container is halved. For this change, which of the following statements hold true regarding the equilibrium constant (Kp) and degree of dissociation (α)?
At a fixed temperature, the volume of the reaction container is halved. For this change, which of the following statements hold true regarding the equilibrium constant (Kp) and degree of dissociation (α)?- a)Neither nor change
- b)Both and change
- c)change but does not change
- d)does not change but change
Correct answer is option 'D'. Can you explain this answer?
Consider the following equilibrium in a closed container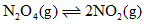
At a fixed temperature, the volume of the reaction container is halved. For this change, which of the following statements hold true regarding the equilibrium constant (Kp) and degree of dissociation (α)?
a)
Neither nor change
b)
Both and change
c)
change but does not change
d)
does not change but change

|
Ramandeep Singh answered |
Basically kp depends on temperature only change in volume doesn't influence it.however degree of dissociation depends upon volume ...so it will change on changing volume.so the answer is ....when the volume of reaction container is halved,equilibrium constant doesn't change but degree of dissociation change.....
At constant temperature, the pressure is directly proportional to the concentration of the gas.- a)true
- b)false
Correct answer is option 'A'. Can you explain this answer?
At constant temperature, the pressure is directly proportional to the concentration of the gas.
a)
true
b)
false
|
|
Vivek Khatri answered |
We have P = CRT e where p is pressure, R is a universal constant and T is the temperature, we derive the equation from the ideal gas equation PV=nRT. So from P = CRT, we can say that at a constant temperature the pressure is directly proportional to the concentration of the gas.
The equilibrium N2(g) + O2(g) ⇌ 2NO(g), is an example of _____________- a)homogeneous chemical equilibrium
- b)heterogeneous chemical equilibrium
- c)neither homogeneous nor heterogeneous
- d)both homogeneous and heterogeneous
Correct answer is option 'A'. Can you explain this answer?
The equilibrium N2(g) + O2(g) ⇌ 2NO(g), is an example of _____________
a)
homogeneous chemical equilibrium
b)
heterogeneous chemical equilibrium
c)
neither homogeneous nor heterogeneous
d)
both homogeneous and heterogeneous
|
|
Vivek Khatri answered |
In homogeneous equilibrium, the reactants and products are present in the same phase or physical state. Nitrogen, Oxygen, and nitrogen monoxide are present in a gaseous state, so it is homogeneous chemical equilibrium.
For the reaction: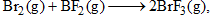 the equilibrium constant at 2000 K and 1.0 bar is 5.25. When the pressure is increased by 8-fold, the equilibrium constant:
the equilibrium constant at 2000 K and 1.0 bar is 5.25. When the pressure is increased by 8-fold, the equilibrium constant: - a)Increases by a factor of 1.86
- b)Decreases by a factor of 1.86
- c)Remains same
- d)Increases by factor of 8
Correct answer is option 'C'. Can you explain this answer?
For the reaction: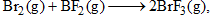 the equilibrium constant at 2000 K and 1.0 bar is 5.25. When the pressure is increased by 8-fold, the equilibrium constant:
the equilibrium constant at 2000 K and 1.0 bar is 5.25. When the pressure is increased by 8-fold, the equilibrium constant:
a)
Increases by a factor of 1.86
b)
Decreases by a factor of 1.86
c)
Remains same
d)
Increases by factor of 8

|
Monika Yadav answered |
Correct answer is 'c' .
because Keq is only affected by temperature and stoichiometry of reaction. not affected by other factors.
because Keq is only affected by temperature and stoichiometry of reaction. not affected by other factors.
A mixture of 2 mole each of helium and an unknown gas (normal boiling point = 0°C) is kept in a 22.4 L flask. If the flask is cooled to 0.1°C, the resultant pressure (in atm) inside the flask is: - a)1.0
- b)2.0
- c)3.0
- d)4.0
Correct answer is option 'D'. Can you explain this answer?
A mixture of 2 mole each of helium and an unknown gas (normal boiling point = 0°C) is kept in a 22.4 L flask. If the flask is cooled to 0.1°C, the resultant pressure (in atm) inside the flask is:
a)
1.0
b)
2.0
c)
3.0
d)
4.0

|
Radha Pani answered |
Putting the formula P= nRT/V....where n is 4 and R is 0.0821 and T is 273.1K and V is 22.4 L ...u will get 4 atm
Chapter doubts & questions for Chemical Equilibrium - Physical Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Equilibrium - Physical Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Physical Chemistry
90 videos|144 docs|67 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily