NEET Exam > NEET Questions > When nitrogen has positive charge why it don'...
Start Learning for Free
When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital?
Most Upvoted Answer
When nitrogen has positive charge why it don't have empty p orbital bu...
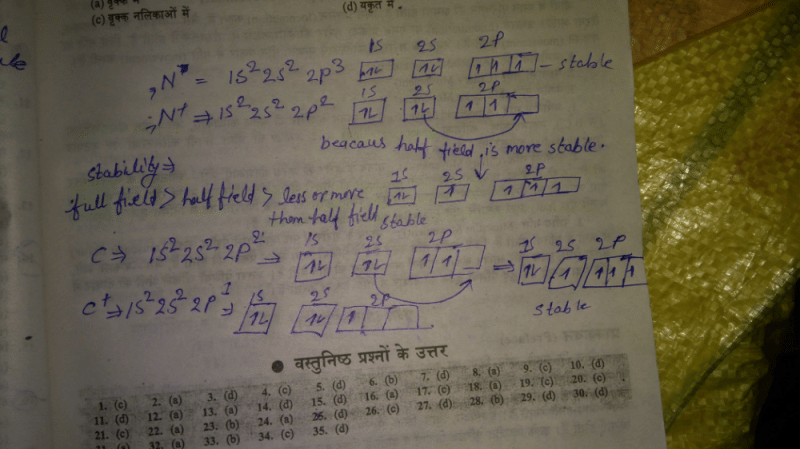
Community Answer
When nitrogen has positive charge why it don't have empty p orbital bu...
Introduction:
When atoms gain or lose electrons, they form charged species called ions. In some cases, the resulting ions may have partially filled or empty orbitals, depending on the electronic configuration of the neutral atom. In this case, we will explore why nitrogen with a positive charge does not have an empty p orbital while carbon with a positive charge does have an empty p orbital.
Explanation:
1. Electronic Configuration:
The electronic configuration of neutral nitrogen (N) is 1s² 2s² 2p³, and neutral carbon (C) is 1s² 2s² 2p². When nitrogen gains a positive charge, it loses three electrons, resulting in 1s² 2s² 2p². On the other hand, when carbon gains a positive charge, it loses two electrons, leading to 1s² 2s² 2p³.
2. Formation of Ions:
When neutral nitrogen forms a cation, it loses electrons from the 2p orbital, specifically from the three p orbitals (px, py, and pz). Due to the loss of these electrons, the 2p orbitals are still partially filled with one electron each, and there is no empty p orbital. Therefore, a positively charged nitrogen ion does not have an empty p orbital.
On the other hand, when neutral carbon forms a cation, it loses electrons from the 2s orbital and one of the 2p orbitals. As a result, there is one remaining electron in the 2p orbital, leaving it empty. Hence, a positively charged carbon ion has an empty p orbital.
3. Stability and Hybridization:
The formation of ions involves the redistribution of electrons, resulting in changes in the electronic configuration. In the case of nitrogen, losing three electrons from the 2p orbital would require a significant amount of energy due to the relatively high stability of a half-filled p orbital. Therefore, nitrogen tends to prefer alternative forms of bonding, such as covalent bonding, rather than losing enough electrons to form a cation.
Carbon, on the other hand, readily forms cations by losing two electrons from the 2s and 2p orbitals. As a result, the empty p orbital in the positively charged carbon ion can participate in bonding with other atoms.
Conclusion:
In summary, when nitrogen gains a positive charge, it does not have an empty p orbital due to the stability associated with a half-filled p orbital. On the contrary, carbon with a positive charge has an empty p orbital, allowing it to form cations and participate in bonding. The electronic configuration and stability considerations play a crucial role in determining whether an ion has empty or partially filled orbitals.
When atoms gain or lose electrons, they form charged species called ions. In some cases, the resulting ions may have partially filled or empty orbitals, depending on the electronic configuration of the neutral atom. In this case, we will explore why nitrogen with a positive charge does not have an empty p orbital while carbon with a positive charge does have an empty p orbital.
Explanation:
1. Electronic Configuration:
The electronic configuration of neutral nitrogen (N) is 1s² 2s² 2p³, and neutral carbon (C) is 1s² 2s² 2p². When nitrogen gains a positive charge, it loses three electrons, resulting in 1s² 2s² 2p². On the other hand, when carbon gains a positive charge, it loses two electrons, leading to 1s² 2s² 2p³.
2. Formation of Ions:
When neutral nitrogen forms a cation, it loses electrons from the 2p orbital, specifically from the three p orbitals (px, py, and pz). Due to the loss of these electrons, the 2p orbitals are still partially filled with one electron each, and there is no empty p orbital. Therefore, a positively charged nitrogen ion does not have an empty p orbital.
On the other hand, when neutral carbon forms a cation, it loses electrons from the 2s orbital and one of the 2p orbitals. As a result, there is one remaining electron in the 2p orbital, leaving it empty. Hence, a positively charged carbon ion has an empty p orbital.
3. Stability and Hybridization:
The formation of ions involves the redistribution of electrons, resulting in changes in the electronic configuration. In the case of nitrogen, losing three electrons from the 2p orbital would require a significant amount of energy due to the relatively high stability of a half-filled p orbital. Therefore, nitrogen tends to prefer alternative forms of bonding, such as covalent bonding, rather than losing enough electrons to form a cation.
Carbon, on the other hand, readily forms cations by losing two electrons from the 2s and 2p orbitals. As a result, the empty p orbital in the positively charged carbon ion can participate in bonding with other atoms.
Conclusion:
In summary, when nitrogen gains a positive charge, it does not have an empty p orbital due to the stability associated with a half-filled p orbital. On the contrary, carbon with a positive charge has an empty p orbital, allowing it to form cations and participate in bonding. The electronic configuration and stability considerations play a crucial role in determining whether an ion has empty or partially filled orbitals.

|
Explore Courses for NEET exam
|

|
Question Description
When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital?.
When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital?.
Solutions for When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? defined & explained in the simplest way possible. Besides giving the explanation of
When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital?, a detailed solution for When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? has been provided alongside types of When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? theory, EduRev gives you an
ample number of questions to practice When nitrogen has positive charge why it don't have empty p orbital but carbon with positive charge have empty p orbital? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















