NEET Exam > NEET Questions > At 27° Ca gas is compressed suddenly such...
Start Learning for Free
At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)
- a)420 K
- b)300K
- c)– 142°C
- d)327°C
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
At 27° Ca gas is compressed suddenly such that its pressure become...
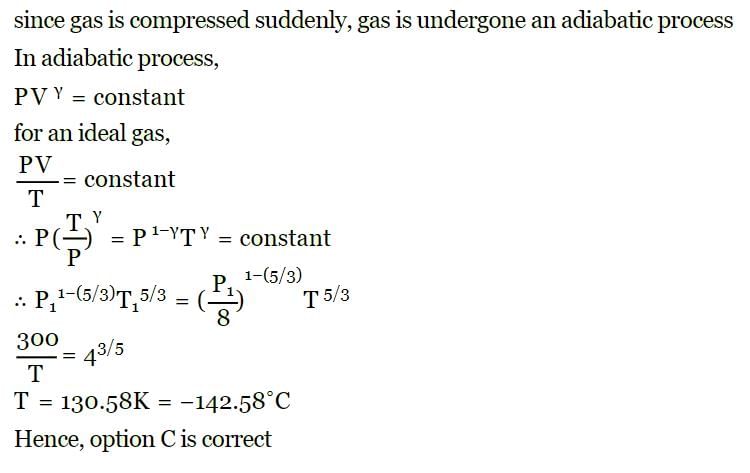
Most Upvoted Answer
At 27° Ca gas is compressed suddenly such that its pressure become...
, it's a time when many people are starting to settle into their careers, building relationships, and possibly even starting a family. Some may still be finishing up their education or exploring different career paths. It's a time of transition and growth, where individuals are discovering more about themselves and what they want out of life. It's also a time when many people start to take their health and wellness more seriously, as they begin to experience the effects of an unhealthy lifestyle. Overall, 27 is a pivotal age where individuals are setting the foundation for their future.
Free Test
FREE
| Start Free Test |
Community Answer
At 27° Ca gas is compressed suddenly such that its pressure become...


|
Explore Courses for NEET exam
|

|
Question Description
At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer?.
At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer?.
Solutions for At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)a)420 Kb)300Kc)– 142°Cd)327°CCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















