Class 12 Exam > Class 12 Questions > Industrial preparation of chloroform employs ...
Start Learning for Free
Industrial preparation of chloroform employs acetone and [1993]
- a)Phosgene
- b)Calcium hypochlorite
- c)Chlorine gas
- d)Sodium chloride.
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Industrial preparation of chloroform employs acetone and [1993]a)Phosg...
By distilling ethanol or acetone with a paste of bleaching powder (laboratory and commercial method).
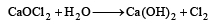
Cl2, so obtained acts as a mild oxidising as well as chlorinating agent
(a)
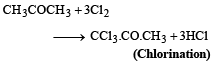
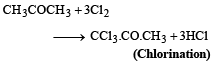
(b)
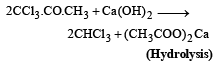
Most Upvoted Answer
Industrial preparation of chloroform employs acetone and [1993]a)Phosg...
Industrial Preparation of Chloroform
Introduction
Chloroform (CHCl3) is a colorless, heavy, volatile liquid with a sweet odor. It was widely used in the past as an anesthetic, but its use has been significantly reduced due to its harmful effects on the liver and nervous system. Chloroform is primarily prepared industrially by reacting acetone with calcium hypochlorite.
Preparation Process
The industrial preparation of chloroform involves the following steps:
1. Acetone Production: Acetone (CH3COCH3) is produced through the dehydrogenation of isopropyl alcohol. This reaction is typically carried out using metal-based catalysts, such as copper or zinc.
2. Chlorination: Acetone is then chlorinated using chlorine gas (Cl2), which leads to the formation of chloroacetone (CH3COCH2Cl). This reaction is an example of an electrophilic substitution reaction.
3. Dehydrochlorination: In the next step, chloroacetone is treated with a strong base, typically sodium hydroxide (NaOH), to remove the hydrogen chloride (HCl) and form dichloromethane (CH2Cl2).
4. Further Chlorination: Dichloromethane is then further chlorinated using chlorine gas to produce chloroform.
5. Purification: The chloroform produced in the previous step is purified by distillation to remove impurities and obtain the final product.
Calcium Hypochlorite as an Intermediate
In the given options, calcium hypochlorite (Ca(ClO)2) is the correct choice. It is used as an intermediate in the industrial preparation of chloroform. Calcium hypochlorite is a strong oxidizing agent that readily reacts with acetone to form chloroacetone, which is then further processed to produce chloroform.
The use of calcium hypochlorite as an intermediate offers several advantages:
1. Availability: Calcium hypochlorite is readily available and can be easily synthesized from calcium hydroxide and chlorine gas.
2. Cost-Effectiveness: Calcium hypochlorite is relatively inexpensive compared to other alternatives, making it a cost-effective option for industrial processes.
3. Efficiency: Calcium hypochlorite efficiently converts acetone into chloroacetone, which is an essential intermediate in the production of chloroform.
4. Controlled Reaction: The reaction between calcium hypochlorite and acetone can be controlled by adjusting the reaction conditions, such as temperature and concentration, to optimize the conversion and yield of chloroform.
Therefore, option B,
Introduction
Chloroform (CHCl3) is a colorless, heavy, volatile liquid with a sweet odor. It was widely used in the past as an anesthetic, but its use has been significantly reduced due to its harmful effects on the liver and nervous system. Chloroform is primarily prepared industrially by reacting acetone with calcium hypochlorite.
Preparation Process
The industrial preparation of chloroform involves the following steps:
1. Acetone Production: Acetone (CH3COCH3) is produced through the dehydrogenation of isopropyl alcohol. This reaction is typically carried out using metal-based catalysts, such as copper or zinc.
2. Chlorination: Acetone is then chlorinated using chlorine gas (Cl2), which leads to the formation of chloroacetone (CH3COCH2Cl). This reaction is an example of an electrophilic substitution reaction.
- CH3COCH3 + Cl2 → CH3COCH2Cl + HCl
3. Dehydrochlorination: In the next step, chloroacetone is treated with a strong base, typically sodium hydroxide (NaOH), to remove the hydrogen chloride (HCl) and form dichloromethane (CH2Cl2).
- CH3COCH2Cl + NaOH → CH2Cl2 + CH3COONa
4. Further Chlorination: Dichloromethane is then further chlorinated using chlorine gas to produce chloroform.
- CH2Cl2 + Cl2 → CHCl3 + HCl
5. Purification: The chloroform produced in the previous step is purified by distillation to remove impurities and obtain the final product.
Calcium Hypochlorite as an Intermediate
In the given options, calcium hypochlorite (Ca(ClO)2) is the correct choice. It is used as an intermediate in the industrial preparation of chloroform. Calcium hypochlorite is a strong oxidizing agent that readily reacts with acetone to form chloroacetone, which is then further processed to produce chloroform.
The use of calcium hypochlorite as an intermediate offers several advantages:
1. Availability: Calcium hypochlorite is readily available and can be easily synthesized from calcium hydroxide and chlorine gas.
2. Cost-Effectiveness: Calcium hypochlorite is relatively inexpensive compared to other alternatives, making it a cost-effective option for industrial processes.
3. Efficiency: Calcium hypochlorite efficiently converts acetone into chloroacetone, which is an essential intermediate in the production of chloroform.
4. Controlled Reaction: The reaction between calcium hypochlorite and acetone can be controlled by adjusting the reaction conditions, such as temperature and concentration, to optimize the conversion and yield of chloroform.
Therefore, option B,

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer?
Question Description
Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer?.
Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer?.
Solutions for Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Industrial preparation of chloroform employs acetone and [1993]a)Phosgeneb)Calcium hypochloritec)Chlorine gasd)Sodium chloride.Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















